Optimising Electrode Montages in Conventional Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation of the Cerebellum for Pain Modulation
Abstract
:1. Introduction
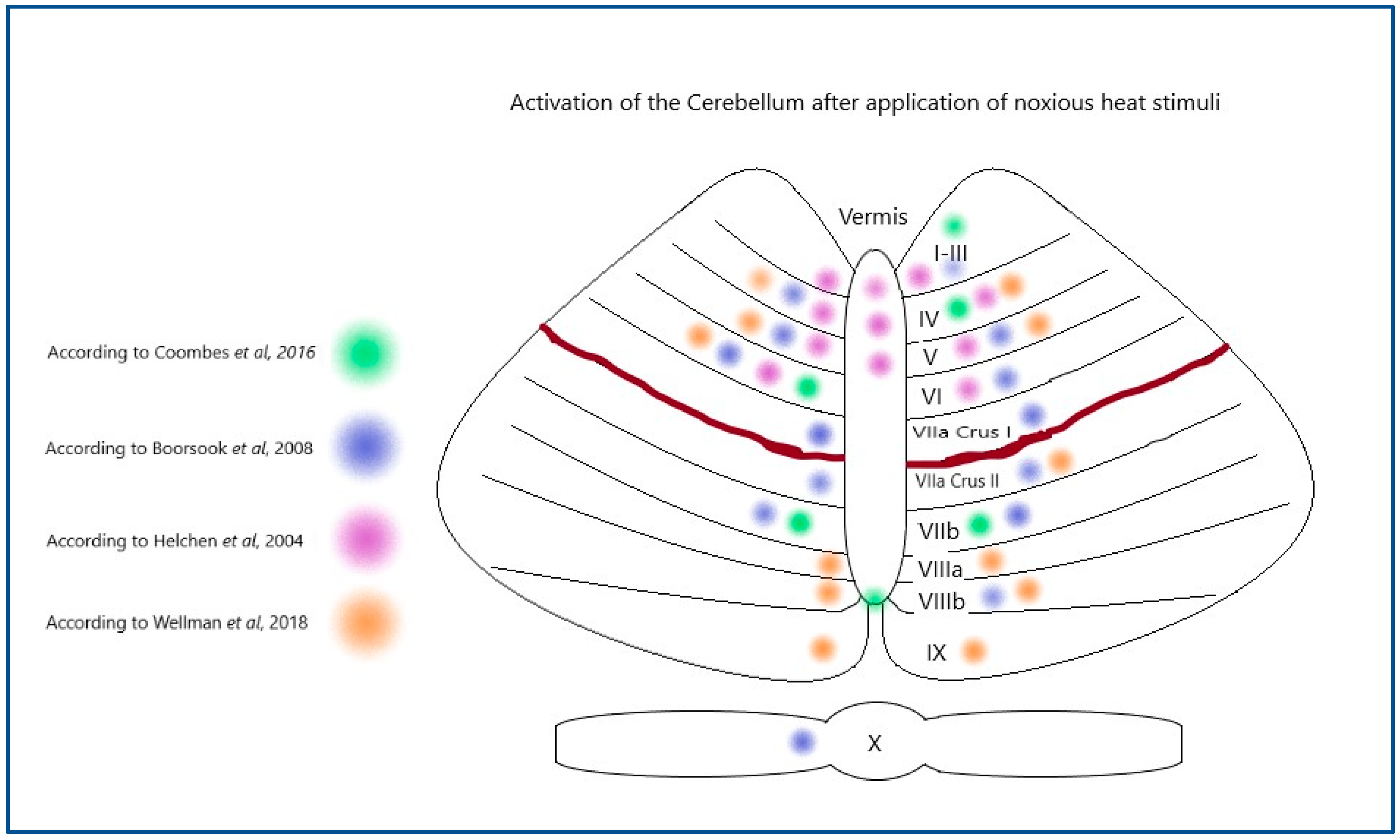
1.1. Activation of the Cerebellum During Pain Stimulation in Humans
1.2. Neurostimulation Targeting in the Cerebellum
1.3. How Might Neurostimulation Targeting Be Made More Reliable?
- How much of an effect does the placement of a saline-soaked sponge cathode have on the current changes caused by ctDCS, as predicted by modelling software?
- How focal is ctDCS predicted to be using modern current modelling software?
- Is ctDCS affecting multiple brain regions, as predicted by modelling software?
- How much of an effect does the placement of the central anode have on the current changes caused by HD-ctDCS, as predicted by modelling software?
- Does amplitude change affect the current changes in ctDCS and HD-ctDCS in a visible way? How much of an effect does the placement of a saline-soaked sponge cathode have on the current changes caused by ctDCS, as predicted by modelling software?
- Are more focal effects predicted for ctDCS or HD-ctDCS by modelling software?
- Do current modelling predictions support the findings of research literature?
- Is the application ctDCS and HD-ctDCS supported as having potential for influencing the experience of pain according to current modelling?
2. Materials and Methods
2.1. Conventional ctDCS
2.2. High-Definition (HD) ctDCS
3. Results
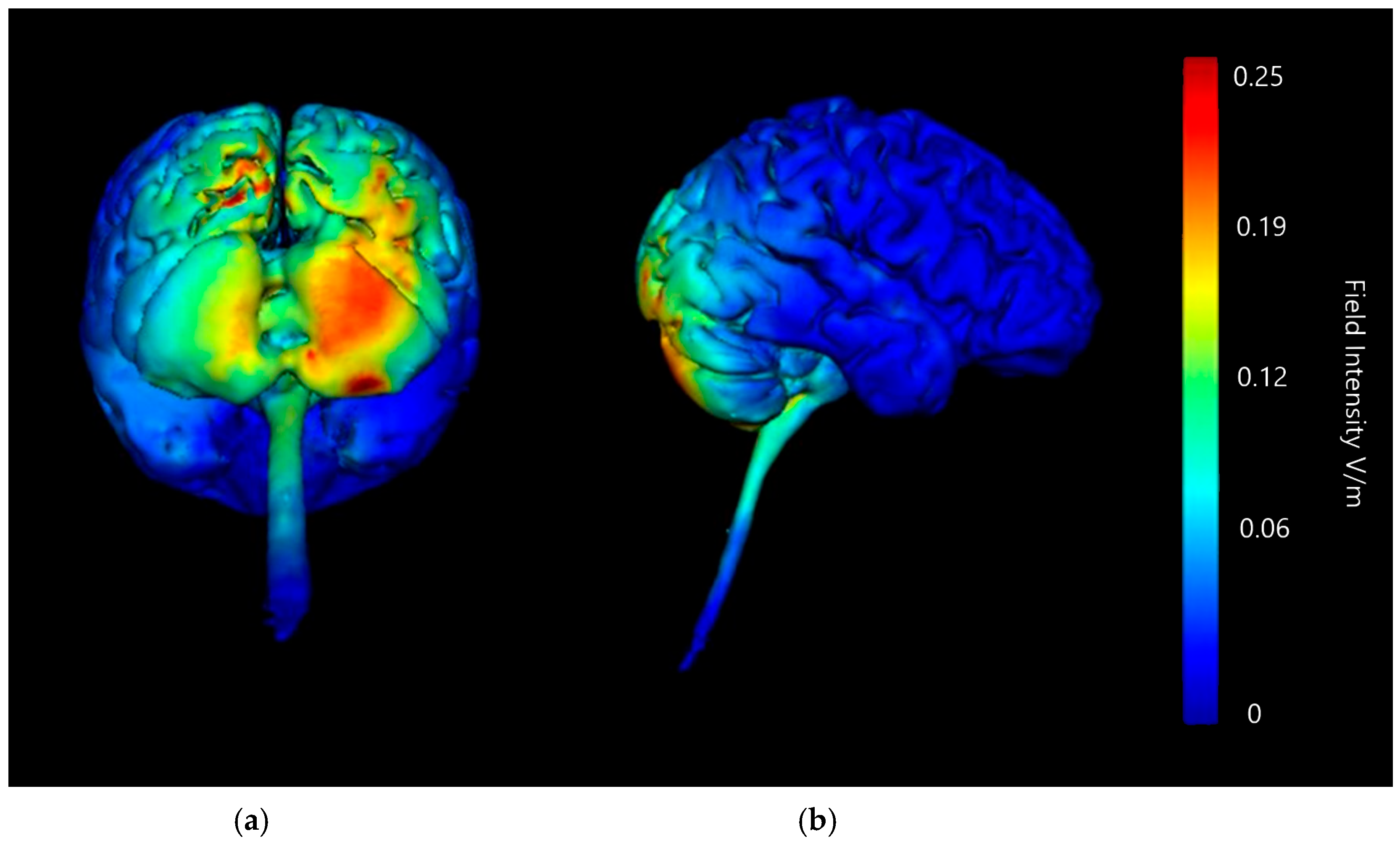
3.1. Anode and Cathode Positioning
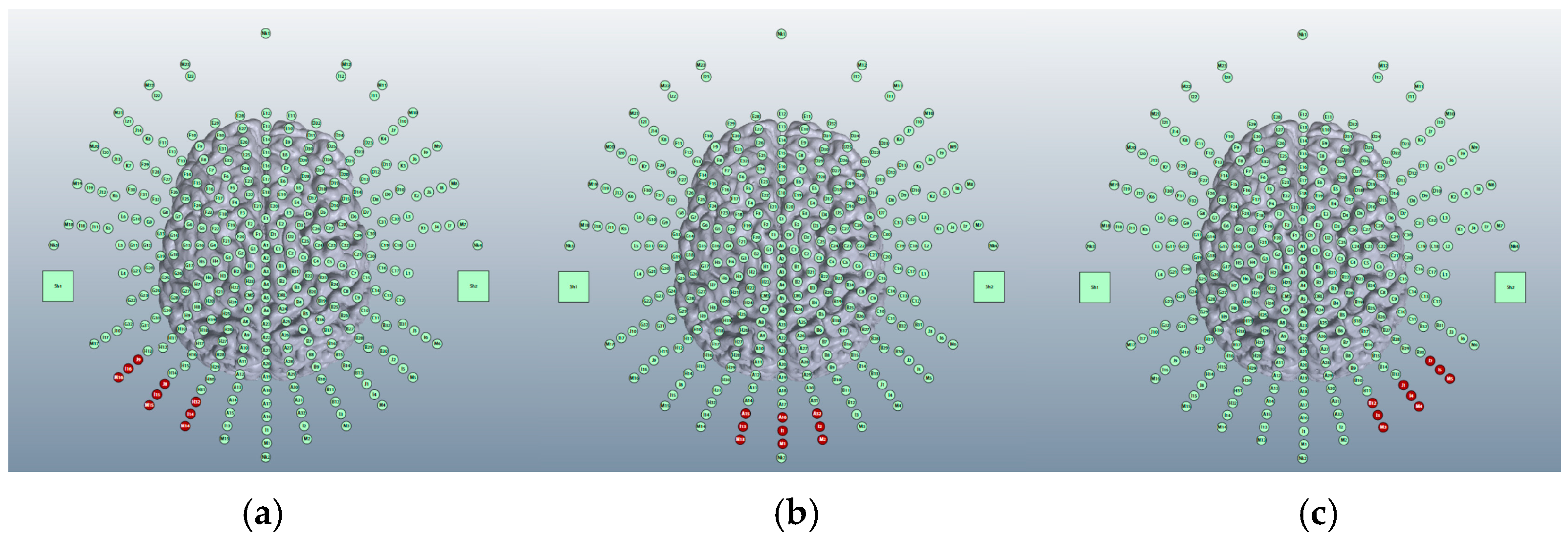
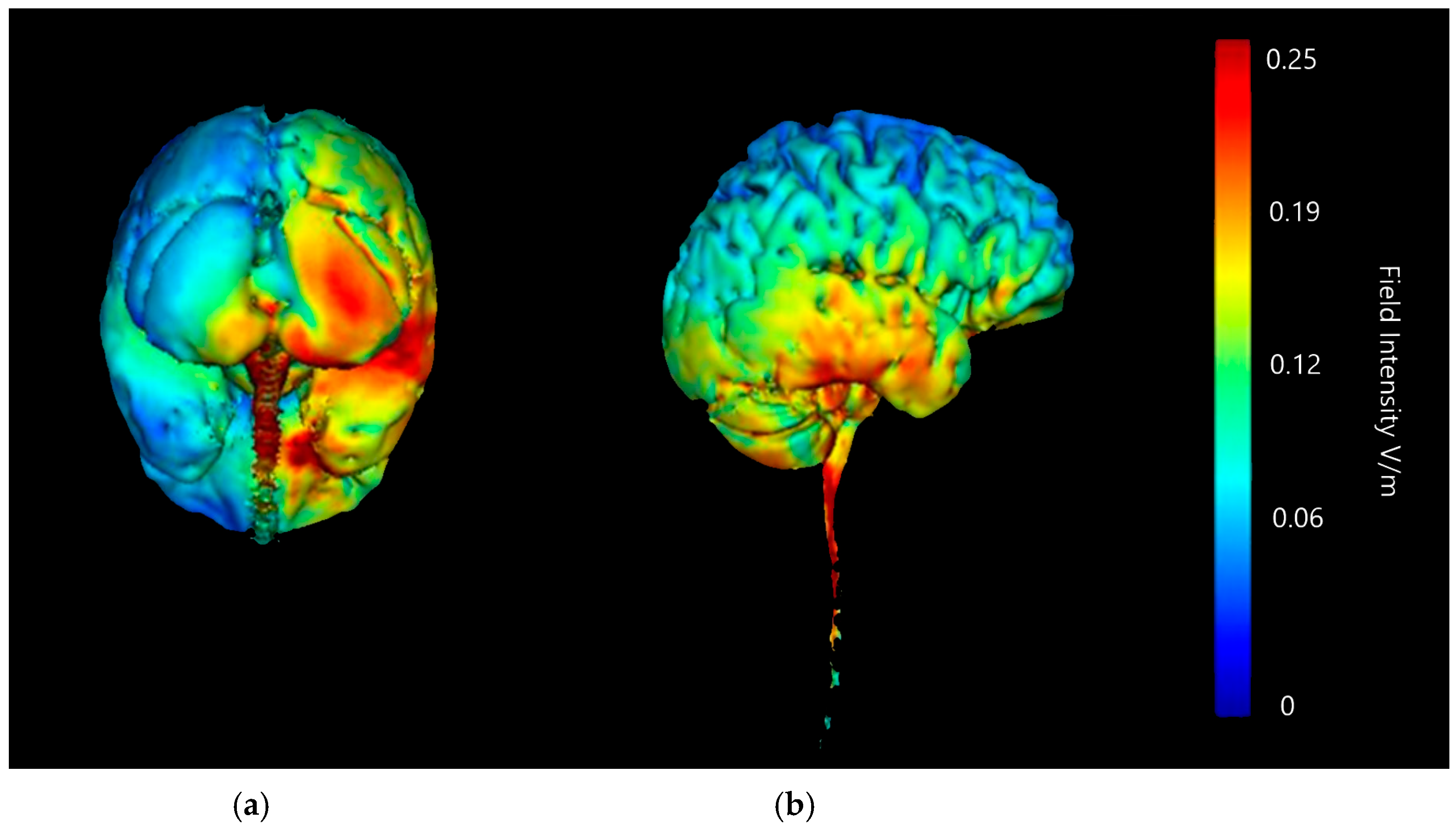
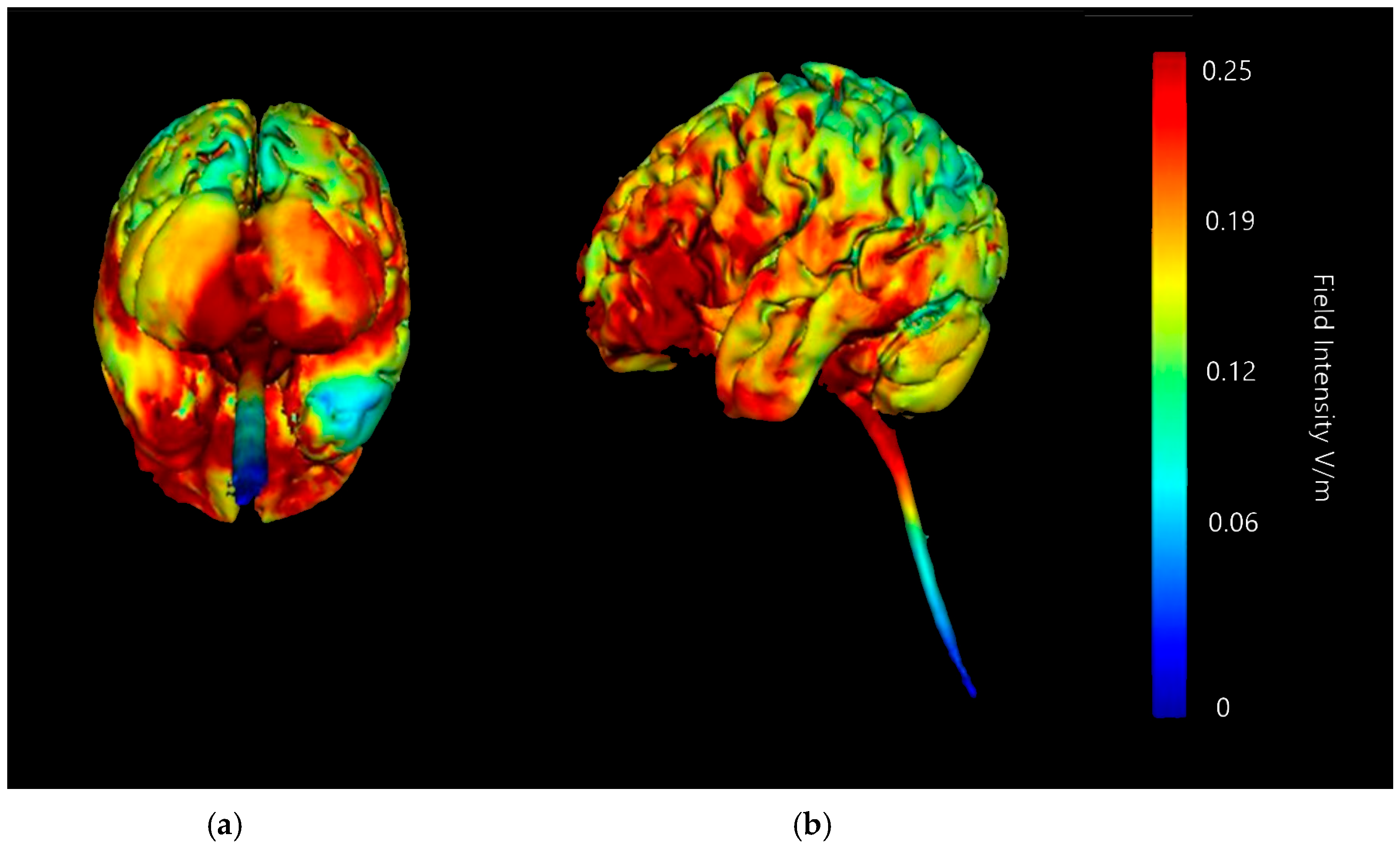
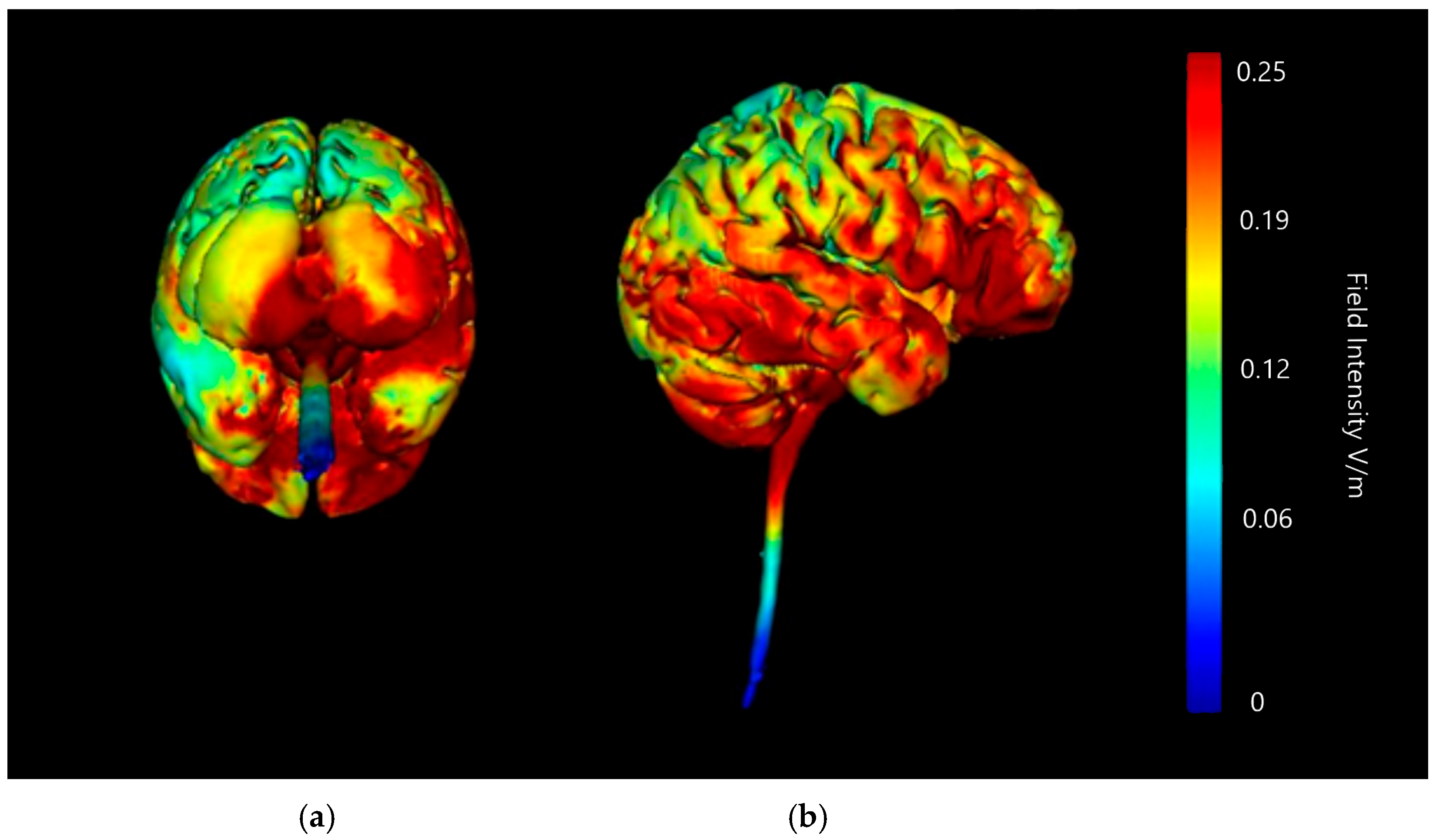
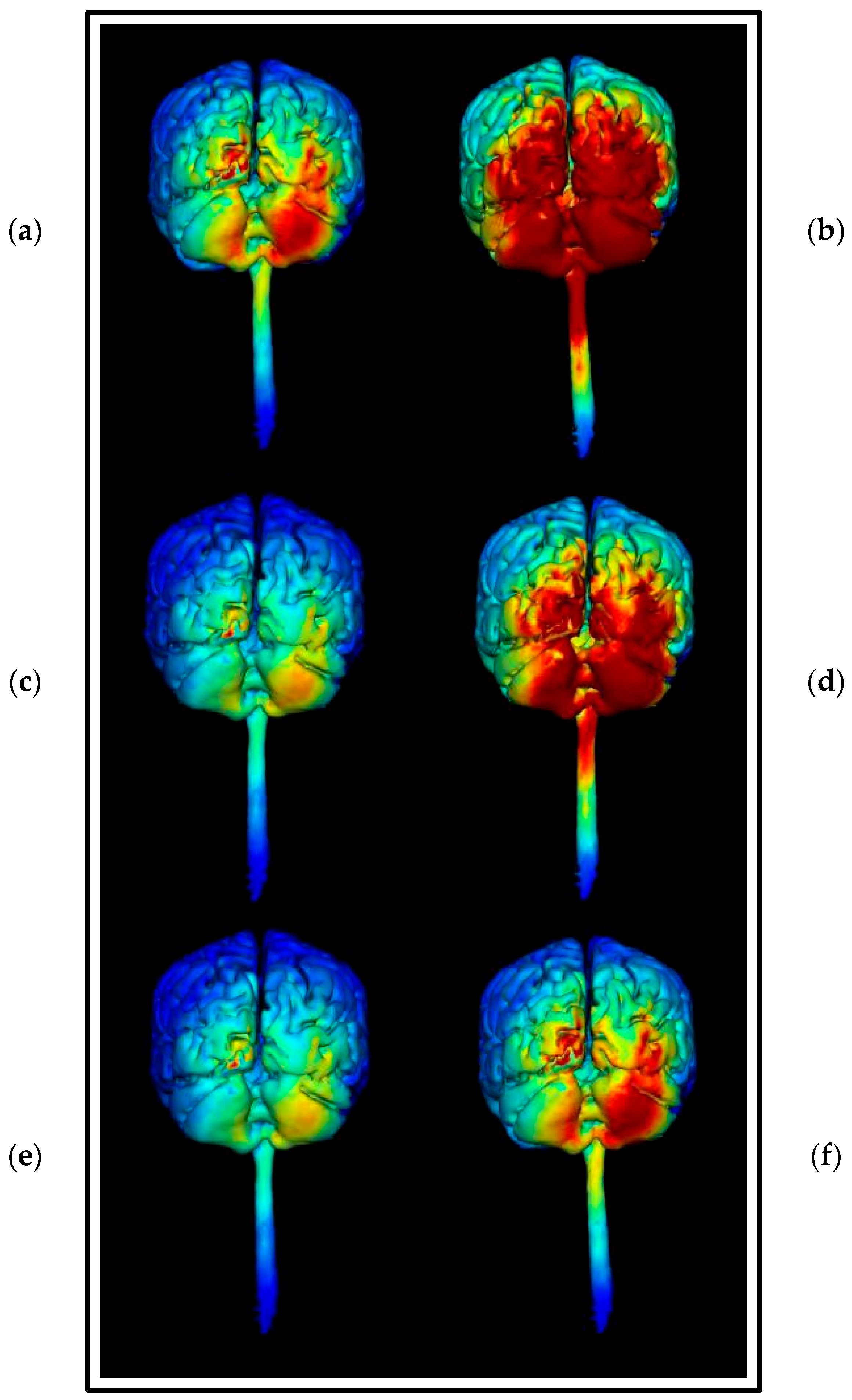
3.1.1. ctDCS Cathode Location
Buccinator Cathode
Supraorbital Cathode
Deltoid Cathode
3.1.2. Image Sensitivity of Cathode Placement Simulations
| Brain Areas | Cerebellar Lobe Diffusion (Left) | Cerebellar Lobe Diffusion (Right) | Spinal Cord Diffusion | Pons/ Medulla Diffusion | Occipital Lobe Diffusion (Left) | Occipital Lobe Diffusion (Right) | Parietal Lobe Diffusion (Left) | Parietal Lobe Diffusion (Right) | Temporal Lobe Diffusion | Frontal Lobe Diffusion (Left) | Frontal Lobe Diffusion (Right) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deltoid anode (left) | Low | High | Low | Low | Medium | Medium | None of note | None of note | None of note | None of note | None of note | Desirable |
| Deltoid anode (right) | Low | High | Very High | Low | None of note | Low | None of note | None of note | None of note | None of note | None of note | Undesirable |
| Buccinator anode (left) | Medium | Low | Very High | Medium | Low | Low | Medium | None of note | None of note | Low | None of note | Undesirable |
| Buccinator anode (right) | Low | High | High | Medium | None of note | Medium | None of note | High | None of note | None of note | Medium | Undesirable |
| Supra orbital (left) | High | Very High | Very High | Very High | Medium | Medium | High | Medium | Medium | Very High | Very High | Undesirable |
| Supra orbital (right) | High | Very High | Very High | Very High | Medium | Medium | Low | Very High | Medium | Very High | Very High | Undesirable |
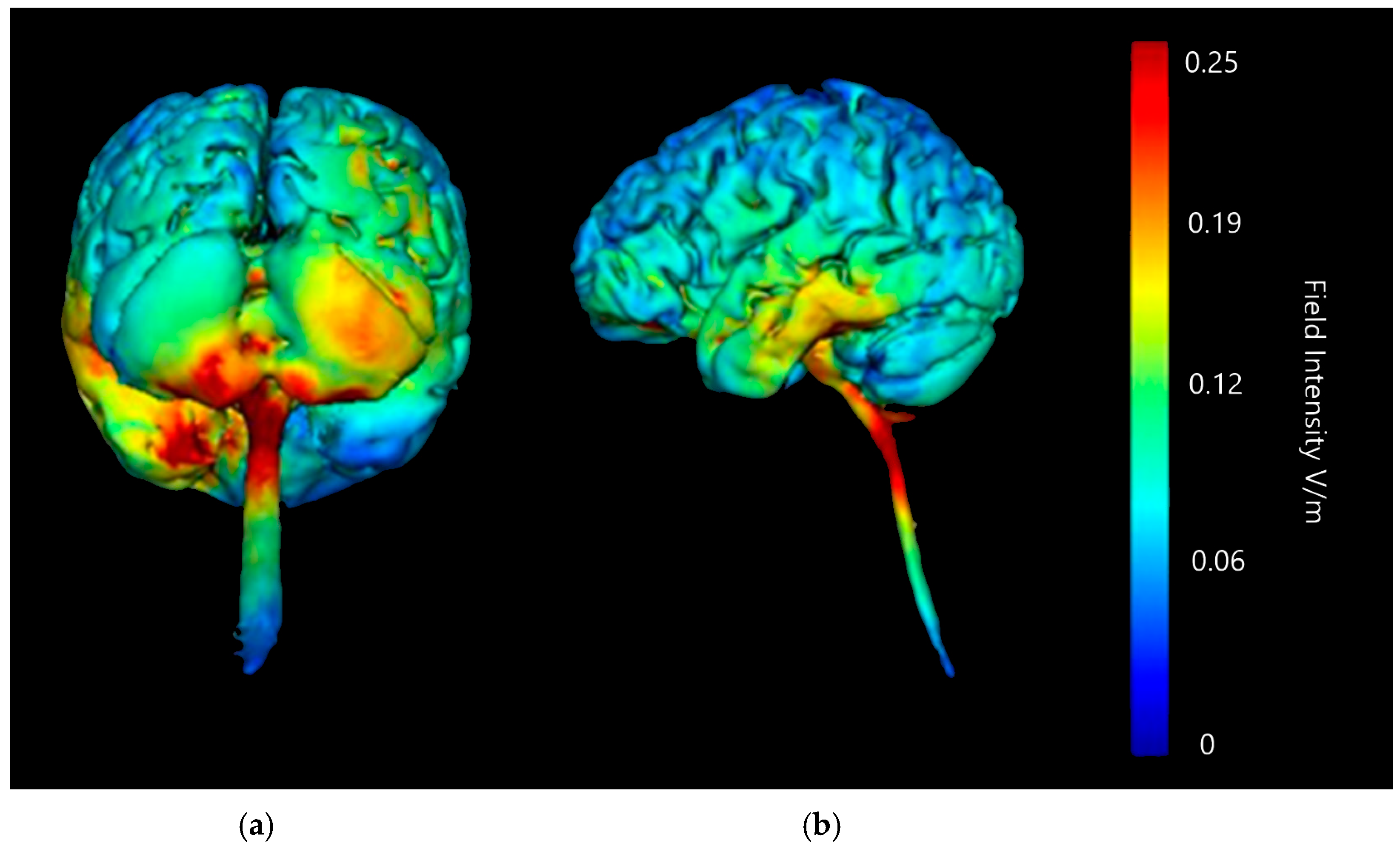
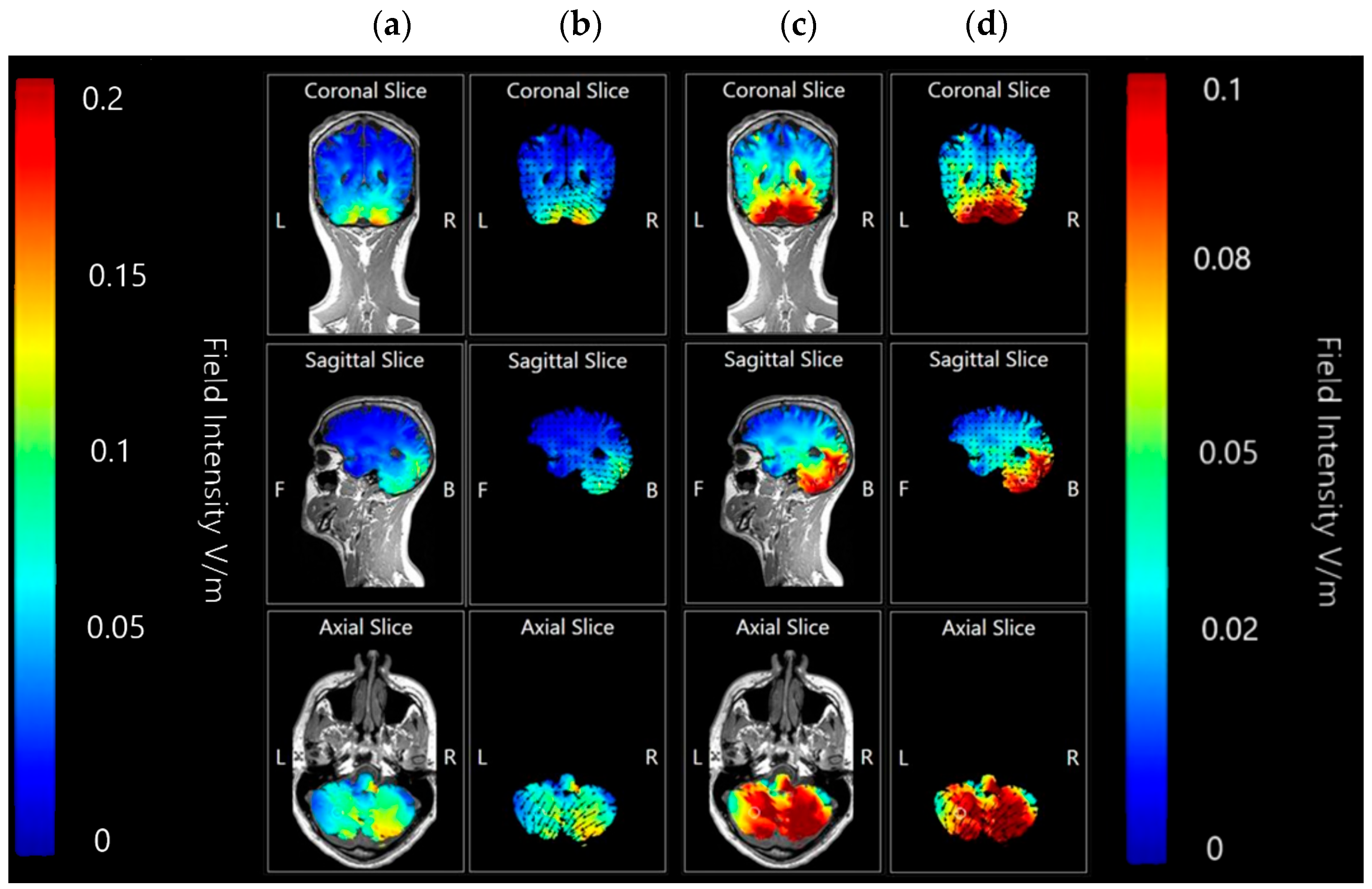
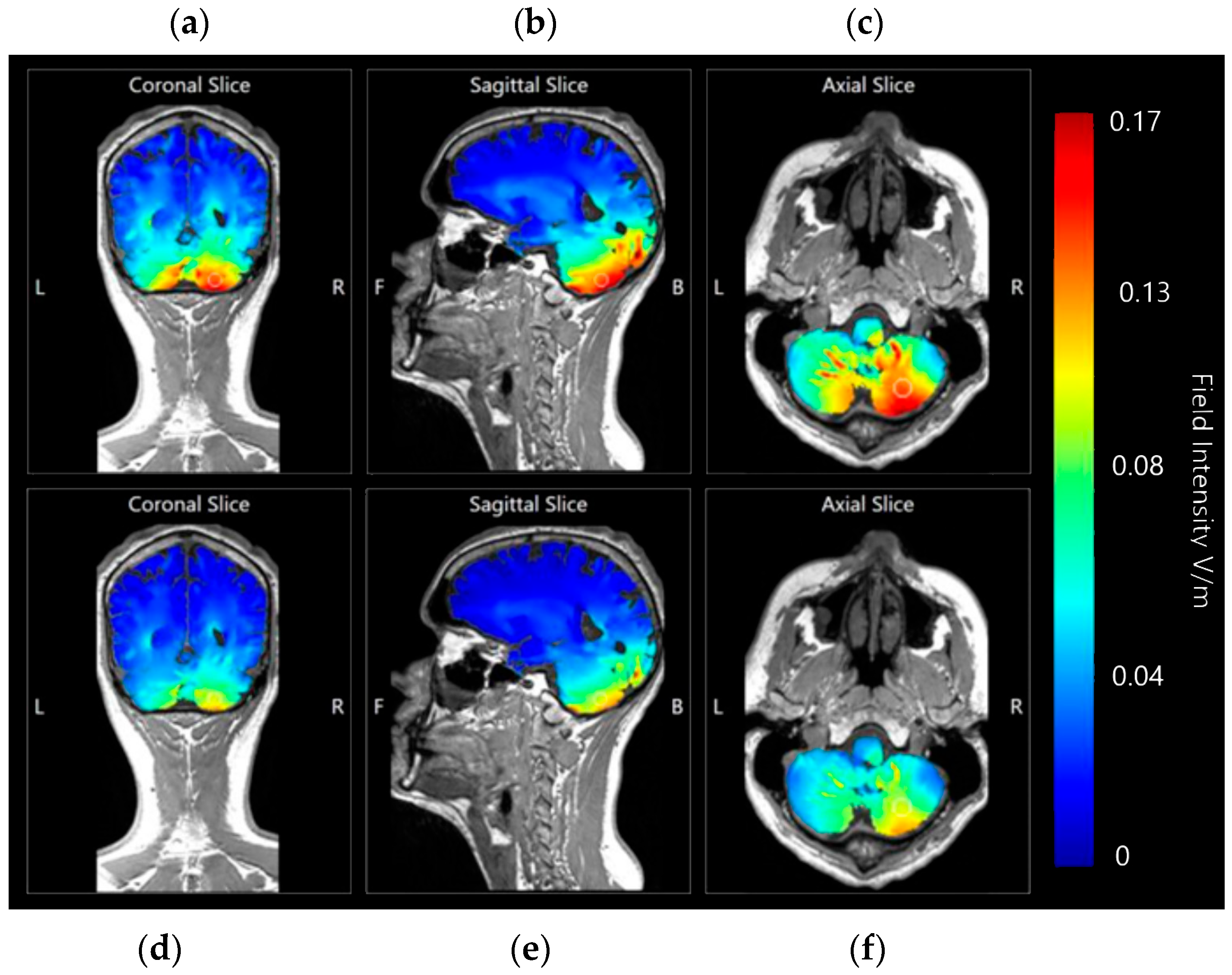
3.1.3. Internal Views—Coronal, Sagittal and Axial
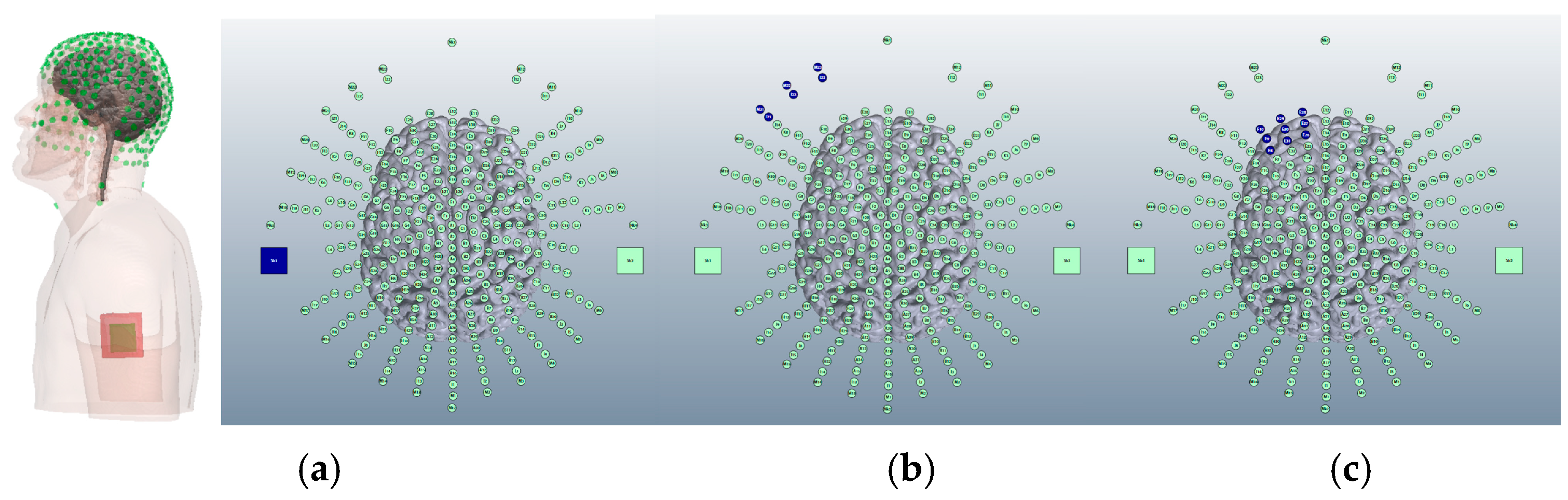
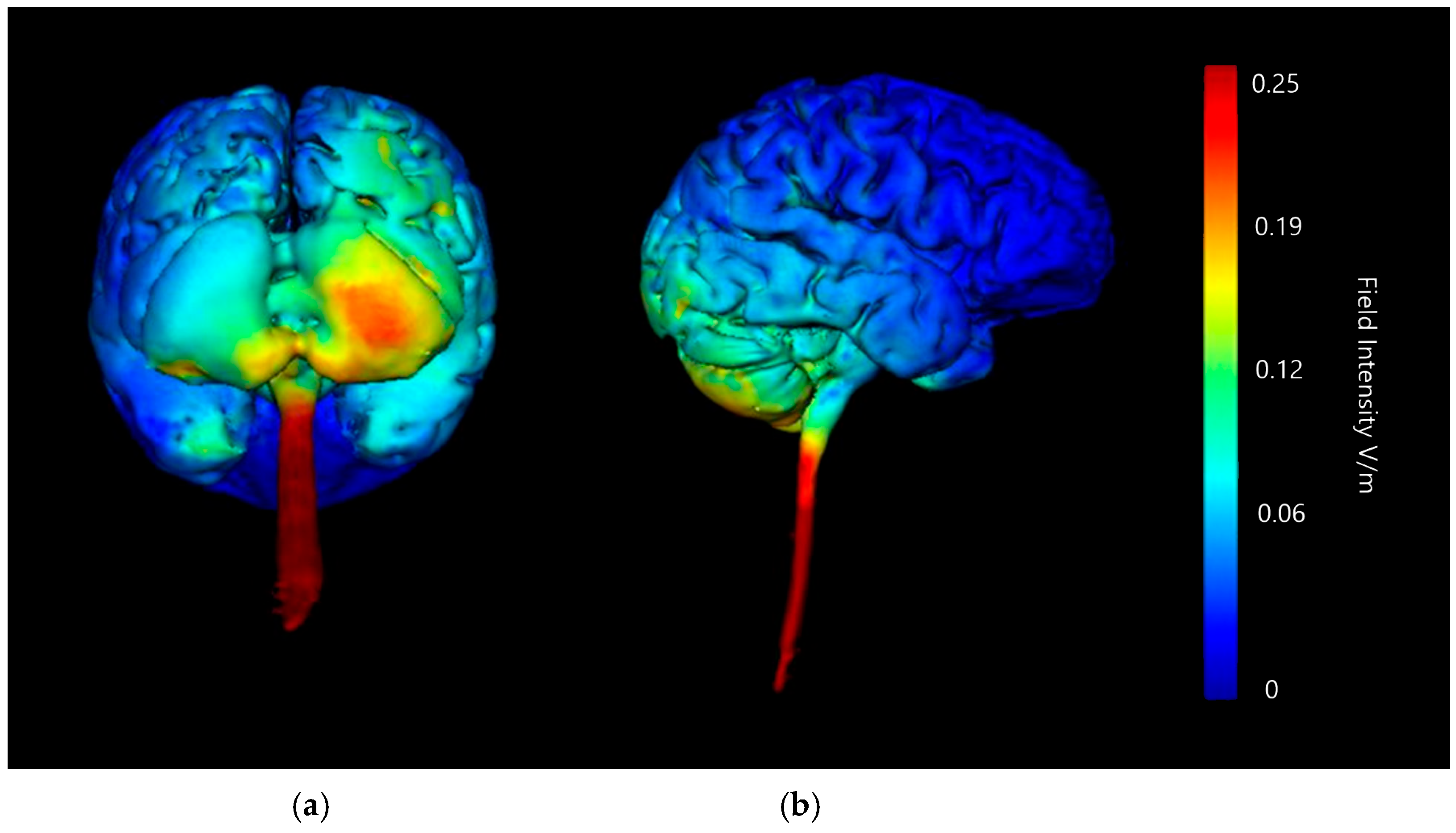
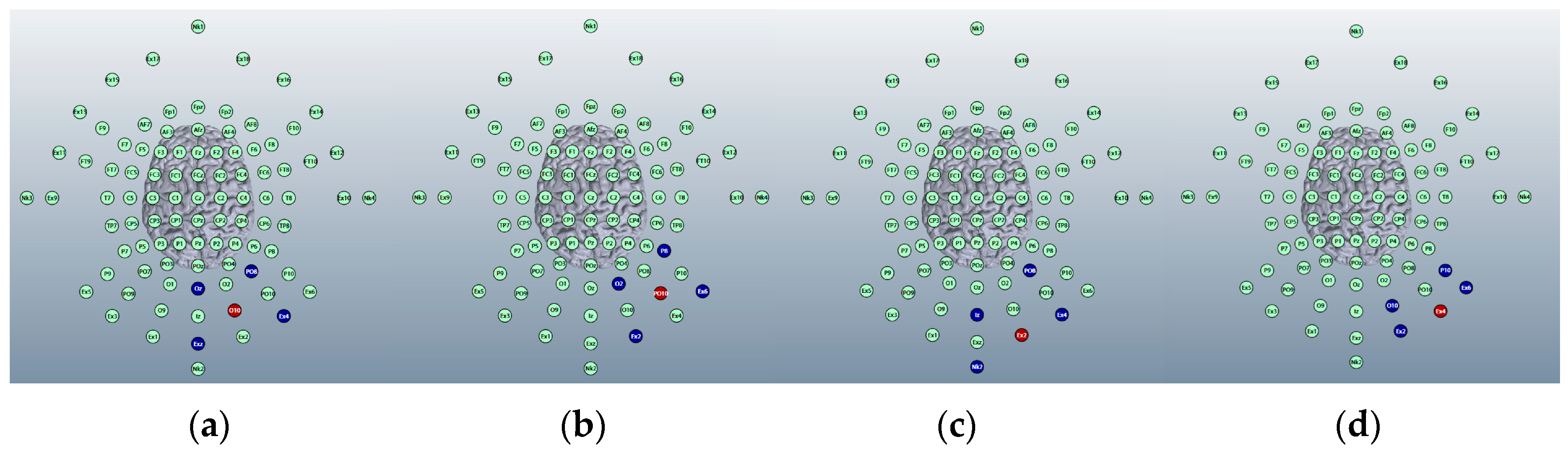
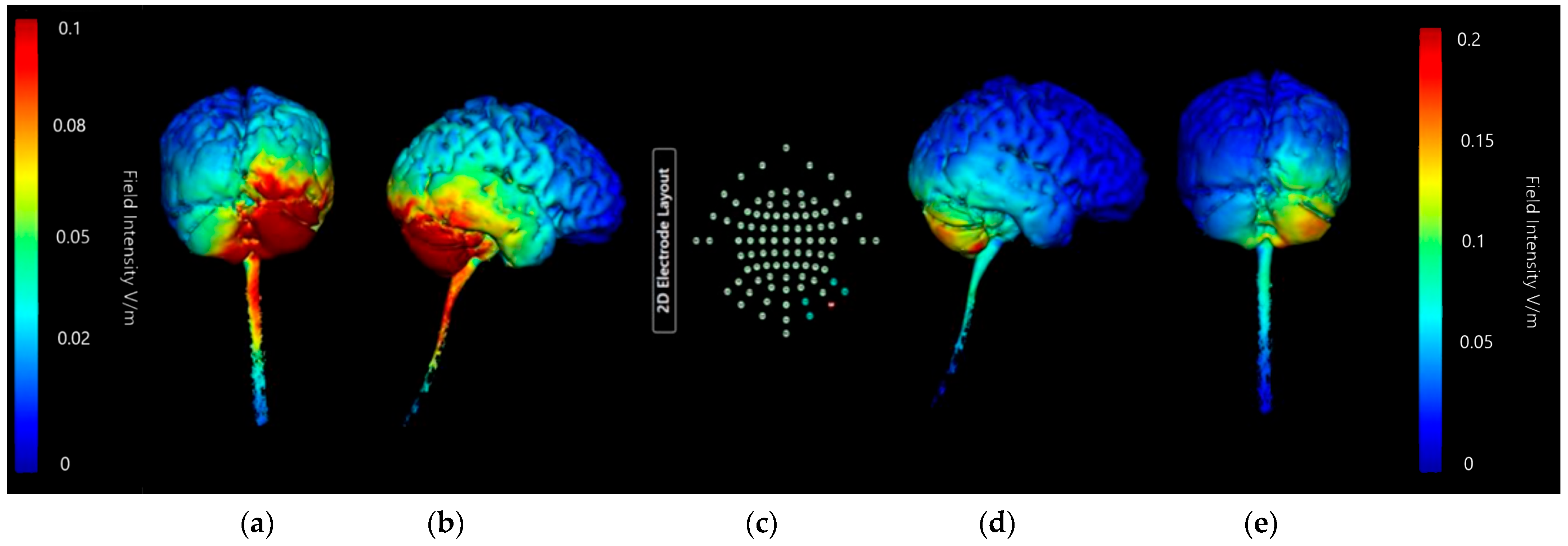
3.1.4. HD-ctDCS Anode and Cathode Montages
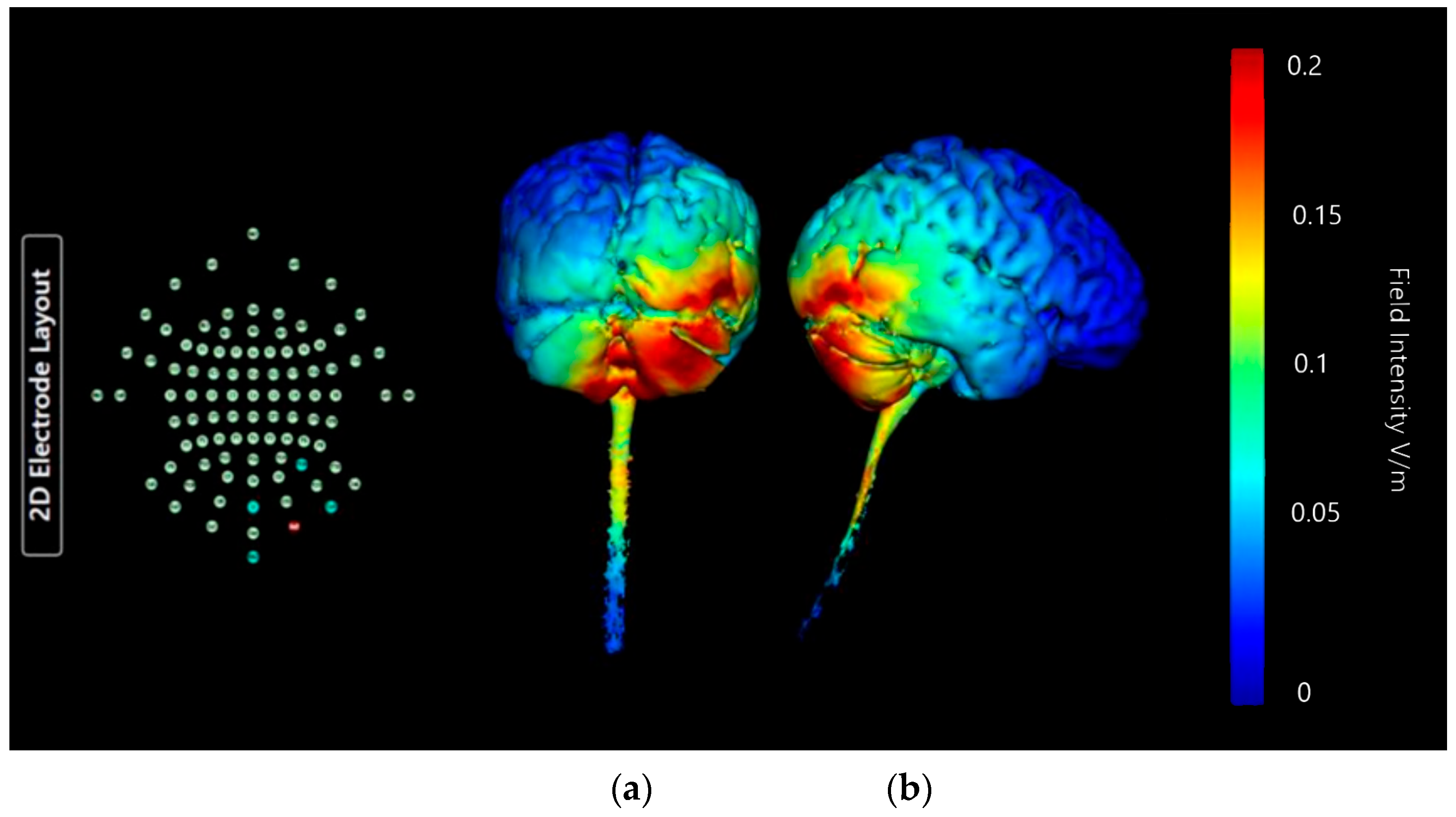
Montage 1: Anode O10, Cathodes Oz, PO8, Exz, and Ex4
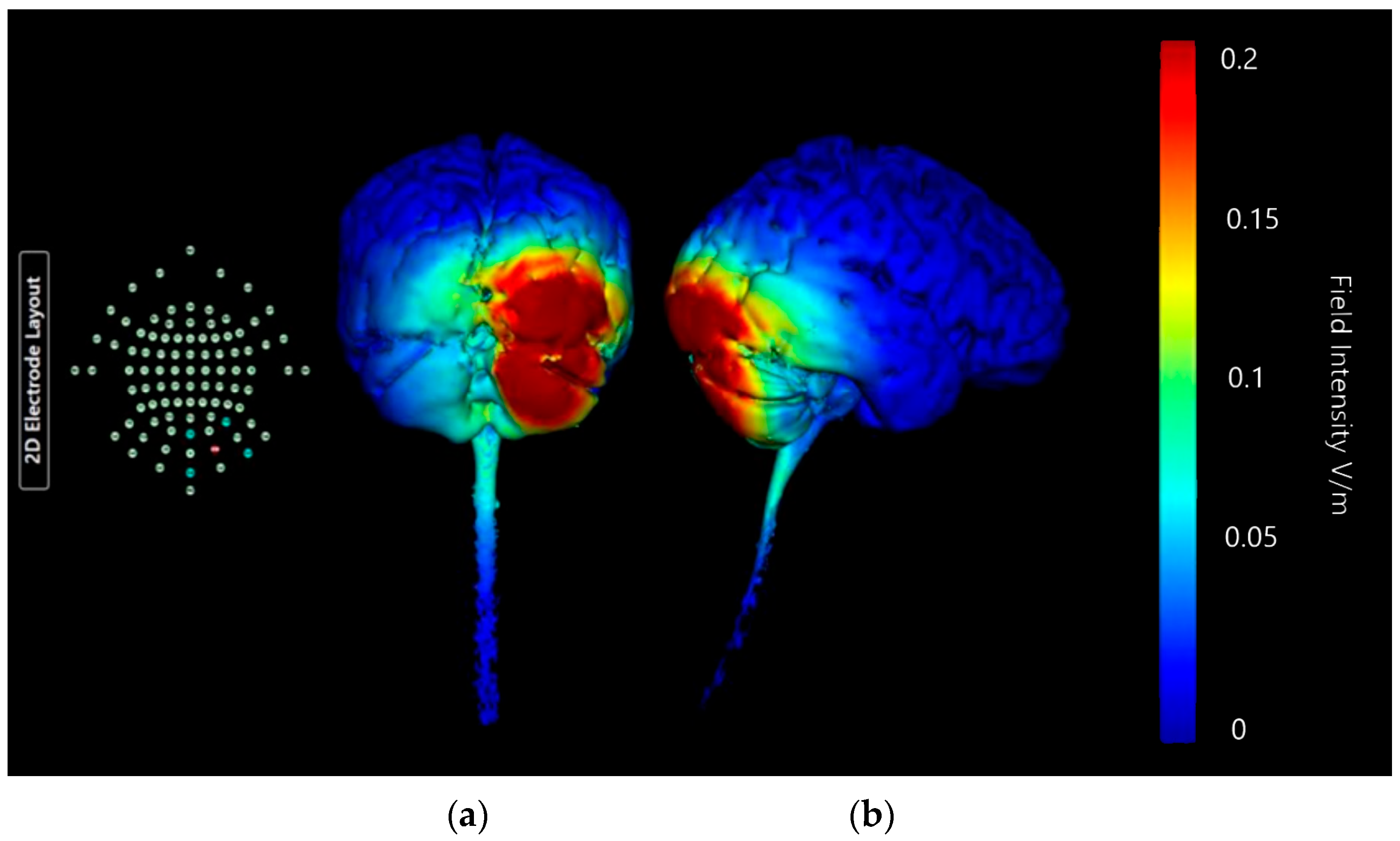
Montage 2: Anode PO10, Cathodes O2, P8, Ex2, and Ex6
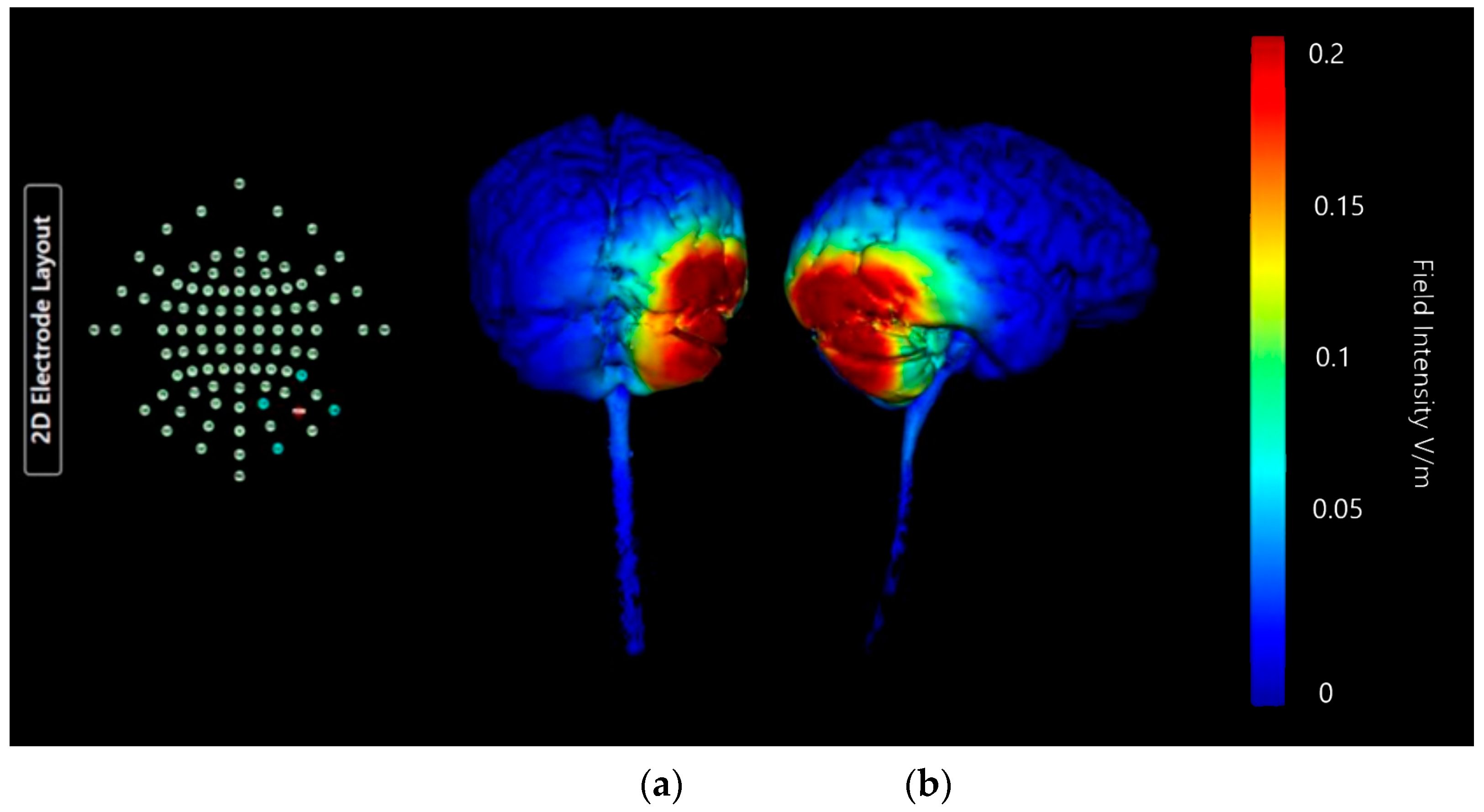
Montage 3: Anode Ex2, Cathodes Iz, Nk2, PO8, and Ex4
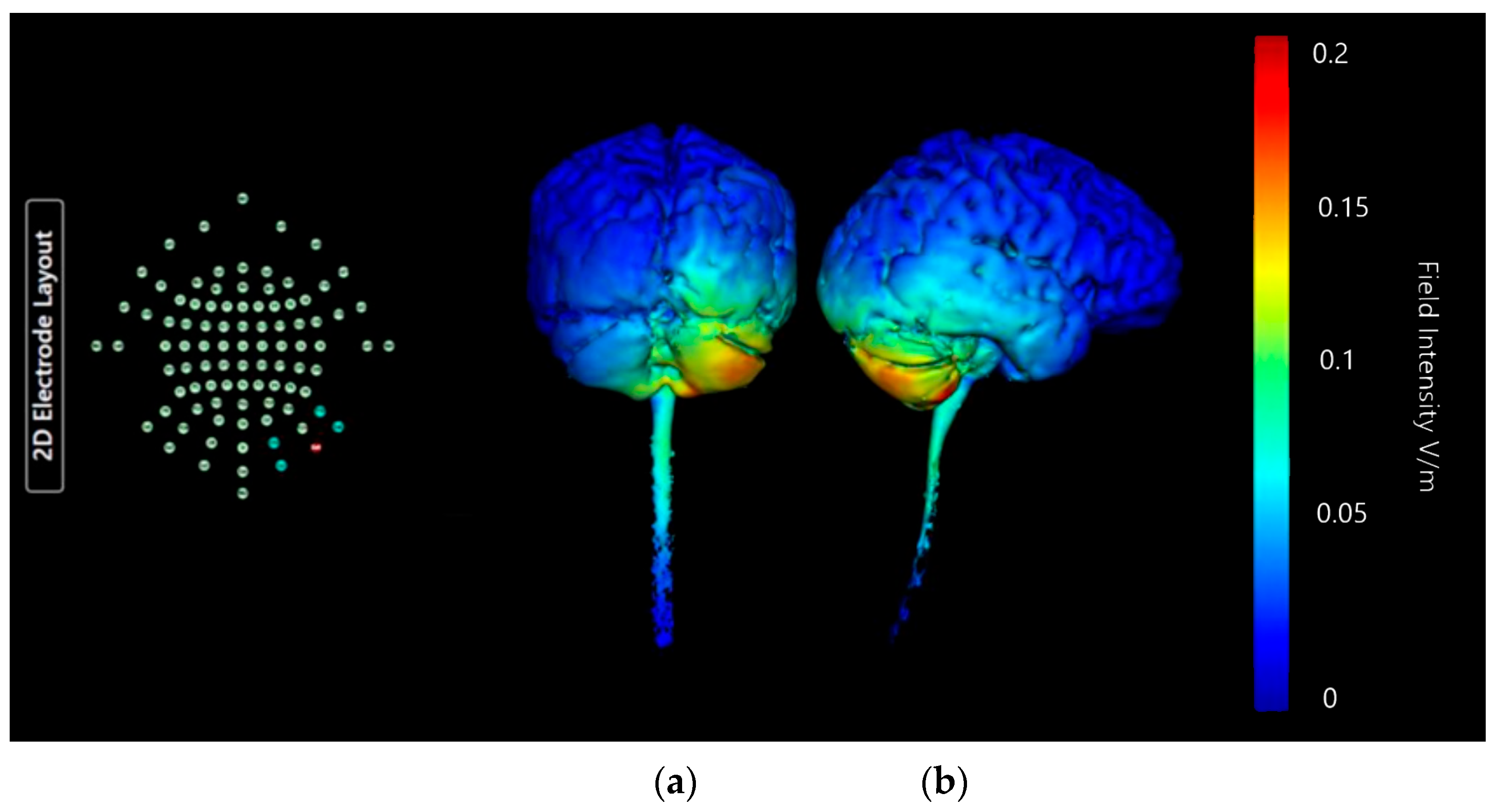
Montage 4: Anode Ex4, Cathodes O10, P10, Ex2, and Ex6
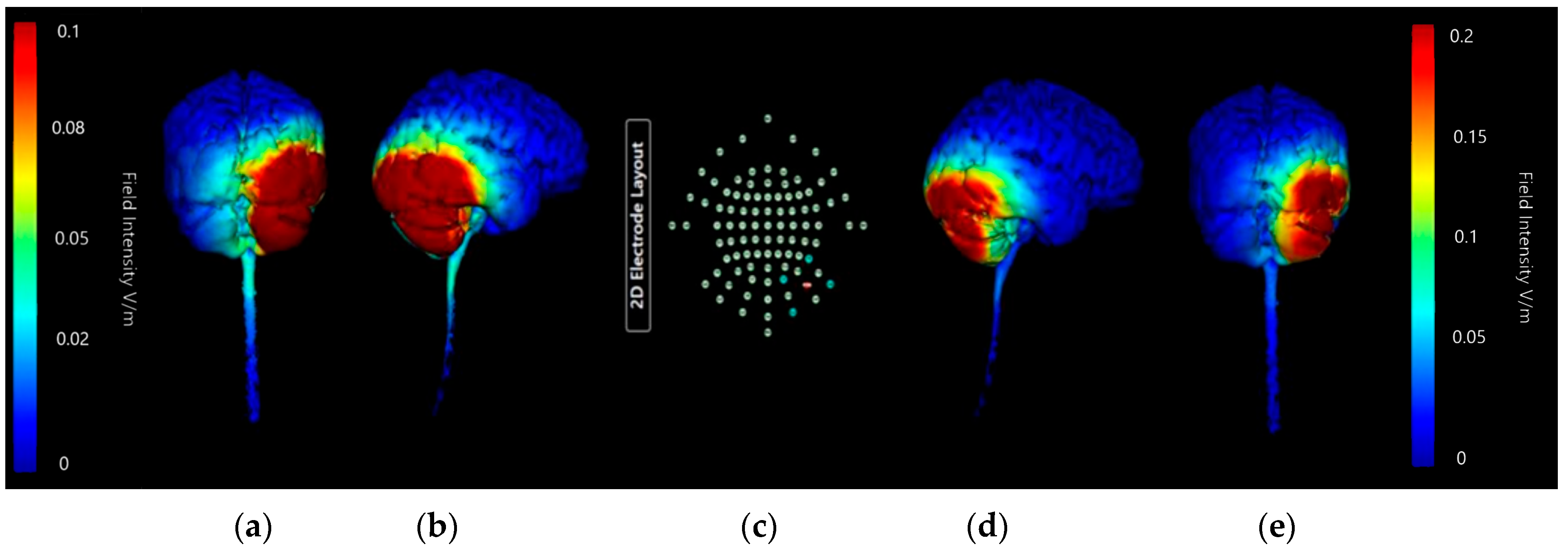
3.1.5. Choice of HD-ctDCS Montage
Comparing Montage 2 (Anode PO10) and Montage 4 (Anode Ex4): Increased Image Sensitivity
3.1.6. Summation
3.2. Current Strength
3.2.1. ctDCS Montage: Right Cerebellar Lobe and Left Deltoid Muscle
3.2.2. Summary of Findings
4. Discussion
4.1. How Is This Applicable to Pain?
4.2. Focality of Stimulation in HD-tDCS
4.3. Applications to Clinical Practice
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| tDCS | Trans-cranial Direct Current Stimulation |
| ctDCS | Cerebellar Trans-cranial Direct Current Stimulation |
| HD-ctDCS | High-Definition Cerebellar Trans-cranial Direct Current Stimulation |
| NIBS | Non-Invasive Brain Stimulation |
| PAG | Peri-aqueductal Grey |
| EEG | Electroencephalogram |
| IASP | International Association for the Study of Pain |
| MRI | Magnetic Resonance Imaging |
| fMRI | Functional Magnetic Resonance Imaging |
| PET | Positron Emission Topography |
| EMG | Electromyogram |
| mA | Milli-Amps |
| V/m | Volt per Metre |
| cm | Centimetre |
| Ag | Silver |
| AgCl | Silver Chloride |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
References
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; IASP: Washington, DC, USA, 2021; Available online: https://www.iasp-pain.org/publications/free-ebooks/classification-of-chronic-pain-second-edition-revised/ (accessed on 3 February 2025).
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Moulton, E.A.; Schmahmann, J.D.; Becerra, L.; Borsook, D. The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev. 2010, 65, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Ivry, R.B.; Sokolov, A.A. Consensus Paper: The Role of the Cerebellum in Perceptual Processes. Cerebellum 2015, 14, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Moulton, E.A.; Tully, S.; Schmahmann, J.D.; Becerra, L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum 2008, 7, 252–272. [Google Scholar] [CrossRef]
- Michelle Welman, F.H.S.; Smit, A.E.; Jongen, J.L.M.; Tibboel, D.; van der Geest, J.N.; Holstege, J.C. Pain Experience is Somatotopically Organized and Overlaps with Pain Anticipation in the Human Cerebellum. Cerebellum 2018, 17, 447–460. [Google Scholar] [CrossRef]
- Coombes, S.A.; Misra, G. Pain and motor processing in the human cerebellum. Pain 2016, 157, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, C.; Mohr, C.; Erdmann, C.; Binkofski, F.; Büchel, C. Neural activity related to self-versus externally generated painful stimuli reveals distinct differences in the lateral pain system in a parametric fMRI study. Hum. Brain Mapp. 2006, 27, 755–765. [Google Scholar] [CrossRef]
- Hofbauer, R.K.; Fiset, P.; Plourde, G.; Backman, S.B.; Bushnell, M.C. Dosedependent Effects of Propofol on the Central Processing of Thermal Pain. Anesthesiology 2004, 100, 386–394. [Google Scholar] [CrossRef]
- Helmchen, C.; Mohr, C.; Erdmann, C.; Binkofski, F. Cerebellar neural responses related to actively and passively applied noxious thermal stimulation in human subjects: A parametric fMRI study. Neurosci. Lett. 2004, 361, 237–240. [Google Scholar] [CrossRef]
- Priori, A.; Ciocca, M.; Parazzini, M.; Vergari, M.; Ferruci, R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J. Physiol. 2014, 592, 3345–3369. [Google Scholar]
- Huang, Y.; Su, Y.; Rorden, C.; Dmochowski, J.; Datta, A.; Parra, L.C. An automated method for high-definition transcranial direct current stimulation modelling. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, San Diego, CA, USA, 28 August–1 September 2012. [Google Scholar] [CrossRef]
- Guiomar, R.; Catoira, B.; Sobral, M.; Castilho, P.; Baeken, C.; Ganho-Avila, A. Cerebellar Transcranial Direct Current Stimulation for Schizophrenia: A Current Modelling Study. Psychiatr. Danub. Abstr. 2022, 34, 10–57. [Google Scholar]
- Tomlinson, S.P.; Davis, N.J.; Bracewell, R.M. Brain stimulation studies of nonmotor cerebellar function: A systematic review. Neurosci. Biobehav. Rev. 2013, 37, 766–789. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Paulus, W.; Nitsche, M.A. Facilitating myoelectric-control with transcranial direct current stimulation: A preliminary study in healthy humans. J. Neuroeng. Rehabil. 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Zuchowski, M.L.; Timmann, D.; Gerwig, M. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 2014, 7, 525–531. [Google Scholar] [CrossRef]
- Ferruci, R.; Brunoni, A.R.; Parazzini, M.; Vergari, M.; Rossi, E.; Fumagalli, M.; Mameli, F.; Rosa, M.; Giannicola, G.; Zago, S.; et al. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 2013, 12, 485–492. [Google Scholar] [CrossRef]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef]
- Grimaldi, G.; Manto, M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann. Biomed. Eng. 2013, 41, 2437–2447. [Google Scholar] [CrossRef]
- Purves, D. Neuroscience, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Castillo-Saavedra, L.; Gebodh, N.; Bikson, M.; Diaz-Cruz, C.; Brandao, R.; Coutinho, L.; Fregni, F. Clinically Effective Treatment of Fibromyalgia Pain with High-Definition Transcranial Direct Current Stimulation: Phase II Open-Label Dose Optimization. J. Pain 2016, 17, 14–26. [Google Scholar] [CrossRef]
- DosSantos, M.F.; Love, T.M.; Martikainen, I.K.; Nascimento, T.D.; Fregni, F.; Cummiford, C.; DaSilva, A.F.M. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. Psychiatry 2012, 3, 31714. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Lima, M.C.; Ferreira, M.J.L.; Wagner, T.; Rigonatti, S.P.; PascualLeone, A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Terney, D.; Kühnl, S.; Paulus, W. Anodal Transcranial Direct Current Stimulation of the Motor Cortex Ameliorates Chronic Pain and Reduces Short Intracortical Inhibition. J. Pain Symptom Manag. 2010, 39, 890–903. [Google Scholar] [CrossRef]
- Bocci, T.; De Carolis, G.; Ferrucci, R.; Paroli, M.; Priori, A.; Valeriani, M.; Sartucci, F. Cerebellar transcranial direct current stimulation (ctDCS) ameliorates phantom limb pain and nonpainful phantom limb sensations. Cerebellum 2019, 18, 527–535. [Google Scholar] [CrossRef]
- Sveva, V.; Cruciania, A.; Mancuso, M.; Santoro, F.; Latorre, A.; Monticone, M.; Rocchi, L. Cerebellar non-invasive brain stimulation; a frontier in chronic pain therapy. J. Pers. Med. 2024, 14, 675. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Leon, C.A.; Sanchez-Garrido Campos, G.; Fernadez, M.; Sanchez-Lopez, A.; Medina, J.F.; Marquez-Ruiz, J. Somatodendritic orientation determines tDCS-induced neuromodulation of Purkinje cell activity in awake mice. bioRxiv 2023. preprint. [Google Scholar]
- Barroso, J.; Branco, P.; Apkarian, A.V. Brain mechanisms of chronic pain: Critical role of translational approach. Transl. Res. 2021, 238, 76–89. [Google Scholar] [CrossRef]
- Coghill, R.C.; Sang, N.C.N.; Maisog, J.M.; Iadorola, M.J. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Moayedi, M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. J. Pain 2017, 18, 1027–1035. [Google Scholar] [CrossRef]
- Silva, A.F.; Zortea, M.; Carvalho, S.; Leite, J.; da Silva Torres, I.L.; Fregni, F.; Caumo, W. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: Randomized clinical trial. Sci. Rep. 2017, 7, 135. [Google Scholar] [CrossRef]
- Saab, C.Y.; Kawasaki, M.; Al-Chaer, E.D.; Willis, W.D. Cerebellar cortical stimulation increases spinal visceral nociceptive responses. J. Neurophysiol. 2001, 85, 2359–2363. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Martins, D.F.; Medeiros, L.F.; Nucci-Martins, C.; Martins, T.C.; Siteneski, A.; Caumo, W.; dos Santos, A.R.S.; Torres, I.L.S. Neurobiological mechanisms of antiallodynic effect of transcranial direct current stimulation (tDCS) in a mice model of neuropathic pain. Brain Res. 2018, 1682, 14–23. [Google Scholar] [CrossRef]
- Saab, C.Y.; Wilis, W.D. The cerebellum: Organization, functions and its role in nociception. Brain Res. 2003, 42, 85–95. [Google Scholar]
- Witney, A. From Mechanisms to Analgesia: Towards the Use of Non-Invasive Neuromodulation for Pain Relief in the Clinic; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Klaus, J.; Schutter, D.J.L.G. Electrode montage-dependent intracranial variability in electric fields induced by cerebellar transcranial direct current stimulation. Sci. Rep. 2021, 11, 22183. [Google Scholar] [CrossRef]
- Batsikadze, G.; Rezaee, Z.; Chang, D.I.; Gerwig, M.; Herlitze, S.; Dutta, A.; Nitsche, M.A.; Timmann, D. Effects of cerebellar transcranial direct current stimulation on cerebellar-brain inhibition in humans: A systematic evaluation. Brain Stimul. 2019, 12, 1177–1186. [Google Scholar] [CrossRef]
- Ferruci, R.; Cortese, F.; Priori, A. Cerebellar tDCS: How to Do It. Cerebellum 2015, 14, 27–30. [Google Scholar] [CrossRef]
- Li, S.; Tang, Y.; Zhou, Y.; Ni, Y. Effects of Transcranial Direct Current Stimulation on Cognitive Function in Older Adults with and without Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Geriatr. Gerontol. 2024, 70, 544–560. [Google Scholar] [CrossRef]




| Montage | Anode Position | Cathode Positions |
|---|---|---|
| 1 | O10 | Oz |
| PO8 | ||
| Exz | ||
| Ex4 | ||
| 2 | PO10 | O2 |
| P8 | ||
| Ex2 | ||
| Ex6 | ||
| 3 | Ex2 | Iz |
| NK2 | ||
| PO8 | ||
| Ex4 | ||
| 4 | Ex4 | O10 |
| P10 | ||
| Ex2 | ||
| Ex6 |
| Cerebellar Lobe Diffusion (Left) | Cerebellar Lobe Diffusion (Right) | Spinal Cord Diffusion | Pons/ Medulla Diffusion | Occipital Lobe Diffusion (Left) | Occipital Lobe Diffusion (Right) | Parietal Lobe Diffusion (Left) | Parietal Lobe Diffusion (Right) | Temporal Lobe Diffusion | Frontal Lobe Diffusion (Left) | Frontal Lobe Diffusion (Right) | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Montage 1: Anode O10 | None of note | High | None of note | None of note | None of note | Very High | None of note | None of note | None of note | None of note | None of note | Potential |
| Montage 2: Anode PO10 | None of note | High | None of note | None of note | None of note | High | None of note | Low | None of note | None of note | None of note | Potential |
| Montage 3: Anode Ex2 | High | High | Medium | Medium | Low | High | None of note | Low | Low | None of note | None of note | Undesirable |
| Montage 4: Anode Ex4 | Very Low | Medium | None of note | None of note | None of note | Low | None of note | Low | None of note | None of note | None of note | High Potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnell Sharp, A.; Witney, A. Optimising Electrode Montages in Conventional Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation of the Cerebellum for Pain Modulation. Brain Sci. 2025, 15, 344. https://doi.org/10.3390/brainsci15040344
Farnell Sharp A, Witney A. Optimising Electrode Montages in Conventional Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation of the Cerebellum for Pain Modulation. Brain Sciences. 2025; 15(4):344. https://doi.org/10.3390/brainsci15040344
Chicago/Turabian StyleFarnell Sharp, Adelais, and Alice Witney. 2025. "Optimising Electrode Montages in Conventional Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation of the Cerebellum for Pain Modulation" Brain Sciences 15, no. 4: 344. https://doi.org/10.3390/brainsci15040344
APA StyleFarnell Sharp, A., & Witney, A. (2025). Optimising Electrode Montages in Conventional Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation of the Cerebellum for Pain Modulation. Brain Sciences, 15(4), 344. https://doi.org/10.3390/brainsci15040344






