Brain Markers of Resilience to Psychosis in High-Risk Individuals: A Systematic Review and Label-Based Meta-Analysis of Multimodal MRI Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review
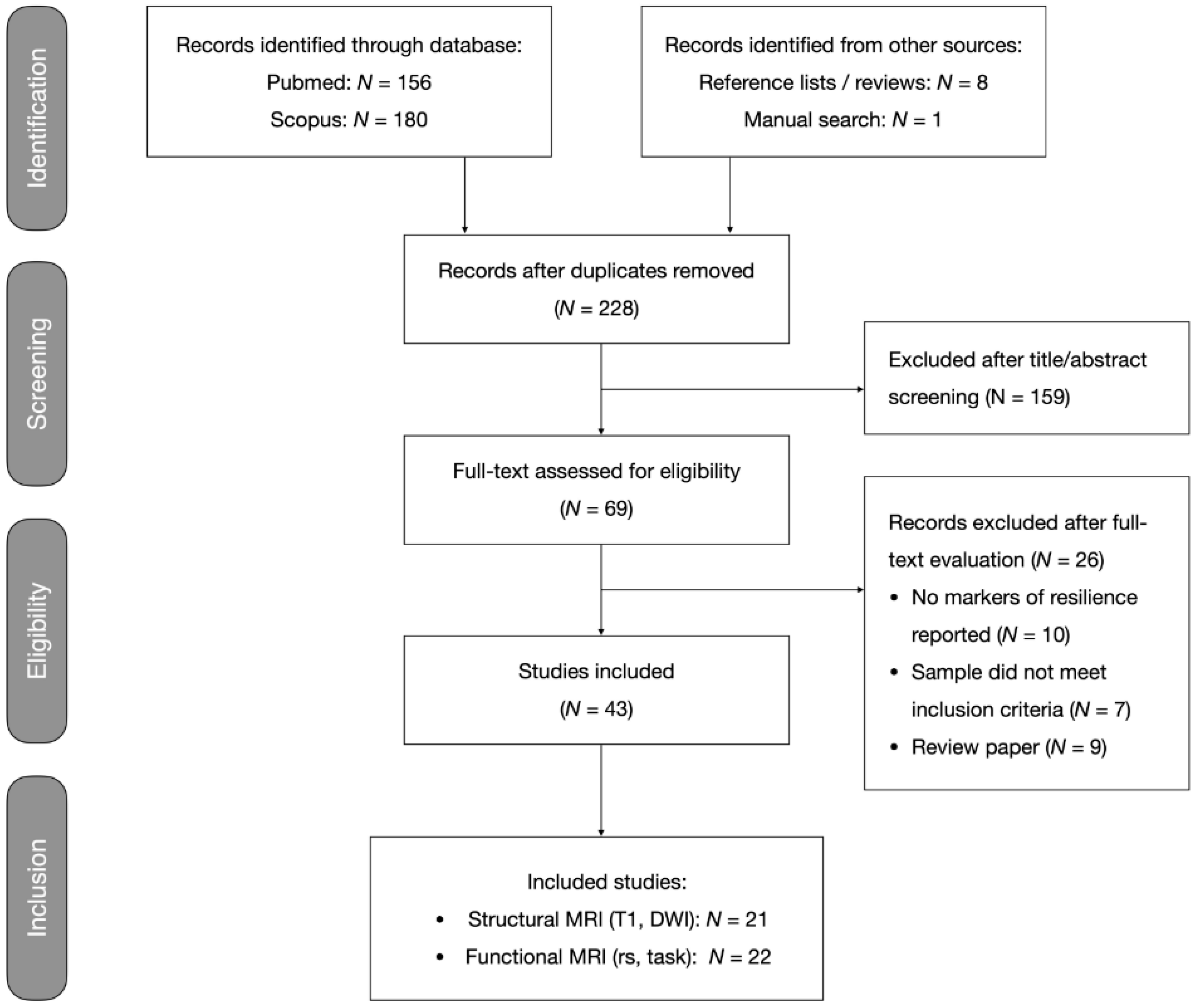
2.1.1. Search and Selection Procedure
2.1.2. Data Extraction
2.1.3. Critical Evaluation
2.2. Label-Based Meta-Analysis
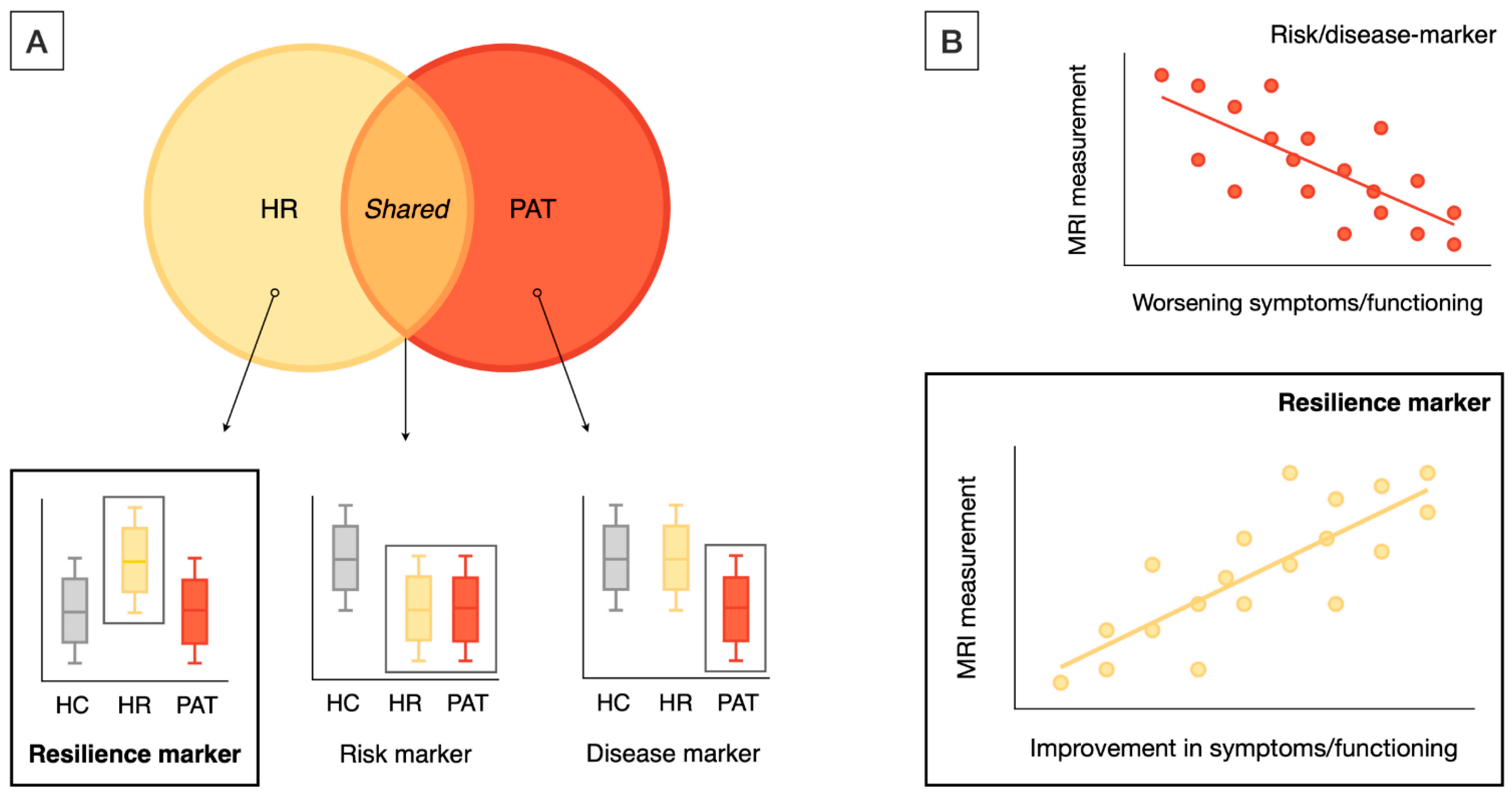
2.2.1. Regional Analysis
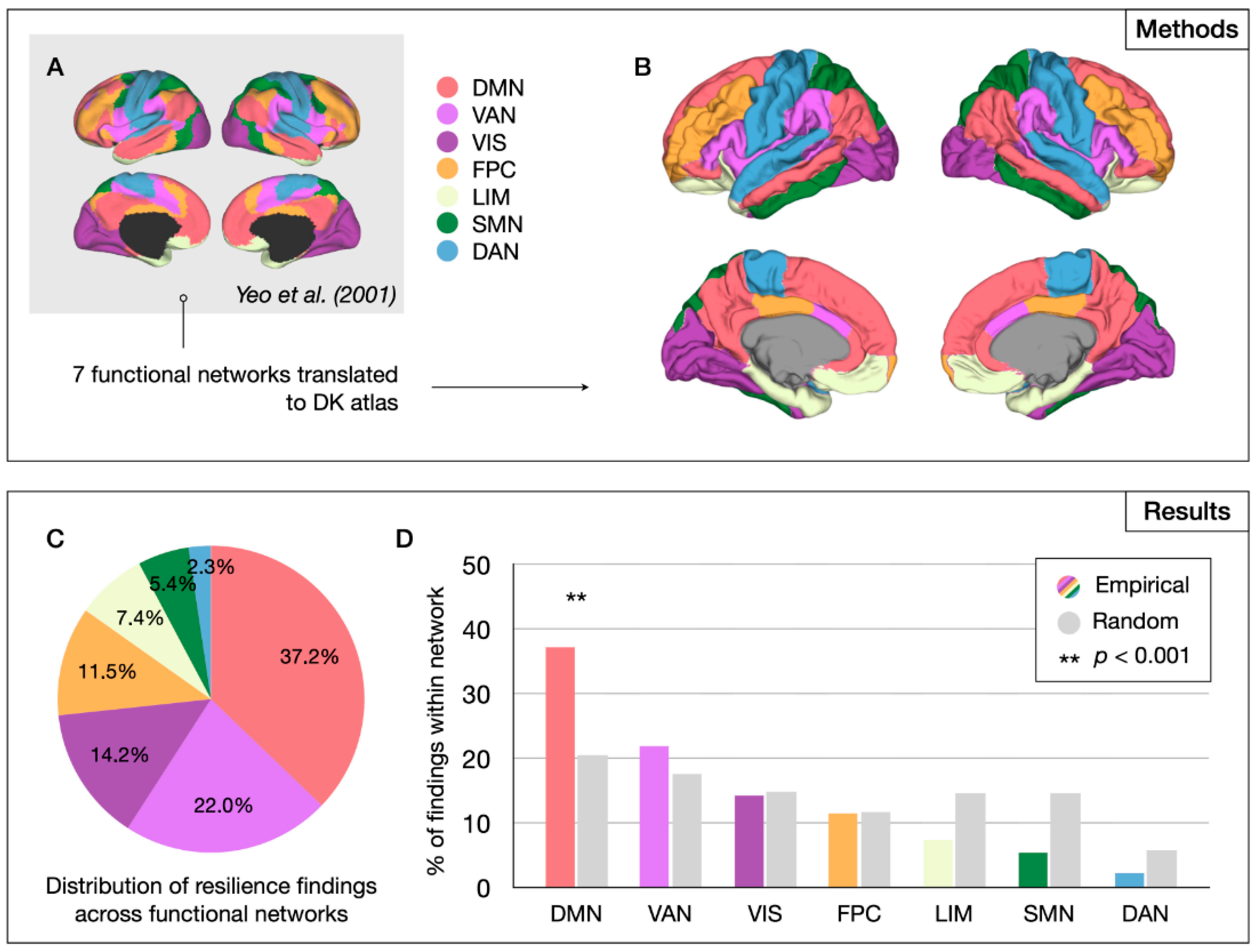
2.2.2. Network-Level Analysis
2.2.3. Graph Theoretical Analysis
2.3. Statistical Analysis
2.3.1. Regional and Network-Level Analyses
2.3.2. Graph Theoretical Meta-Analysis
3. Results
3.1. Systematic Review
3.2. Label-Based Meta-Analysis
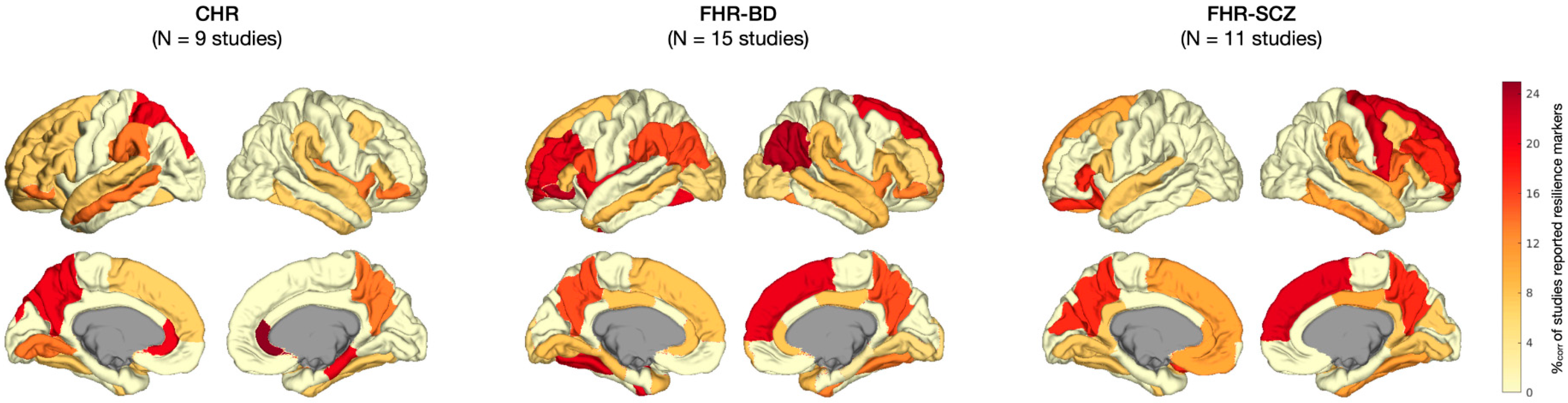
3.2.1. Regional Results
3.2.2. Network-Level Results
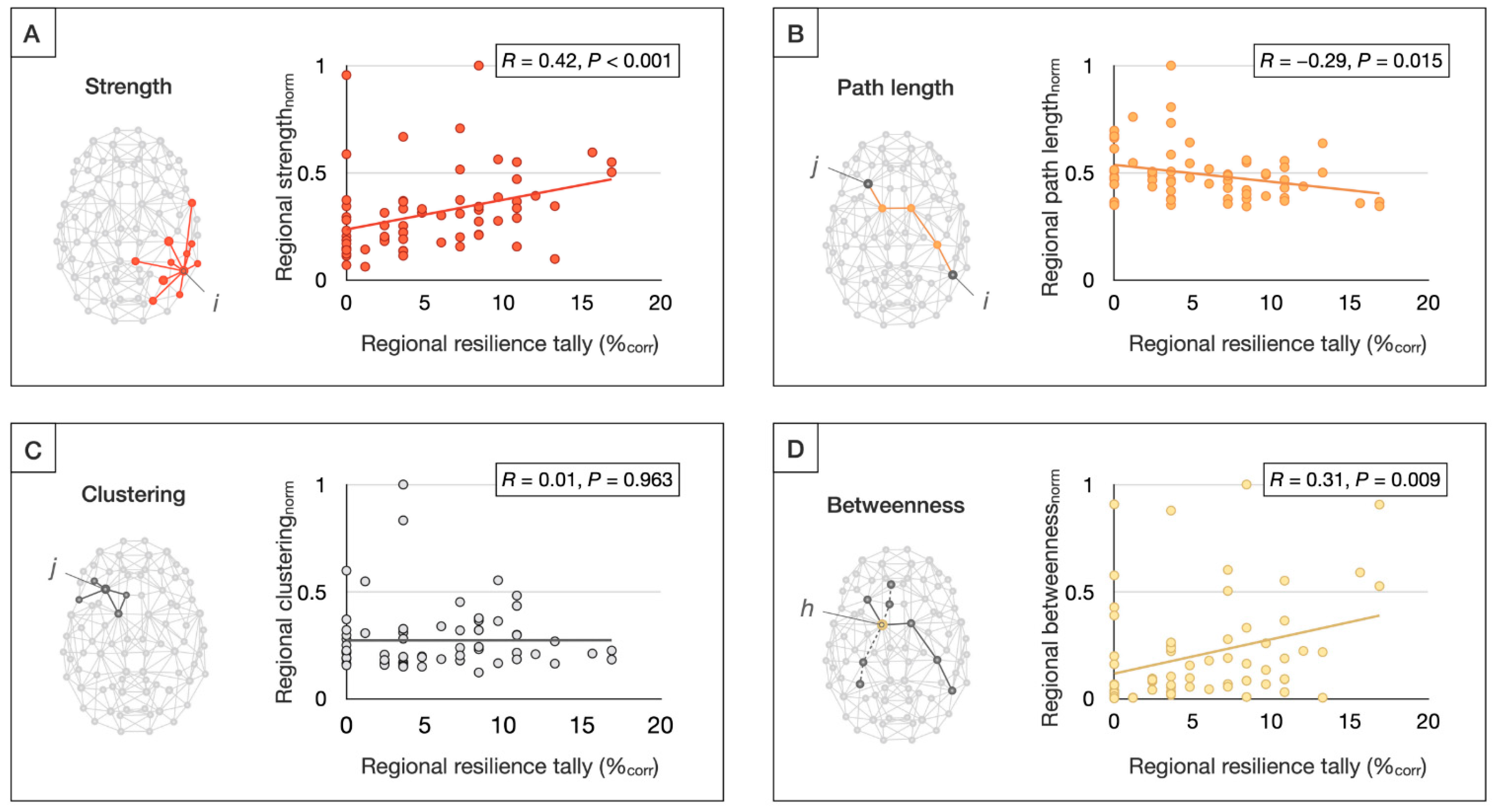
3.2.3. Graph Theoretical Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHR | Clinical high risk |
| DK | Desikan–Killiany (atlas) |
| DMN | Default mode network |
| FHR | Familial high risk |
| FG | Fusiform gyrus |
| HC | Healthy control |
| HR | High risk |
| IFG | Inferior frontal gyrus |
| MNI | Montreal Neurological Institute |
| MRI | Magnetic resonance imaging |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| Rs-fMRI | Resting-state functional MRI |
Appendix A
| Paper | Participants Details | MRI Acquisition | MRI Analysis | Statistical Analysis | Main Resilience Findings |
|---|---|---|---|---|---|
| Fornito et al., 2008 [87] | 35 resilient CHR (UHR-NP, 20 yr, 57% M) 35 non-resilient CHR (UHR-P, 19 yr, 60% M) 33 HC (21 yr, 64% M) | 1.5T GE Signa: spoiled-GRE recoil sequence, TR 14.3 ms, TE 3.3 ms, FA 30°, FOV 24 cm, and voxel size 0.938 mm2 × 1.5 mm | Skull-stripping and alignment to N27 template with FSL; FreeSurfer for segmentation and cortical surface reconstruction. | ANOVA to test regional GM volume, SA, and CT; Bonferroni-adjusted α of p < 0.0167 (α/3) for 3 pairwise comparisons (i.e., HC, resilient CHR, and non-resilient CHR). | Resilient CHR showed increased the cortical thickness of the dorsal limbic ACC and a trend-level increase in the rostral limbic ACC compared with HC, and increased the thickness of the rostral limbic ACC and subcallosal paralimbic ACC (trend-level) compared with non-resilient CHR. |
| Habets et al., 2008 [88] | 32 FHR (FDR, 36 yr, 44% M) 31 SCZ (31 yr, 48% M) 27 HC (36 yr, 44% M) | 1.5T Philips Gyro-scan NT-I1: T2- and proton-density (PD)-weighted images (dual-echo FSE sequence, TR 4 s, TE1 20 ms, TE2 100 ms, FOV 22 cm, 60 slices, 3 mm thick, and interleaved, with 0.3 mm gap) | Brain mask generated from PD images, proportions of different tissues were determined for voxels within mask, and transformation of maps into standard space using AFNI. | ANCOVA modeling the effect of cognitive scores on GM volumes fitted for each voxel, permutation testing with β/SE(β) > 2 voxel threshold, and cluster thresholds limiting false positive tests to < 1 per map. | In addition to risk-associated cerebellar gray matter deficits shared with SCZ, FHR showed increased gray matter density of a cortical region, specifically the SFG. However, FHR showed a correlation between poorer executive performance and density of the SFG, cingulate gyrus, and cerebellum. |
| Kempton et al., 2009 [89] | 50 FHR (FDR, 34 yr, 48% M, incl. 14 with MDD, 31 yr, 36% M) 30 BD (39 yr, 50% M) 52 HC (35 yr, 52% M) | 1.5T GE Signa: T1 images (3D spoiled GRE sequence, TR 18 ms, TE 5.1 ms, FA 20°, and voxel size 0.94 mm2 × 1.5 mm) | SMP5 for VBM with unified segmentation. | Regional GM volumes were assessed for group effect using ANCOVA with ICV as the covariate, using a voxel-threshold of p < 0.001 (uncorrected) and cluster threshold ≥ 5. | BD patients, healthy FHR, and FHR with MDD all showed increased left insula volume (i.e., marker of BD risk); only healthy FHR showed an increased left cerebellar (vermal) volume compared with HC and BD, which is suggestive of an association with resilience. |
| Greenstein et al., 2011 [90] | 80 FHR (sibs, 17 yr, 46% M) 94 COS (17 yr, 54% M) 110 HC (17 yr, 58% M) | 1.5T GE Signa: T1 images (spoiled GRE sequence, TR 24 ms, TE 5 ms, FA 45°, FOV 24 cm, and contiguous 1.5 mm axial slices and 2.0 mm in the coronal plane) | MR images registered to standard space using linear transformation and BRAINS2 software package to parcellate the cerebellum. | Polynomial mixed model regression to assess the cerebellar development; t-tests at two time points (mean age, last scan after age 20) to assess group effects (alpha p < 0.01). | FHR and COS showed divergent trajectories of the total cerebellar volume; only adolescent FHR had a greater superior vermis volume. Across the follow-up (2 yr intervals up to 12 years), the developmental trajectory of the superior vermis in FHR converged with HC. |
| Frangou, 2012 $ [91] | 48 FHR (FDR, 37 yr, 50% M) 47 BD (46 yr, 45% M) 71 HC (40 yr, 51% M) | 1.5T GE Neuro-optimized Signa: T1 scan (TR 1.8 s, TE 5.1 ms, TI 450 ms, FOV 4 × 18 cm, FA 20°, and voxel size 0.9 mm2 × 1.5 mm) | SPM5 for voxel-based morphometry using unified segmentation. | An ANCOVA model was used to assess the effect of the group on regional GM volumes, correcting for the ICV, with an uncorrected p < 0.001 voxel threshold and cluster threshold of 5. | In addition to putative risk markers (e.g., increased insula volume), FHR showed unique changes (relative to patients and HC), including a higher cerebellar vermis volume. Functional MRI findings from this study were also reported by Pompei et al., 2011 [92], as listed in Table A3. |
| van Erp et al., 2012 [93] | 14 FHR (co-twins, 44 yr, 36% M) 18 BD (44 yr, 44% M, incl. 10 lithium-treated and 8 non-treated) 32 HC (twins, 47 yr, 53% M) | 1.0T Siemens: T1 images (MPRAGE sequence, TR 11.4 ms, TE 4.4 ms, FOV 25 cm, and voxel size 0.98 mm2 × 1.2 mm) | Preprocessing using MNI tools (mritotal, N3). MultiTracer for hippocampal tracing; 3D radial distance mapping for hippocampal shape analysis and group comparison. | Mixed model regression to assess the hippocampal volume, SA, length, and thickness for group effects using permutation to assess the significance of statistical maps and correct for multiple comparisons. | Compared with controls, FHR co-twins showed a larger hippocampal thickness than controls along the border of the cornu ammonis and anterior subiculum, especially in the right hemisphere. These regions overlapped partly with areas that showed increased thickening in BD patients, possibly secondary to lithium-treatment mimicking resilience effects. |
| Eker et al., 2014 [94] | 28 FHR (sibs, 35 yr, 40% M) 28 BD (36 yr, 58% M) 30 HC (35 yr, 33% M) | 3.0T Siemens Magnetom Verio: T1 images (MPRAGE sequence, TR 1.6 s, TE 221 ms, TI 900 ms, FA 9°, FOV 25.6 cm, and voxel size = 1 mm3 mm). FLAIR and T2 scan to exclude brain lesions. | SPM8 for preprocessing and VBM analyses using DARTEL. | ANCOVA to assess group effects, correcting for age, sex, and ICV. Main analysis with FWE-corrected p < 0.05 and cluster > 5; exploratory analyses with uncorrected p < 0.001 and cluster > 50 in a priori selected areas. | In exploratory analyses, FHR had more gray matter in the left DLPFC than HC and BD. As this effect was unique to FHR and observed in the context of reduced gray matter in the left OFC in both FHR and BD, increased DLPFC gray matter density in FHR may reflect a compensatory response. |
| Chakravarty et al., 2015 [95] | 71 FHR (sibs, 19 yr, 48% M) 86 COS (18 yr, 58% M) 81 HC (17 yr, 67% M) | 1.5T GE Signa: T1 images (spoiled GRE sequence, TR 24 ms, TE 5 ms, FA 45°, FOV 24 cm, and axial contiguous 1.5 mm thick slices) | Striatum segmentation with MAGeT Brain algorithm; marching cubes method for surface reconstruction. | Shape measurements (vertex-wise) analyzed using mixed-model regression, adjusting for family ties using an FDR-corrected alpha. | FHR and COS both showed striatal shape changes, with outward displacement of the ventral striatum and inward displacement along the anterior head; in FHR, these striatal shape abnormalities normalized in early adulthood. |
| Goghari et al., 2015 [96] | 26 FHR (FDR, 41 yr, 35% M) 25 SCZ (41 yr, 52% M) 23 HC (40 yr, 48% M) | 3T GE Discovery MR750: T1 images (MPRAGE, TR 7.4 ms, TE 3.1 ms, FA 11°, FOV 25.6 cm, and 236 1 mm thick coronal slices) | FreeSurfer v5.1.0 for cortical reconstruction and parcellation (acc. to DK atlas). | ANCOVAs to assess group effects; univariate tests for lobes showing significant effects without further multiple comparison correction. | FHR showed greater cortical thickness of the bilateral caudal MFG, IFG (opercular and triangular part), STG, isthmus-cingulate gyrus, precuneus, cuneus, LG, left FG, and lateral OFC compared with HC and SCZ. |
| Sariçiçek et al., 2015 [97] | 25 FHR (FDR, 32 yr, 46% M) 28 BD (36 yr, 26% M) 29 HC (34 yr, 28% M) | 1.5T Philips Achieva: T1 images (FFE sequence, TR 25 ms, TE 6 ms, FA 8°, FOV 24 cm, axial slices, and 1 mm thickness) | VBM analyses in SPM8 using DARTEL. | ANCOVA for VBM group comparisons, with ICV and education as covariates; thresholds: cluster p < 0.05, voxel p < 0.01, and cluster size > 205. | Relative to HC, both FHR and BD had reduced volume of the cerebellum (incl. vermis) and increased volume of the bilateral IFG, but only FHR showed increased gray matter volume of the left SMG and parahippocampal gyrus. |
| Zalesky et al., 2015 [98] | 86 FHR (sibs, 49% M) 109 COS (57% M) 102 HC (59% M) (age range 12 to 24 yr, mean age not reported) | 1.5T GE Signa: T1 images (spoiled GRE sequence, TR 24 ms, TE 5 ms, FA 45°, FOV 24 cm, and contiguous 1.5 mm thick axial slices) | Neural net classifier to segment registered and corrected images, surface deformation algorithm, cortical thickness measured in native space, and network mapping on lobar and regional level (DK atlas). | Initial broad analysis to identify lobes with connectivity deficits, followed by localized approach focused on significant regions from the broad analysis. No further multiple comparison correction. | Risk-associated (i.e., shared with COS) deficits in CT correlations between the left occipital (pericalcarine gyrus and FG) and temporal (STG) lobes, normalized by mid-adolescence in FHR. Protracted adult-onset normalization in COS correlated with symptom improvement. In addition, FHR showed increased correlations between the right cingulate and right temporal and parietal lobes. |
| Chang et al., 2016 [99] | 31 FHR (offspr, 18 yr, 68% M) 60 SCZ (18 yr, 48% M) 71 HC (21 yr, 38% M) | 3.0T GE Signa HDX: T1 images (FSPGR sequence, TR 7.2 ms, TE 3.2 ms, FA 13°, FOV 24 cm, 176 slices, and voxels 1 mm3) | DARTEL in SPM8 for preprocessing, including segmentation, registration, and normalization to MNI template. | GM comparisons in full-factorial design, with age and sex as covariates and using voxel threshold of p < 0.01 in AlphaSim-corrected (cluster > 444 voxels), post hoc 2-sample t-tests. | FHR showed increased gray matter volumes of the right cerebellum (anterior and posterior lobe), FG, ITG, SMG, and precentral gyrus compared with both HC and SCZ (according to Table and Figure 2 as reported in Chang et al. (2016), text states differently). |
| de Wit et al., 2016 # [100] | 16 resilient CHR (UHR-remitted, 15 yr, 76% M) 19 non-resilient CHR (UHR-non-remitted, 16 yr, 56% M) 48 HC (16 yr, 60% M) (ages at the baseline, with 6 year follow-up) | 1.5T Philips: T1 images (FFE sequence, TR 30 ms, TE 4.6 ms, FA 30°, FOV 25.6 cm, and contiguous coronal slices of 1.5 mm) | FreeSurfer v5.1.0 for preprocessing and to compute the gray matter volume, CT, SA, and gyrification. Longitudinal FreeSurfer pipeline for between-session comparisons. | Effects of age, group, and their interaction were assessed using a linear mixed model; multiple comparison correction was not specified. | Resilient CHR showed an increased CT of the bilateral caudal MFG and FG; left SFG; rostral MFG; orbital IFG; lateral OFC; MTG; banks of the STS, SMG, SPG, and precuneus; right ITG and parahippocampal gyrus; and large volumes of the left precuneus lateral OFC and pallidum. There were also smaller decreases over time in the CT and volume of several areas. |
| Katagiri et al., 2018 [101] | 37 resilient CHR (ARMS-N, 24 yr, 30% M, incl. 14 med naive) 5 non-resilient CHR (ARMS-P, 18 yr, 20% M) 16 HC (23 yr, 50% M) | 1.5T Toshiba Excelart Vantage: T1 images (TR 24.4 ms, TE 5.5 ms, FA 35°, FOV 25 cm, and 35 sagittal slices of 2 mm) | FreeSurfer v5.2.0 with default processing settings for computing longitudinal corpus callosum volumes. | ANOVA testing CC sub-volume group effects; linear regression between longitudinal volume changes and symptoms (all p < 0.05, uncorrected). | While resilient CHR showed a reduction in the mid-anterior, central, and mid-posterior CCs at the baseline, subsequent volume increases in the central CC were associated with improvements in negative symptoms over the follow-up. |
| Katagiri et al., 2019 [80] | 37 resilient CHR (ARMS-N, 24 yr, 30% M, incl. 14 med naive) 5 non-resilient CHR (ARMS-P, 18 yr, 20% M) 16 HC (23 yr, 50% M) | 1.5T Toshiba Excelart Vantage: T1 images (TR 24.4 ms, TE 5.5 ms, FA 35°, FOV 25 cm, and 35 sagittal slices of 2 mm) | FreeSurfer v5.2.0 for preprocessing with standard settings for computing longitudinal striatal volumes. | ANOVAs testing striatal volume for group effects, and multiple regression to correlate the volume changes with symptoms (all p < 0.05, uncorrected). | There were no group differences in the baseline striatal volumes in resilient versus non-resilient CHR. Improvements in the positive symptoms were correlated with an increased right nucleus accumbens volume in resilient CHR. |
| Yalin et al., 2019 [102] | 24 FHR (FDR, 32 yr, 46% M) 27 BD (36 yr, 37% M) 29 HC (33 yr, 38% M) | 1.5T Philips Tesla Achieva: T1 images (FFE sequence, TR 8.7 ms, TE 4 ms, FA 8°, FOV 23 × 22 cm, and 1 mm thick slices) | FreeSurfer v5.3.0 for preprocessing and computing the regional cortical thickness and surface area (DK atlas). | Regional CT and SA group effects testedwith a GEE model to account for family ties, covarying for age, sex, and ICV (for SA), with a Bonferroni correction. | Exploratory analyses showed a significant increase in the right STG SA in FHR siblings relative to HC, whereas BD showed a trend-level increase in the SA of the STG (and a significant increase in the SA of the left IFG, triangular part). |
| Paper | Participants Details | MRI Acquisition | MRI Analysis | Statistical Analysis | Main Resilience Findings |
|---|---|---|---|---|---|
| Hoptman et al., 2008 [104] | 22 FHR (FDR, 20 yr, 32% M) 23 SCZ (37 yr, 70% M) 37 HC (23 yr, 46% M) | 1.5T Siemens Vision: T1 scan (MRPAGE, TR 11.6 ms, TE 4.9 ms, FA 8°, FOV 30.7 cm, 172 slices, and voxels 1.2 mm3); DWI scan (TR 6 s, TE 100 ms, FOV 32 cm, b-value = 1000 s/mm2, 8 non-collinear gradients, NEX 7, voxels 2.5 mm2, 19 slices of 5 mm, and no gap) | FA calculated with in-house-developed software. T1-scans skull-stripped with FreeSurfer, registered to distortion corrected b = 0 images; b = 0 and FA maps transformed to Talairach space. | Voxelwise ANOVA with age and sex as covariates, extracting clusters of >100 contiguous voxels with p < 0.05 (incl. at least 1 voxel with p < 0.001). A lower threshold of 50 contiguous voxels was used in the follow-up analysis. | FHR showed an increased FA in the left subgenual ACC, right MFG and SFG, and pontine tegmental white matter, which may represent areas that offer protection against disease onset in those at familial high risk for psychosis. |
| Kim et al., 2012 [105] | 22 FHR (FDR $, 23 yr, 36% M) 15 SCZ (23 yr, 53% M) 26 HC (22 yr, 50% M) | 1.5T Siemens Avanto: DWI scan (TR 5.9 s, TE 96 ms, FOV 23 cm, axial slices of 2 mm, no gap, voxels 1.8 mm2 × 4 mm, b-value 1000 s/mm2, and 12 non-collinear directions) | FSL preprocessing. Callosal boundaries traced with Moore–Neighbor algorithm, CC segmented into 200 equidistance surface points, and FA extracted from each. | FA of each surface point assessed for group effects with ANOVA, using age and sex as covariates and using random permutation with p < 0.01 to control the type 1 error rate. | While SCZ showed the decreased FA of CC splenium and genu (trend), FHR had an increased FA of the genu, which may reflect compensatory WM changes counteracting the influence of the genetic vulnerability to psychosis. |
| Boos et al., 2013 [58] | 123 FHR (sibs, 27 yr, 46% M) 126 SCZ (27 yr, 80% M) 109 HC (27 yr, 50% M) | 1.5T Philips Achieva: T1 scan (SPGR sequence, TR 30 ms, TE 4.6 ms, FA 30°, FOV 25.6 cm, 160–180 contiguous slices, and voxels 1 mm2 × 1.2 mm); DWI scan (32 diffusion-weighted volumes, b-factor 1000 s/mm2, 8 b = 0 volumes, TR 9.8 s, TE 88 ms, FA 90°, FOV 24 cm, 60 slices of 2.5 mm, and no gap) | DTI scans realigned, distortion-corrected, and transformed with ANIMAL software. Fiber reconstruction with FACT algorithm and the mean FA extracted per tract. | Mixed models to test 8 WM bundles for group effects in the FA while covarying for age, sex, handedness, interactions, and family ties; exploratory study without multiple comparison correction (results not significant after a correction). | FHR showed a higher FA of the bilateral arcuate fascicles relative to HC and SCZ, while the arcuate FA was negatively associated with symptom severity in SCZ. Together, these effects are suggestive of compensatory changes in the arcuate in FHR, which may guard against symptoms. |
| Goghari et al., 2014 [106] | 24 FHR (FDR, 40 yr, 42% M) 25 SCZ (41 yr, 52% M) 27 HC (41 yr, 48% M) | 3T GE: DWI scan (HARDI, 60 gradient directions with b-value 1300 s/mm2, and no other details provided) | ExploreDTI for preprocessing and deterministic tractography; seed-based tracking of fornix, in-house software for along-tract analysis. | Multiple ANCOVAs to assess the effects of group on the FA, MD, RD, and AD separately for fornix body and fimbria without multiple comparison correction; along-tract analyses were FDR corrected. | Along-tract analyses showed local increases in the FA in the right fimbria of the fornix in FHR compared with HC and SCZ, which may represent a compensatory mechanism to guard against psychosis. |
| Katagiri et al., 2015 [107] | 34 resilient CHR (ARMS-N, 24 yr, 26% M, incl. 11 untreated) 7 non-resilient CHR (ARMS-P, 21 yr, 14% M) 16 HC (23 yr, 50% M) | 1.5T Toshiba Excelart Vantage: DWI scan (single-shot EPI, TR 7.7 s, TE 100 ms, FOV 26 cm, voxels 1.02 mm2 × 5 mm, 30 axial slices along 6 gradient directions, b-value 1000 s/mm2, and unweighted b = 0 images) | Preprocessing in the FSL including distortion correction, masking, tensor fitting, and registration to standard space. TBSS for the between-group FA analysis. | Group comparisons with t-tests using randomize function in the FSL and threshold-free cluster enhancement method, with p < 0.05 and FWE correction for multiple comparisons. | At the baseline, CHR (relative to HC) showed a reduced FA of the left anterior CC. Resilient CHR showed improved subthreshold positive symptoms at one-year follow-up in association with an increase in the FA in the left anterior CC (genu). |
| Paper | Participants Details | Task | MRI Acquisition | MRI Analysis | Statistical Analysis | Main Resilience Findings |
|---|---|---|---|---|---|---|
| Working Memory (WM) | ||||||
| Fusar-Poli et al., 2010 [108] | 15 CHR (ARMS, 24 yr, 53% M, incl. 13 resilient CHR) 15 HC (25 yr, 60% M) | Paired association learning task | 1.5T Signa (GE): T2 * scan (no MR sequence reported, TR 2 s, TE 40 ms, FA 90°, 14 axial planes, 38 slices of 3 mm, and 0.3 mm gap) and high-res IR-prepped dataset (TR 1.6 s, TE 80 ms, and TI 180 ms) | Processing in SPM5; functional volumes realigned to the first volume and corrected for motion artifacts (no further details). | Full factorial model 2nd-level analysis to assess the cognitive load, group, and interaction effects. Pairwise t-tests to assess the longitudinal changes with voxel-wise threshold p < 0.05 with FWE correction. | At baseline, hypoactivation of the left precuneus, SPF, and MTG was observed in CHR, as well as a failure to activate parietal areas with increasing task difficulty. Improved clinical status at follow-up correlated with a longitudinal compensatory activation increase in the left LG and SPL. |
| Fusar-Poli et al., 2011 [109] | 15 CHR (ARMS, 24 yr, 53% M, incl. 13 resilient CHR) 15 HC (25 yr, 60% M) | N-back task (0-, 1-, or 2-back) | 1.5T Signa (GE): T2 * scan (GRE sequence, TR 2 s, TE 40 ms, FA 90°, 14 axial slices of 3 mm, and 0.3 mm gap); high-resolution inversion recovery dataset (TR 1.6 s, TE 80 ms, and TI 180 ms); T1 scan (SPGR sequence, TR 0.3 s, FA 20°, and 128 axial slices of 1.5 mm) | Functional images with SPM5; T1 scan with VBM5. Biological para-metric mapping for VBM-fMRI integration. Volumes corrected for motion artifacts, with no further details. | 2nd-level analysis to test group effects in task activation using independent sample t-test, with whole-brain voxel-wise p < 0.05 and a FWE correction. Pairwise t-tests to explore longitudinal change and assess the effect of the functional outcome. | At baseline, CHR showed reduced task-related activation of the MFG, SMG, and IPL and lower GM volumes of the middle and medial frontal gyri, insula, and ACC. Between the baseline and follow-up, CHR showed longitudinal increase in activation of the right parahippocampal gyrus and ACC, which was correlated with functional improvement. |
| Choi et al., 2012 [110] | 17 FHR (FDR #, 21 yr, 53% M) 21 CHR (UHR, 22 yr, 57% M) 15 SCZ (23 yr, 53% M) 16 HC (21 yr, 56% M) | Spatial delayed-response task | 1.5T Siemens Avanto: functional images (multi-slice EPI, TR 2.34 s, TE 41 ms, FA 90°, FOV 21 cm, and 25 axial interleaved slices); T1 scan for co-registration and anatomical localization (176 contiguous axial slices, with no other details) | Preprocessing in SPM2; volumes realigned to correct for interscan movement and stereotactically normalized. | 2-sample t-test for between-group analysis with uncorrected voxel threshold p < 0.001, with cluster size > 15. Correlation analysis with behavioral performance and clinical variables. | FHR showed higher activity of DLPFC (BA9), VLPFC (BA 44), and left thalamus during WM encoding and maintenance. CHR showed a negative correlation between the thalamus activity and symptoms. Increased WM-related activation of PFC and thalamus may constitute a compensatory mechanism in FHR. |
| Smieskova et al., 2012 [111] | 16 resilient CHR (ARMS-LT, 25 yr, 69% M) 17 non-resilient CHR (ARMS-ST, 25 yr, 77% M) 21 SCZ (29 yr, 76% M) 20 HC (27 yr, 50% M) | N-back task (0-, 1-, and 2-back) | 3T Siemens Magnetom Verio: functional images (EPI sequence, TR 2.5 s, TE 28 ms, FOV 22.8 cm, voxels 3 mm3, 38 slices, 0.5 mm gap, and 126 volumes) and T1 scan (MPRAGE, TR 2 s, TE 3.4 ms, voxels 1 mm3, and TI 1 s) | Processing in SPM8 for functional and VBM8 for structural images. Functional volumes realigned to the first volume and corrected for motion artifacts. | ANCOVA to assess effect of group on task activation, covarying for age, sex, and voxel-wise GMV to assess significance as the cluster level using random-field theory (threshold p < 0.05 with an FWE correction). | Resilient CHR (i.e., CHR-LT) had a higher activation of bilateral precuneus and right IFG/insula than SCZ and CHR-ST, with an intact N-back performance and reaction times. Insular and IFG activation were associated with GM volumes in these regions in CHR-LT and may thus reflect resilience-related processes. |
| Stäblein et al., 2018 [112] | 22 FHR (FDR, 43 yr, 36% M) 25 SCZ (37 yr, 68% M) 25 HC (35 yr, 48% M) | Masked change detection task | 3T Siemens Magnetom: T2 * scan (GRE-EPI, TR 2 s, TE 30 ms, FA 90°, FOV 19.2 cm, voxels 3 mm3, 30 slices with 0.6 mm gap, and 456 volumes in 2 runs during 1 session); and T1 scan for co-registration (MPRAGE, 160 sagittal slices, TR 2.25 s, TE 2.6 ms, FA 9°, FOV 25.6 cm, and voxels 1 mm3) | BrainVoyager QX v2.8.4; 3D head motion correction; datasets with motion exceeding 3 mm in each direction were discarded. | 2nd-level random effects repeated measures ANOVA; statistical maps FDR-corrected with cluster size > 160 and voxel threshold p < 0.01; Monte-Carlo simulation to assess cluster-level type 1 errors with p < 0.05 false positive rate. | FHR showed an increased right insula and precentral gyrus activity without behavioral deficits and a shift from decreased frontal activity at short intervals to increased activity at longer intervals, suggesting that WM consolidation may have been slowed in FHR, which allowed for the deployment of compensatory neuronal resources during encoding to support the WM performance. |
| Cognitive Control | ||||||
| Pompei et al., 2011 [92] | 25 healthy FHR (FDR, 35 yr, 52% M) 14 depressed FHR (FDR, 31 yr, 36% M) 39 BD (39 yr, 49% M) 48 HC (36 yr, 52% M) | Stroop Color Word Test (SCWT) | 1.5T GE Neuro-optimized Signa: T2 * (EPI sequence, TR 3.5 s, TE 40 ms, FA 90°, voxels 3.75 mm2 × 7 mm, 18 non-contiguous axial slices, and 0.7 mm gap); T1 scan (TR 1.8 s, TE 5.1 ms, TI 450 ms, FOV 4 × 18 cm, FA 20°, and voxels 0.9 mm2 × 1.5 mm) | SPM 5 for preprocessing and PPI analysis during SCWT; motion correction methods not reported. | One-sample t-test random effects analysis (p < 0.0001 voxel and p < 0.05 cluster thresholds) to compute the contrast images from the within-group PPI analysis. Interaction between the PPI and group analyzed to test the group effects. | Alongside putative risk markers, resilient FHR showed increased decoupling between the right VLPFC and bilateral insula and additional coupling between the right VLPFC and bilateral DLPFC, which was hypothesized to reflect an adaptive functional change associated with resilience to BD. |
| Emotion Recognition/Processing | ||||||
| Spilka et al., 2015 [113] | 27 FHR (FDR, 41 yr, 37% M) 28 SCZ (41 yr, 54% M) 27 HC (41 yr, 48% M) | Passive facial emotion perception task | 3T GE Discovery MR750: fMRI (EPI sequence, TR 2.5 s, TE 30 ms, FA 77°, FOV 22 cm, and 40 slices of 3.4 mm); T1 scan (TR 7.4 ms, TE 3.1 ms, TI 650 ms, FOV 25.6 cm, and 236 1 mm slices) | FSL v5.0.6 for preprocessing and analysis; motion parameters included as regressors of non-interest. | Subject-specific effects into mixed-effects model using unpaired t-tests for between-group comparisons, with a voxel threshold of z > 2.3 and cluster p < 0.05 with an RFT correction. | In addition to the hypoactivation of face-processing areas, FHR showed hyperactivation of frontal emotion-processing areas (left triangular IFG and OFC), possibly reflecting compensatory cortical recruitment to maintain intact facial emotion perception. |
| Sepede et al., 2015 [114] | 22 FHR (FDR, 32 yr, 32% M) 23 BD (35 yr, 39% M) 24 HC (33 yr, 33% M) | IAPS-based emotional task (identifying vegetable items inside neutral or negative pictures) | 1.5T Philips Achieva: T2 * (EPI sequence, TR 3 s, TE 50 ms, FA 90°, voxel size 4 mm3, 30 transaxial slices, and no gap); T1 scan (3D sequence, TR 25 ms, TE 4.7 ms, FA 30°, and voxels 1 mm3) | BrainVoyager QX 2.2 for processing; motion correction as part of pre-processing, with no details provided. | Group effects tested with random effect GLM, controlling for performance and mood symptoms and using voxel p < 0.001 and cluster > 4 to account for multiple comparisons. | BD patients showed reduced accuracy in target detection, while FHR performed similar to HC. Compared with both HC and BD, FHR showed hyperactivation of right LG and reduced activation of right SFG and pre-SMA; may reflect resilience markers. |
| Tseng et al., 2015 [115] | 13 FHR (FDR, 14 yr, 62% M) 27 BD (14 yr, 56% M) 37 HC (15 yr, 43% M) | Face encoding task | 3T GE: T2 * (single-shot EPI-GRE, TR 2 s, TE 40 ms, FOV 24 cm, voxels 3.75 mm2, and 23 contiguous slices of 5 mm); T1 (MPRAGE, TR 11.4 ms, TE 4.4 ms, TI 300 ms, FOV 25.6 cm, and 180 1 mm sagittal slices) | Preprocessing using SPM8 including motion (no details provided), slice timing correction, and normalization to MNI space. | Whole-brain ANOVA with group as the between-subject variable, using p < 0.001 (uncorrected) and cluster size > 10, without further mention of multiple comparisons. | Both BD and FHR showed hypo-activation of the left MFG during correctly vs. incorrectly recognized faces, but BD showed additional hypoactivation while FHR showed hyperactivation of the right parahippocampal gyrus, suggesting a possible compensatory process. |
| Dima et al., 2016 [116] | 25 FHR (FDR, 40 yr, 54% M) 41 BD (44 yr, 52% M) 46 HC (40 yr, 49% M) | Facial affect recognition paradigm | 1.5T GE Sigma: T2 * images (no MR sequence reported, TR 2 s, TE 40 ms, FA 70°, voxels 3.75 mm2 × 7.7 mm, and 450 volumes) and T1 scan (IR-prepped SPGR sequence, TR 18 ms, TE 5.1 ms, FA 20°, and voxels 0.94 mm2 × 1.5 mm) | SPM8 for preprocessing, conventional fMRI analysis, and DCM analysis; no motion correction reported. | Group effects tested using ANOVA with symptoms score as the covariate using FWE-corrected p < 0.05 and cluster size > 20; ANOVA or Kruskal–Wallis used to test the DCM output. | During face affect recognition, both BD and FHR showed higher fronto-limbic connectivity, but only FHR showed additional hyperconnectivity between the FG and IOG, suggesting additional recruitment in the affect-processing network as an adaptive neural response to emotional faces. |
| Welge et al., 2016 [117] | 32 healthy FHR (offspr, 15 yr, 28% M) 32 depressed FHR (offspr, 14 yr, 19% M) 32 BD (16 yr, 41% M) 32 HC (15 yr, 34% M) | Continuous performance task with emotional and neutral distractors (CPT-END) | 4.0T Varian Unity INOVA: T2 * images (GRE-EPI, TR 3 s, TE 29 ms, FOV 20.8 cm, FA 75°, and 5 mm thick slices) and T1 scan for anatomical localization (30 contiguous axial slices of 5 mm, with no further details) | Preprocessing and analysis in AFNI; small movement corrected via realignment; excessive motion or warped volumes removed. | A Bayesian hierarchical model was used to limit type 1 errors for pairwise comparisons between 4 clinical groups in 16 predefined ROIs. | All FHR showed greater task-related activation in left BA 44 (IFG, opercular part) relative to HC and healthy FHR showed higher activation in right BA 10 (FP) relative to BD and depressed FHR, possibly reflecting a compensatory response relevant to resilience to BD. |
| Spilka and Goghari, 2017 [118] | 27 FHR (FDR, 41 yr, 37% M) 28 SCZ (41 yr, 54% M) 27 HC (41 yr, 48% M) | Facial emotion discrimination under a target emotion condition; age discrimination task | 3T GE Discovery MR750: T2 * (GRE sequence, TR 2.5 s, TE 30 ms, FOV 22 cm, voxels 3.4 mm3, 40 interleaved slices, and 206 vols); T1 (MPRAGE, TR 7.4 ms, TE 3.1 ms, FOV 25.6 cm, and 236 1 mm slices) | Preprocessing and analysis in FSL v5.0.6; time-series plots of estimated head motion were inspected, with >3.5 mm excluded. | Between-group comparisons using unpaired non-parametric t-tests, with a voxel p < 0.001 threshold and FWE-corrected p < 0.05 cluster threshold. | FHR showed higher deactivation of bilateral precuneus and right PCC during age discrimination and of left cuneus during emotion discrimination, possibly reflecting inhibition of internally generated thought to maximize external attention toward task stimuli. |
| Wiggins et al., 2017 [119] | 22 FHR (FDR, 16 yr, 59% M) 36 BD (18 yr, 58% M) 41 HC (17 yr, 51% M) | Face Emotion Labeling Task (identifying emotions on faces with different intensities of emotions) | 3T GE MR750: fMRI (single-shot EPI-GRE, TR 2.3 s, TE 25 ms, FA 50°, FOV 24 cm, voxel size 2.5 mm2 × 2.6 mm, and 47 contiguous axial slices); T1 scan for spatial normalization (FA 15°, FOV 24 cm, and 124 axial slices of 1.2 mm) | AFNI for processing and mixed model analysis. Motion parameters included in the baseline model. TR pairs with the >1 mm frame-wise displacement censored. | Whole-brain linear mixed model with group as between-subjects factor and emotion/intensity as within-subject factors using voxel a p < 0.005 threshold and cluster ≥ 39, equivalent to an FDR-corrected p < 0.05. | In addition to changes shared with BD (risk markers), FHR showed hyperactivation of the bilateral PCC/precuneus, IFG, SFG, temporo-parietal areas, TP/insula, and left FG and hypoactivation of the left angular gyrus; this may reflect increased neural sensitivity to social cues by compensating for deficits in executive areas. |
| Nimarko et al., 2019 [120] | 27 resilient FHR (BD offspr, 13 yr, 56% M) 23 non-resilient FHR (BD offspring, 14 yr, 30% M) 24 HC (15 yr, 42% M) | Implicit emotion perception task (viewing images of happy, fearful, or calm expressions) | 3T GE Signa: fMRI (spiral in–out pulse sequence, TR 2 s, TE 30 ms, FA 80°, FOV 22 cm, 30 axial slices of 4 mm, and 1 mm gap); T1 scan for normalization (FSPGR sequence, TR 8.5 ms, TE 3.32 ms, TI 400 ms, FA 15°, FOV 25.6 cm, and 186 axial slices of 1 mm) | FSL featuring motion correction with MCFLIRT if mean displacements > 2 mm or more than 1/3 of the volumes had DVARS values > 75th percentile plus 1.5 times the interquartile range. | Group effects tested with whole-brain voxel-wise t-tests corrected for age and sex using voxel threshold z > 2.3 and cluster p < 0.05 and corrected for multiple comparisons. | Resilient FHR showed right precuneus and left IFG hypoactivation relative to non-resilient FHR, and higher connectivity between the left IFG and the left MFG, MTG, and insula for fear > calm contrast, and between the left IPL and left precuneus/LG, and the right SMG and left FG for happy > calm, which was associated with improved pro-social behavior and functioning. |
| Theory of Mind | ||||||
| Brüne et al., 2011 [121] | 10 CHR $ (26 yr, 70% M, incl. 1 converter at 1yr follow-up) 22 SCZ (27 yr, 68% M) 26 HC (29 yr, 64% M) | Theory of mind task | 1.5T Siemens Magnetom Symphony: functional images (single-shot EPI, TR 3 s, TE 60 ms, FOV 22 cm, voxels 3.5 × 3 mm3, FA 90°, 30 trans-axial slices, 0.3 mm gap, and 157 scans; T1-scan (MPRAGE, TR 1.8 s, TE 3.87 ms, FOV 25.6 cm, voxels 1 mm3, and 160 sagittal slices) | Preprocessing and analysis in SPM5 using MarsBaR toolbox to derive ROIs; no motion correction reported. | Second-level analysis to locate ToM regions with uncorrected p < 0.05 and cluster size > 10. Group effects in ToM-area activation assessed with two-sample t-test with p < 0.05 and cluster size > 10. | CHR activated the ToM network (PFC, PCC, and temporoparietal cortex) more strongly than SCZ and (in part) HC. Specifically, CHR showed increased activation of the left IFG, bilateral STG and SMG, left MTG, and HG. This may suggest the compensatory overactivation of brain regions critical for empathic responses during mental state attribution. |
| Willert et al., 2015 [122] | 21 FHR (FDR, 31 yr, 33% M) 24 BD (45 yr, 50% M) 81 HC (36 yr, 49% M) | Theory of mind task | 3T Siemens Trio: functional images (EPI sequence, TR 2 s, TE 30 ms, FA 80°, FOV 19.2 cm, 28 slices of 4 mm, and 240 volumes) | Preprocessing in SPM8 with gPPI (generalized form of context-dependent PPI), with 6 regressors modeling head motion included in 1st-level analyses. | ANCOVAs to test the activation and connectivity of 4 predefined ROIs corrected for age, sex, education, and task response, with an FWE correction for the number of ROIs. | BD patients showed reduced TPJ activation and reduced fronto-TPJ connectivity, while FHR showed increased activation of the right MTG and stronger connectivity between the right MTG and MPFC, suggesting compensatory MTG recruitment during mental state attribution in FHR. |
| Paper | Participants Details | MRI Acquisition | MRI Analysis | Statistical Analysis | Main Resilience Findings |
|---|---|---|---|---|---|
| Anticevic et al., 2014 [123] | 21 FHR (offspr, 20 yr, 47% M) 48 SCZ (28 yr, 44% M, incl. 20 chronic and 28 early course) 96 HC (29 yr, 45% M) | 3T GE Signa HDX: T2 * images (GRE-EPI, TR 2 s, TE 30 ms, FOV 24 cm, 35 axial slices of 3 mm, and 200 volumes) and T1 scan (FSPGR, TR 7.1 ms, TE 3.2 ms, FA 13°, FOV 24 cm, 176 slices of 1 mm, and no gap) | FreeSurfer to segment amygdala seed; group comparisons of connectivity seed maps with FSL; rigid-body motion correction, volumes with a single FD > 1 functional voxel were excluded. | 2nd-level ANOVA to assess the effects of the group, with whole-brain type 1 error correction via threshold-free cluster enhancement using 10,000 permutations. | While the SCZ patients showed reduced amygdala connectivity with the OFC, FHR showed increased connectivity between the amygdala and a brainstem region around the noradrenergic arousal nuclei implicated in stress responses, which may reflect either a risk or resilience mechanism in young FHR. |
| Guo et al., 2014 [124] | 28 FHR (sibs, 26 yr, 54% M) 28 SCZ (23 yr, 54% M) 60 HC (27 yr, 58% M) | 1.5T GE Signa Twinspeed: functional images (GRE-EPI, TR 2 s, TE 40 ms, FA 90°, FOV 24 cm, 20 transverse slices of 5 mm, and resolution of 3.75 mm2) | Preprocessing using SPM8 and DPARSF; functional scans realigned to the middle volume; head motion parameters were regressed out of the data. | One-way ANOVA for group effects in mean connectivity and distance; connectivity strength of 4005 node pairs tested using Bonferroni-corrected independent t-tests to localize the strongest effects. | FHR and SCZ showed proportional decreases in long-range relative to short-range connectivity, but only FHR showed the strengthening of existing long-range links, which is suggestive of a compensatory process. Moreover, FHR showed the higher strength of the short- and long-range salience network, short-range subcortical, and long-range frontal network (trend-level) connections. |
| Doucet et al., 2017 [125] | 64 FHR (sibs, 32 yr, 42% M) 78 BD (34 yr, 33% M) 41 HC (33 yr, 32% M) | 3T Siemens Allegra: T2 * images (single shot GRE-EPI, TR 1.5 s, TE 27 ms, FOV 24 cm, FA 60°, and 3.43 × 5 mm3 voxel size); T1 scan (MPRAGE, TR 2.2 s, TE 4.13 ms, TI 766 ms, FA 13°, and voxel 0.8 mm3) | Preprocessing with SPM12 and REST toolbox; graph analyses with Brain Connectivity Toolbox; average motion regressed from graph metrics. | Permutation analysis to assess group effects in global connectivity and modularity metrics at an FDR-corrected p < 0.05 and regional degree and participation using p < 0.05 following permutation testing. | BD and FHR showed lower cohesiveness of sensorimotor network, with associated reduction in integration of DMN regions (MPFC, hippocampus) in BD, while FHR showed increased participation coefficients of the ventral ACC, angular gyrus, and SMA and the nodal degree of the L IFG (orbital part), suggesting possible resilience markers. |
| Duan et al., 2019 [126] | 89 FHR (FDR, 25 yr, 58% M) 137 SCZ (24 yr, 39% M) 210 HC (26 yr, 38% M) | 3T GE Signa HD: functional images (GRE-EPI, TR 2 s, TE 30 ms, FA 90°, FOV 24 cm, 35 slices of 3 mm, and no gap) | Preprocessing SPM12 and DPARFS; PAGANI toolkit for network reconstruction and analyses; removed subjects with excessive motion (>3 mm or 3°). | ANCOVA to test group effects, with age and sex as covariates and an FDR-corrected p < 0.05 or voxel-wise p < 0.001 and p < 0.05 cluster threshold, with an RFT correction. | SCZ showed increased medium- and long-range distance strengths of the orbital IFG, while FHR showed a reduced distance strength of this region, possibly representing an adaptive response to maintain segregation/integration balance of the functional brain network in FHR. |
| Ganella et al., 2018 [127] | 16 FHR (FDR, 58 yr, 13% M) 42 SCZ (41 yr, 70% M, i.e., treatment resistant) 42 HC (39 yr, 59% M) | 3T Siemens Avanto Magnetom TIM Trio: T2 * images (EPI sequence, TR 2.4 s, TE 40 ms, and voxel size 3.3 × 3.5 mm3) and T1 scan (MPRAGE, TR 1.98 s, TE 4.3 ms, FA 15°, FOV 25 cm, and 176 sagittal slices of 1 mm) | Preprocessing using FSL and SPM8; head motion controlled with Friston 24-parameter model; rs-volumes with FD > 0.5 mm excluded. | ANCOVA analysis with age and sex as covariates was used to test each pairwise connection for group effects, with the NBS analysis using a p < 0.01 primary threshold, and an FWE-corrected p < 0.05 sub-network threshold. | Functional connections that showed group differences were classified as resilience, risk, or illness related. A minority (~5%) of connections classified as resilience mainly involved reduced connectivity between temporal (i.e., TP) and subcortical regions (posterior cingulum). |
| Guo et al., 2020 [128] | 28 FHR (FDR, 26 yr, 54% M) 28 SCZ (25 yr, 54% M) 60 HC (27 yr, 58% M) | 1.5T GE Signa Twinspeed: functional images (GRE-EPI, TR 2 s, TE 40 ms, FA 90°, and FOV 24 cm) and T1 scan (20 contiguous 5 mm thick transverse slices with 1 mm gap) | Preprocessing with SPM8 and DPARSF; variance explained by head motion differences was removed in primary and secondary correlation analyses. | ANOVA was used to assess group effects on connectivity and graph metrics using an FDR-correction with post hoc pairwise t-tests, correcting for age, sex, and motion effects. | FHR demonstrated greater global functional connectivity diversity than HC and SCZ, and a higher level of global degree, clustering coefficient, and global efficiency compared with the other groups. |
Appendix B
Appendix B.1. Summary of Resilience-Associated Findings in Subcortical Structures
Appendix B.1.1. Cerebellum
Appendix B.1.2. Corpus Callosum
Appendix B.1.3. Basal Ganglia, Thalamus, Hippocampus, and Amygdala
Appendix C
References
- Newman, R. APA’s Resilience Initiative. Prof. Psychol. Res. Pract. 2005, 36, 227–229. [Google Scholar] [CrossRef]
- DeLuca, J.S.; Rakhshan Rouhakhtar, P.; Klaunig, M.J.; Akouri-Shan, L.; Jay, S.Y.; Todd, T.L.; Sarac, C.; Andorko, N.D.; Herrera, S.N.; Dobbs, M.F.; et al. Psychosis-like experiences and resilience: A systematic and critical review of the literature. Psychol. Serv. 2022, 19 (Suppl. S1), 120–138. [Google Scholar] [CrossRef]
- Loss, G.A.B. Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive After Extremely Aversive Events? Am. Psychol. 2004, 59, 20–28. [Google Scholar] [CrossRef]
- Brodsky, A.E.; Cattaneo, L.B. A Transconceptual Model of Empowerment and Resilience: Divergence, Convergence and Interactions in Kindred Community Concepts. Am. J. Community Psychol. 2013, 52, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Luthar, S.S.; Cicchetti, D.; Becker, B. The Construct of Resilience: A Critical Evaluation and Guidelines for Future Work. Child. Dev. 2000, 71, 543–562. [Google Scholar] [CrossRef]
- Masten, A.S. Resilience in individual development: Successful adaptation despite risk and adversity. In Educational Resilience in Inner-City America: Challenges and Prospects; Wang, M.C., Gordon, E.W., Eds.; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 1994; pp. 3–25. [Google Scholar]
- Ungar, M.; Liebenberg, L. Assessing Resilience Across Cultures Using Mixed Methods: Construction of the Child and Youth Resilience Measure. J. Mix. Methods Res. 2011, 5, 126–149. [Google Scholar] [CrossRef]
- Guimond, S.; Mothi, S.S.; Makowski, C.; Chakravarty, M.M.; Keshavan, M.S. Altered amygdala shape trajectories and emotion recognition in youth at familial high risk of schizophrenia who develop psychosis. Transl. Psychiatry 2022, 12, 202. [Google Scholar] [CrossRef]
- Frangou, S. Neuroimaging Markers of Risk, Disease Expression, and Resilience to Bipolar Disorder. Curr. Psychiatry Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Cannon, T.D. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci. 2015, 19, 744–756. [Google Scholar] [CrossRef]
- Cannon, T.D.; Yu, C.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cornblatt, B.A.; Heinssen, R.; Jeffries, C.D.; Mathalon, D.H.; McGlashan, T.H.; et al. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am. J. Psychiatry 2016, 173, 980–988. [Google Scholar] [CrossRef]
- Di Stefano, V.; D’Angelo, M.; Monaco, F.; Vignapiano, A.; Martiadis, V.; Barone, E.; Fornaro, M.; Steardo, L.; Solmi, M.; Manchia, M. Decoding Schizophrenia: How AI-Enhanced fMRI Unlocks New Pathways for Precision Psychiatry. Brain Sci. 2024, 14, 1196. [Google Scholar] [CrossRef] [PubMed]
- Caballero, N.; Machiraju, S.; Diomino, A.; Kennedy, L.; Kadivar, A.; Cadenhead, K.S. Recent Updates on Predicting Conversion in Youth at Clinical High Risk for Psychosis. Curr. Psychiatry Rep. 2023, 25, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Damme, K.S.; Ered, A.; Capizzi, R.; Frosch, I.; Ellman, L.M.; Mittal, V.A. Neuroimaging Markers of Resiliency in Youth at Clinical High Risk for Psychosis: A Qualitative Review. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Masten, A.; Best, K.; Garmezy, N. Resilience and development: Contributions from the study of children who overcome adversity. Dev. Psychopathol. 1990, 2, 425–444. [Google Scholar] [CrossRef]
- Rutter, M. Resilience as a dynamic concept. Dev. Psychopathol. 2012, 24, 335–344. [Google Scholar] [CrossRef]
- Masten, A.S.; Cicchetti, D. Resilience in development: Progress and transformation. In Developmental Psychopathology, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 4, pp. 271–333. [Google Scholar]
- Garmezy, N.; Masten, A.S.; Tellegen, A. The Study of Stress and Competence in Children: A Building Block for Developmental Psychopathology. Child. Dev. 1984, 55, 97–111. [Google Scholar] [CrossRef]
- Rutter, M. Resilience in the Face of Adversity. Protective Factors and Resistance to Psychiatric Disorder. Br. J. Psychiatry 1985, 147, 598–611. [Google Scholar] [CrossRef]
- Luthar, S.S.; Lyman, E.L.; Crossman, E.J. Resilience and Positive Psychology. In Handbook of Developmental Psychopathology; Springer: Boston, MA, USA, 2014; pp. 125–140. [Google Scholar] [CrossRef]
- Stress, N.G. Competence, and Development: Continuities in the Study of Schizophrenic Adults, Children Vulnerable to Psychopathology, and the Search for Stress-Resistant Children. Am. J. Orthopsychiatry 1987, 57, 159–174. [Google Scholar]
- de Godoy, L.L.; Alves, C.A.P.F.; Saavedra, J.S.M.; Studart-Neto, A.; Nitrini, R.; Leite, C.d.C.; Bisdas, S. Understanding brain resilience in superagers: A systematic review. Neuroradiology 2021, 63, 663–683. [Google Scholar] [CrossRef]
- Garo-Pascual, M.; Gaser, C.; Zhang, L.; Tohka, J.; Medina, M.; Strange, B.A. Brain structure and phenotypic profile of superagers compared with age-matched older adults: A longitudinal analysis from the Vallecas Project. Lancet Healthy Longev. 2023, 4, e374–e385. [Google Scholar] [CrossRef]
- Radua, J.; Mataix-Cols, D. Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.R.; McMillan, K.M.; Lancaster, J.L.; Kochunov, P.; Turkeltaub, P.E.; Pardo, J.V.; Fox, P.T. A comparison of label-based review and ALE meta-analysis in the stroop task. Hum. Brain Mapp. 2005, 25, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Scarpazza, C.; Lattanzi, G.M.; Antoniades, M.; Di Fabio, F.; Sartori, G.; Eickhoff, S.B.; McGuire, P.; Tognin, S. Systematic review and multi-modal meta-analysis of magnetic resonance imaging findings in 22q11.2 deletion syndrome: Is more evidence needed? Neurosci. Biobehav. Rev. 2019, 107, 143–153. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- McGlashan, T.; Walsh, B.; Woods, S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Yung, A.R.; Yuen, H.P.; McGorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’Olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; Stanford, C.; et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Yung, A.R.; McGorry, P.D.; McFarlane, C.A.; Jackson, H.J.; Patton, G.C.; Rakkar, A. Monitoring and Care of Young People at Incipient Risk of Psychosis. Schizophr. Bull. 1996, 22, 283–303. [Google Scholar] [CrossRef]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, T.D.; Wolf, D.H.; Loughead, J.; Ruparel, K.; Elliott, M.A.; Hakonarson, H.; Gur, R.C.; Gur, R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage 2012, 60, 623–632. [Google Scholar] [CrossRef]
- Power, J.D.; Schlaggar, B.L.; Petersen, S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 2015, 15, 536–551. [Google Scholar] [CrossRef]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Collin, G.; Kahn, R.; de Reus, M.; Cahn, W.; van den Heuvel, M. Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr. Bull. 2014, 40, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.P.; Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 2011, 31, 15775–15786. [Google Scholar] [CrossRef]
- Groot, C.; Van Loenhoud, A.C.; Barkhof, F.; Van Berckel, B.N.; Koene, T.; Teunissen, C.C.; Scheltens, P.; van der Flier, W.M.; Ossenkoppele, R. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology 2018, 90, e149–e156. [Google Scholar] [CrossRef]
- Guo, L.-H.; Alexopoulos, P.; Wagenpfeil, S.; Kurz, A.; Pernecky, R. Brain size and the compensation of Alzheimer’s disease symptoms: A longitudinal cohort study. Alzheimer’s Dement. 2013, 9, 580–586. [Google Scholar] [CrossRef]
- Negash, S.; Xie, S.; Davatzikos, C.; Clark, C.M.; Trojanowski, J.Q.; Shaw, L.M.; Wolk, D.A.; Arnold, S.E. Cognitive and functional resilience despite molecular evidence of Alzheimer’s disease pathology. Alzheimer’s Dement. 2013, 9, e89–e95. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, E.J.; Gefen, T.; Shi, J.; Samimi, M.; Bigio, E.; Weintraub, S.; Geula, C.; Mesulam, M.-M. Youthful Memory Capacity in Old Brains: Anatomic and Genetic Clues from the Northwestern SuperAging Project. J. Cogn. Neurosci. 2013, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, M.S.; Eack, S.M.; Wojtalik, J.A.; Prasad, K.M.; Francis, A.N.; Bhojraj, T.S.; Greenwald, D.P.; Hogarty, S.S. A broad cortical reserve accelerates response to cognitive enhancement therapy in early course schizophrenia. Schizophr. Res. 2011, 130, 123–129. [Google Scholar] [CrossRef]
- Selemon, L.D.; Zecevic, N. Schizophrenia: A tale of two critical periods for prefrontal cortical development. Transl. Psychiatry 2015, 5, e623-11. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Mehta, U.M.; Padmanabhan, J.L.; Shah, J. Dysplasticity, metaplasticity, and schizophrenia: Implications for risk, illness, and novel interventions. Dev. Psychopathol. 2015, 27, 615–635. [Google Scholar] [CrossRef]
- Grafman, J. Conceptualizing functional neuroplasticity. J. Commun. Disord. 2000, 33, 345–356. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Thermenos, H.W.; Milanovic, S.; Tsuang, M.T.; Faraone, S.V.; McCarley, R.W.; Shenton, M.E.; Green, A.I.; Nieto-Castanon, A.; LaViolette, P.; et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. USA 2009, 106, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.L.; Woods, S.W.; Kiehl, K.A.; Calhoun, V.D.; Pearlson, G.D.; Roach, B.J.; Ford, J.M.; Srihari, V.H.; McGlashan, T.H.; Mathalon, D.H. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front. Psychiatry 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, L.L.; Chen, Y.; Yin, H.; Tan, Q.; Hu, D. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci. Rep. 2015, 5, 14655. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zong, X.-F.; Mann, J.J.; Zheng, J.-J.; Liao, Y.-H.; Li, Z.-C.; He, Y.; Chen, X.-G.; Tang, J.-S. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci. Bull. 2017, 33, 73–84. [Google Scholar] [CrossRef]
- Rodríguez-Cano, E.; Alonso-Lana, S.; Sarró, S.; Fernández-Corcuera, P.; Goikolea, J.M.; Vieta, E.; Maristany, T.; Salvador, R.; McKenna, P.J.; Pomarol-Clotet, E. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Park, K.; Kim, C.; Ryu, S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. NeuroImage Clin. 2019, 22, 101805. [Google Scholar] [CrossRef] [PubMed]
- Collin, G.; Nieto-Castanon, A.; Shenton, M.E.; Pasternak, O.; Kelly, S.; Keshavan, M.S.; Seidman, L.J.; McCarley, R.W.; Niznikiewicz, M.A.; Li, H.; et al. Brain Functional Connectivity Data Enhance Prediction of Clinical Outcome in Youth at Risk for Psychosis. Neuroimage Clin. 2020, 26, 102108. [Google Scholar] [CrossRef]
- Kasanova, Z.; Hernaus, D.; Vaessen, T.; van Amelsvoort, T.; Winz, O.; Heinzel, A.; Pruessner, J.; Mottaghy, F.M.; Collip, D.; Myin-Germeys, I. Early-Life Stress Affects Stress-Related Prefrontal Dopamine Activity in Healthy Adults, but Not in Individuals with Psychotic Disorder. PLoS ONE 2016, 11, e0150746. [Google Scholar] [CrossRef]
- Prakash, R.S.; Shankar, A.; Tripathi, V.; Yang, W.F.; Fisher, M.; Bauer, C.C.; Betzel, R.; Sacchet, M.D. Mindfulness Meditation and Network Neuroscience: Review, Synthesis, and Future Directions. Biol. Psychiatry Cogn. Neurosci. Neuroimaging, 2024; in press. [Google Scholar] [CrossRef]
- DeTore, N.R.; Luther, L.; Deng, W.; Zimmerman, J.; Leathem, L.; Burke, A.S.; Nyer, M.B.; Holt, D.J. Efficacy of a transdiagnostic, prevention-focused program for at-risk young adults: A waitlist-controlled trial. Psychol. Med. 2023, 53, 3490–3499. [Google Scholar] [CrossRef]
- Bauer, C.C.; Okano, K.; Ghosh, S.S.; Lee, Y.J.; Melero, H.; Angeles, C.d.L.; Nestor, P.G.; del Re, E.C.; Northoff, G.; Niznikiewicz, M.A.; et al. Real-time fMRI neurofeedback reduces auditory hallucinations and modulates resting state connectivity of involved brain regions: Part 2: Default mode network -preliminary evidence. Psychiatry Res. 2020, 284, 112770. [Google Scholar] [CrossRef]
- Hadar, R.; Bikovski, L.; Soto-Montenegro, M.L.; Schimke, J.; Maier, P.; Ewing, S.; Voget, M.; Wieske, F.; Götz, T.; Desco, M.; et al. Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Mol. Psychiatry 2018, 23, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Boos, H.B.; Mandl, R.C.; van Haren, N.E.; Cahn, W.; van Baal, G.C.M.; Kahn, R.S.; Pol, H.E.H. Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur. Neuropsychopharmacol. 2013, 23, 295–304. [Google Scholar] [CrossRef]
- Catani, M.; Mesulam, M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 2009, 44, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Skeide, M.A.; Brauer, J.; Friederici, A.D. Brain Functional and Structural Predictors of Language Performance. Cereb. Cortex 2016, 26, 2127–2139. [Google Scholar] [CrossRef]
- Glasser, M.F.; Rilling, J.K. DTI Tractography of the Human Brain’s Language Pathways. Cereb. Cortex 2008, 18, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Hubl, D.; Koenig, T.; Strik, W.; Federspiel, A.; Kreis, R.; Boesch, C.; Maier, S.E.; Schroth, G.; Lovblad, K.; Dierks, T. Pathways that make voices: White matter changes in auditory hallucinations. Arch. Gen. Psychiatry 2004, 61, 658–668. [Google Scholar] [CrossRef]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef]
- Calvo, N.; Anderson, J.A.E.; Berkes, M.; Freedman, M.; Craik, F.I.M.; Bialystok, E. Gray Matter Volume as Evidence for Cognitive Reserve in Bilinguals With Mild Cognitive Impairment. Alzheimer Dis. Assoc. Disord. 2023, 37, 7–12. [Google Scholar] [CrossRef]
- Collin, G.; Keshavan, M.S. Connectome development and a novel extension to the neurodevelopmental model of schizophrenia. Dialogues Clin. Neurosci. 2018, 20, 101–110. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Kahn, R.S.; Goñi, J.; High-cost, O.S. high-capacity backbone for global brain communication. Proc. Natl. Acad. Sci. USA 2012, 109, 11372–11377. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Sporns, O. An Anatomical Substrate for Integration among Functional Networks in Human Cortex. J. Neurosci. 2013, 33, 14489–14500. [Google Scholar] [CrossRef] [PubMed]
- de Reus, M.A.; van den Heuvel, M.P. Simulated rich club lesioning in brain networks: A scaffold for communication and integration? Front. Hum. Neurosci. 2014, 8, 647. [Google Scholar] [CrossRef]
- Heuvel, M.P.v.D.; Sporns, O.; Collin, G.; Scheewe, T.; Mandl, R.C.W.; Cahn, W.; Goñi, J.; Pol, H.E.H.; Kahn, R.S. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 2013, 70, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Collin, G.; Scholtens, L.H.; Kahn, R.S.; Hillegers, M.H.J.; van den Heuvel, M.P. Affected Anatomical Rich Club and Structural-Functional Coupling in Young Offspring of Schizophrenia and Bipolar Disorder Patients. Biol. Psychiatry 2017, 82, 746–755. [Google Scholar] [CrossRef]
- Hafeman, D.M.; Chang, K.D.; Garrett, A.S.; Sanders, E.M.; MPhillips, L. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012, 14, 375–410. [Google Scholar] [CrossRef]
- Sassi, R.B.; Nicoletti, M.; Brambilla, P.; Mallinger, A.G.; Frank, E.; Kupfer, D.J.; Keshavan, M.S.; Soares, J.C. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci. Lett. 2002, 329, 243–245. [Google Scholar] [CrossRef]
- Keshavan, M.; Anderson, S.; Pettegrew, J. Changes in caudate volume with neuroleptic treatment. Lancet 1994, 344, 1434. [Google Scholar] [CrossRef]
- Chopra, S.; Fornito, A.; Francey, S.M.; O’donoghue, B.; Cropley, V.; Nelson, B.; Graham, J.; Baldwin, L.; Tahtalian, S.; Yuen, H.P.; et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study. Neuropsychopharmacology 2021, 46, 1494–1501. [Google Scholar] [CrossRef]
- Orlov, N.D.; Giampietro, V.; O’Daly, O.; Lam, S.L.; Barker, G.J.; Rubia, K.; McGuire, P.; Shergill, S.S.; Allen, P. Real-time fMRI neurofeedback to down- regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: A proof-of-concept study. Transl. Psychiatry 2018, 4, 46. [Google Scholar] [CrossRef]
- Gupta, T.; Kelley, N.J.; Pelletier-baldelli, A.; Mittal, V.A. Transcranial Direct Current Stimulation, Symptomatology, and Cognition in Psychosis: A Qualitative Review. Front. Behav. Neurosci. 2018, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Garmezy, N. Children at risk: The search for the antecedents of schizophrenia, part II: Ongoing research programs, issues, and intervention. Schizophr. Bull. 1974, 1, 55–125. [Google Scholar] [CrossRef]
- Ćurčić-Blake, B.; Ford, J.M.; Hubl, D.; Orlov, N.D.; Sommer, I.E.; Waters, F.; Allen, P.; Jardri, R.; Woodruff, P.W.; David, O.; et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog. Neurobiol. 2017, 148, 1–20. [Google Scholar] [CrossRef]
- DeVylder, J.E.; Muchomba, F.M.; Gill, K.E.; Ben-David, S.; Walder, D.J.; Malaspina, D.; Corcoran, C.M. Symptom trajectories and psychosis onset in a clinical high-risk cohort: The relevance of subthreshold thought disorder. Schizophr. Res. 2014, 159, 278–283. [Google Scholar] [CrossRef]
- Katagiri, N.; Pantelis, C.; Nemoto, T.; Tsujino, N.; Saito, J.; Hori, M.; Yamaguchi, T.; Funatogawa, T.; Mizuno, M. Longitudinal changes in striatum and sub-threshold positive symptoms in individuals with an ‘at risk mental state’ (ARMS). Psychiatry Res. Neuroimaging 2019, 285, 25–30. [Google Scholar] [CrossRef]
- Levy, D.L.; Coleman, M.J.; Sung, H.; Ji, F.; Matthysse, S.; Mendell, N.R.; Titone, D. The genetic basis of thought disorder and language and communication disturbances in schizophrenia. J. Neurolinguist. 2010, 23, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Castanon, A. Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020. [Google Scholar]
- Strik, W.; Dierks, T.; Hubl, D.; Horn, H. HaIIucinations, Thought Disorders, and the Language Domain in Schizophrenia. Clin. EEG Neurosci. 2008, 39, 91–94. [Google Scholar] [CrossRef]
- Vita, A.; Dieci, M.; Giobbio, G.M.; Caputo, A.; Ghiringhelli, L.; Comazzi, M.; Garbarini, M.; Mendini, A.P.; Morganti, C.; Tenconi, F.; et al. Language and thought disorder in schizophrenia: Brain morphological correlates. Schizophr. Res. 1995, 15, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fornito, A.; Yung, A.R.; Wood, S.J.; Phillips, L.J.; Nelson, B.; Cotton, S.; Velakoulis, D.; McGorry, P.D.; Pantelis, C.; Yücel, M. Anatomic Abnormalities of the Anterior Cingulate Cortex Before Psychosis Onset: An MRI Study of Ultra-High-Risk Individuals. Biol. Psychiatry 2008, 64, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Habets, P.; Krabbendam, L.; Hofman, P.; Suckling, J.; Oderwald, F.; Bullmore, E.; Woodruff, P.; Van Os, J.; Marcelis, M. Cognitive Performance and Grey Matter Density in Psychosis: Functional Relevance of a Structural Endophenotype. Neuropsychobiology 2008, 58, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kempton, M.J.; Haldane, M.; Jogia, J.; Grasby, P.M.; Collier, D.; Frangou, S. Dissociable Brain Structural Changes Associated with Predisposition, Resilience, and Disease Expression in Bipolar Disorder. J. Neurosci. 2009, 29, 10863–10868. [Google Scholar] [CrossRef]
- Greenstein, D.; Lenroot, R.; Clausen, L.; Chavez, A.; Vaituzis, A.; Tran, L.; Gogtay, N.; Rapoport, J. Cerebellar development in childhood onset schizophrenia and non-psychotic siblings. Psychiatry Res. Neuroimaging 2011, 193, 131–137. [Google Scholar] [CrossRef]
- Frangou, S. Brain structural and functional correlates of resilience to Bipolar Disorder. Front. Hum. Neurosci. 2012, 5, 184. [Google Scholar] [CrossRef]
- Pompei, F.; Dima, D.; Rubia, K.; Kumari, V.; Frangou, S. Dissociable functional connectivity changes during the Stroop task relating to risk, resilience and disease expression in bipolar disorder. Neuroimage 2011, 57, 576–582. [Google Scholar] [CrossRef]
- van Erp, T.G.; Thompson, P.M.; Kieseppä, T.; Bearden, C.E.; Marino, A.C.; Hoftman, G.D.; Haukka, J.; Partonen, T.; Huttunen, M.; Kaprio, J.; et al. Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Hum. Brain Mapp. 2012, 33, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Eker, C.; Simsek, F.; Yılmazer, E.E.; Kitis, O.; Cinar, C.; Eker, O.D.; Coburn, K.; Gonul, A.S. Brain regions associated with risk and resistance for bipolar I disorder: A voxel-based MRI study of patients with bipolar disorder and their healthy siblings. Bipolar Disord. 2014, 16, 249–261. [Google Scholar] [CrossRef]
- Chakravarty, M.M.; Rapoport, J.L.; Giedd, J.N.; Raznahan, A.; Shaw, P.; Collins, D.L.; Lerch, J.P.; Gogtay, N. Striatal Shape Abnormalities as Novel Neurodevelopmental Endophenotypes in Schizophrenia: A Longitudinal Study. Hum. Brain Mapp. 2015, 1469, 1458–1469. [Google Scholar] [CrossRef]
- Goghari, V.M.; Truong, W.; Spilka, M.J. A magnetic resonance imaging family study of cortical thickness in schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 660–668. [Google Scholar] [CrossRef]
- Sarıçiçek, A.; Yalın, N.; Hıdıroğlu, C.; Çavuşoğlu, B.; Taş, C.; Ceylan, D.; Zorlu, N.; Ada, E.; Tunca, Z.; Özerdem, A. Neuroanatomical correlates of genetic risk for bipolar disorder: A voxel-based morphometry study in bipolar type I patients and healthy first degree relatives. J. Affect. Disord. 2015, 186, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zalesky, A.; Pantelis, C.; Cropley, V.; Fornito, A.; Cocchi, L.; McAdams, H.; Clasen, L.; Greenstein, D.; Rapoport, J.L.; Gogtay, N. Delayed Development of Brain Connectivity in Adolescents With Schizophrenia and Their Unaffected Siblings. JAMA Psychiatry 2015, 72, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Womer, F.Y.; Bai, C.; Zhou, Q.; Wei, S.; Jiang, X.; Geng, H.; Zhou, Y.; Tang, Y.; Wang, F. Voxel-based morphometry in individuals at genetic high risk for schizophrenia and patients with schizophrenia during their first episode of psychosis. PLoS ONE 2016, 11, e0163749. [Google Scholar] [CrossRef]
- de Wit, S.; Wierenga, L.M.; Oranje, B.; Ziermans, T.B.; Schothorst, P.F.; van Engeland, H.; Kahn, R.S.; Durston, S. Brain development in adolescents at ultra-high risk for psychosis: Longitudinal changes related to resilience. Neuroimage Clin. 2016, 12, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, N.; Pantelis, C.; Nemoto, T.; Tsujino, N.; Saito, J.; Hori, M.; Yamaguchi, T.; Funatogawa, T.; Mizuno, M. Symptom recovery and relationship to structure of corpus callosum in individuals with an ‘at risk mental state’. Psychiatry Res. Neuroimaging 2018, 272, 1–6. [Google Scholar] [CrossRef]
- Yalin, N.; Saricicek, A.; Hidiroglu, C.; Zugman, A.; Direk, N.; Ada, E.; Cavusoglu, B.; Er, A.; Isik, G.; Ceylan, D.; et al. Clinical Cortical thickness and surface area as an endophenotype in bipolar disorder type I patients and their first-degree relatives. NeuroImage Clin. 2019, 22, 101695. [Google Scholar] [CrossRef]
- McGlashan, S.W.; Miller, T.H.; Woods, T.J. Structured Interview for Prodromal Syndromes (SIPS)—Version 3.0; PRIME Research Clinic, Yale School of Medicine: New Haven, CT, USA, 2001. [Google Scholar]
- Hoptman, M.; Nierenberg, J.; Bertisch, H.; Catalano, D.; Ardekani, B.; Branch, C.; Delisi, L. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr. Res. 2008, 106, 115–124. [Google Scholar] [CrossRef]
- Kim, S.N.; Park, J.S.; Jang, J.H.; Jung, W.H.; Shim, G.; Park, H.Y.; Hwang, J.Y.; Choi, C.-H.; Kang, D.-H.; Lee, J.-M.; et al. Increased white matter integrity in the corpus callosum in subjects with high genetic loading for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 50–55. [Google Scholar] [CrossRef]
- Goghari, V.M.; Billiet, T.; Sunaert, S.; Emsell, L. A diffusion tensor imaging family study of the fornix in schizophrenia. Schizophr. Res. 2014, 159, 435–440. [Google Scholar] [CrossRef]
- Katagiri, N.; Pantelis, C.; Nemoto, T.; Zalesky, A.; Hori, M.; Shimoji, K.; Saito, J.; Ito, S.; Dwyer, D.B.; Fukunaga, I.; et al. A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an ‘at risk mental state’ (ARMS). Schizophr. Res. 2015, 162, 7–13. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Broome, M.; Matthiasson, P.; Woolley, J.; Johns, L.; Tabraham, P.; Bramon, E.; Valmaggia, L.; Williams, S.; McGuire, P. Spatial working memory in individuals at high risk for psychosis: Longitudinal fMRI study. Schizophr. Res. 2010, 123, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Broome, M.; Matthiasson, P.; Woolley, J.; Johns, L.; Tabraham, P.; Bramon, E.; Valmaggia, L.; Williams, S.; McGuire, P. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: Longitudinal VBM-fMRI study. J. Psychiatr. Res. 2011, 45, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Park, J.-Y.; Jung, M.H.; Jang, J.H.; Kang, D.-H.; Jung, W.H.; Han, J.Y.; Choi, C.-H.; Hong, K.S.; Kwon, J.S. Phase-specific brain change of spatial working memory processing in genetic and ultra-high risk groups of schizophrenia. Schizophr. Bull. 2012, 38, 1189–1199. [Google Scholar] [CrossRef]
- Smieskova, R.; Allen, P.; Simon, A.; Aston, J.; Bendfeldt, K.; Drewe, J.; Gruber, K.; Gschwandtner, U.; Klarhoefer, M.; Lenz, C.; et al. Different duration of at-risk mental state associated with neurofunctional abnormalities. A multimodal imaging study. Hum. Brain Mapp. 2012, 33, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Stäblein, M.; Storchak, H.; Ghinea, D.; Kraft, D.; Knöchel, C.; Prvulovic, D.; Bittner, R.A.; Reif, A.; Oertel-Knöchel, V. Visual working memory encoding in schizophrenia and first-degree relatives: Neurofunctional abnormalities and impaired consolidation. Psychol. Med. 2018, 49, 75–83. [Google Scholar] [CrossRef]
- Spilka, M.J.; Arnold, A.E.; Goghari, V.M. Functional activation abnormalities during facial emotion perception in schizophrenia patients and nonpsychotic relatives. Schizophr. Res. 2015, 168, 330–337. [Google Scholar] [CrossRef]
- Sepede, G.; De Berardis, D.; Campanella, D.; Perrucci, M.G.; Ferretti, A.; Salerno, R.M.; Di Giannantonio, M.; Romani, G.L.; Gambi, F. Neural correlates of negative emotion processing in bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 60, 1–10. [Google Scholar] [CrossRef]
- Tseng, W.-L.; Bones, B.; Kayser, R.; Olsavsky, A.; Fromm, S.; Pine, D.; Leibenluft, E.; Brotman, M. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur. Psychiatry 2015, 30, 94–98. [Google Scholar] [CrossRef]
- Dima, D.; Roberts, R.E.; Frangou, S. Connectomic markers of disease expression, genetic risk and resilience in bipolar disorder. Transl. Psychiatry 2016, 6, e706–e707. [Google Scholar] [CrossRef]
- Welge, J.A.; Saliba, L.J.; Strawn, J.R.; Eliassen, J.C.; Patino, L.R.; Adler, C.M.; Weber, W.; Schneider, M.R.; Barzman, D.H.; Strakowski, S.M.; et al. Neurofunctional Differences Among Youth With and at Varying Risk for Developing Mania. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 980–989. [Google Scholar] [CrossRef]
- Spilka, M.J.; Goghari, V.M. Similar patterns of brain activation abnormalities during emotional and non-emotional judgments of faces in a schizophrenia family study. Neuropsychologia 2017, 96, 164–174. [Google Scholar] [CrossRef]
- Wiggins, J.L.; Brotman, M.A.; Adleman, N.E.; Kim, P.; Wambach, C.G.; Reynolds, R.C.; Chen, G.; Towbin, K.; Pine, D.S.; Leibenluft, E. Neural Markers in Pediatric Bipolar Disorder and Familial Risk for Bipolar Disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2017, 56, 67–78. [Google Scholar] [CrossRef]
- Nimarko, A.F.; Garrett, A.S.; Carlson, G.A.; Singh, M.K. Neural correlates of emotion processing predict resilience in youth at familial risk for mood disorders. Dev. Psychopathol. 2019, 31, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M.; Özgürdal, S.; Ansorge, N.; von Reventlow, H.G.; Peters, S.; Nicolas, V.; Tegenthoff, M.; Juckel, G.; Lissek, S. An fMRI study of ‘theory of mind’ in at-risk states of psychosis: Comparison with manifest schizophrenia and healthy controls. Neuroimage 2011, 55, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Willert, A.; Mohnke, S.; Erk, S.; Schnell, K.; Romanczuk-Seiferth, N.; Quinlivan, E.; Schreiter, S.; Spengler, S.; Herold, D.; Wackerhagen, C.; et al. Alterations in neural Theory of Mind processing in euthymic patients with bipolar disorder and unaffected relatives. Bipolar Disord. 2015, 17, 880–891. [Google Scholar] [CrossRef]
- Anticevic, A.; Tang, Y.; Cho, Y.T.; Repovs, G.; Cole, M.W.; Savic, A.; Wang, F.; Krystal, J.H.; Xu, K. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr. Bull. 2014, 40, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Palaniyappan, L.; Yang, B.; Liu, Z.; Xue, Z.; Feng, J. Anatomical distance affects functional connectivity in patients with schizophrenia and their siblings. Schizophr. Bull. 2014, 40, 449–459. [Google Scholar] [CrossRef]
- Doucet, G.E.; Bassett, D.S.; Yao, N.; Glahn, D.C.; Frangou, S. The Role of Intrinsic Brain Functional Connectivity in Vulnerability and Resilience to Bipolar Disorder. Am. J. Psychiatry 2017, 174, 1214–1222. [Google Scholar] [CrossRef]
- Duan, J.; Xia, M.; Womer, F.Y.; Chang, M.; Yin, Z.; Zhou, Q.; Zhu, Y.; Liu, Z.; Jiang, X.; Wei, S.; et al. Dynamic changes of functional segregation and integration in vulnerability and resilience to schizophrenia. Hum. Brain Mapp. 2019, 40, 2200–2211. [Google Scholar] [CrossRef]
- Ganella, E.P.; Seguin, C.; Bartholomeusz, C.F.; Whittle, S.; Bousman, C.; Wannan, C.M.; Di Biase, M.A.; Phassouliotis, C.; Everall, I.; Pantelis, C.; et al. Risk and resilience brain networks in treatment-resistant schizophrenia. Schizophr. Res. 2018, 193, 284–292. [Google Scholar] [CrossRef]
- Guo, S.; He, N.; Liu, Z.; Linli, Z.; Tao, H.; Palaniyappan, L. Brain-Wide Functional Dysconnectivity in Schizophrenia: Parsing Diathesis, Resilience, and the Effects of Clinical Expression. Can. J. Psychiatry 2020, 65, 21–29. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collin, G.; Goldenberg, J.E.; Chang, X.; Qi, Z.; Whitfield-Gabrieli, S.; Cahn, W.; Wang, J.; Stone, W.S.; Keshavan, M.S.; Shenton, M.E. Brain Markers of Resilience to Psychosis in High-Risk Individuals: A Systematic Review and Label-Based Meta-Analysis of Multimodal MRI Studies. Brain Sci. 2025, 15, 314. https://doi.org/10.3390/brainsci15030314
Collin G, Goldenberg JE, Chang X, Qi Z, Whitfield-Gabrieli S, Cahn W, Wang J, Stone WS, Keshavan MS, Shenton ME. Brain Markers of Resilience to Psychosis in High-Risk Individuals: A Systematic Review and Label-Based Meta-Analysis of Multimodal MRI Studies. Brain Sciences. 2025; 15(3):314. https://doi.org/10.3390/brainsci15030314
Chicago/Turabian StyleCollin, Guusje, Joshua E. Goldenberg, Xiao Chang, Zhenghan Qi, Susan Whitfield-Gabrieli, Wiepke Cahn, Jijun Wang, William S. Stone, Matcheri S. Keshavan, and Martha E. Shenton. 2025. "Brain Markers of Resilience to Psychosis in High-Risk Individuals: A Systematic Review and Label-Based Meta-Analysis of Multimodal MRI Studies" Brain Sciences 15, no. 3: 314. https://doi.org/10.3390/brainsci15030314
APA StyleCollin, G., Goldenberg, J. E., Chang, X., Qi, Z., Whitfield-Gabrieli, S., Cahn, W., Wang, J., Stone, W. S., Keshavan, M. S., & Shenton, M. E. (2025). Brain Markers of Resilience to Psychosis in High-Risk Individuals: A Systematic Review and Label-Based Meta-Analysis of Multimodal MRI Studies. Brain Sciences, 15(3), 314. https://doi.org/10.3390/brainsci15030314






