The Bulb, the Brain and the Being: New Insights into Olfactory System Anatomy, Organization and Connectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Brain Specimen
2.2. White Matter Dissection
2.3. HCP Database Subjects
2.4. Multimodal Testing Data
2.5. Fiber Tracking Analysis
2.6. In Vivo Explorative White Matter Analysis
2.7. Regional-Based Analysis (Cohort 1)
2.8. Atlas-Based Analysis (Cohort 2)
2.9. Statistical Analysis of Tractography Data and Multimodal Testing Scores
3. Results
3.1. Ex Vivo Measurement of White Matter Structures
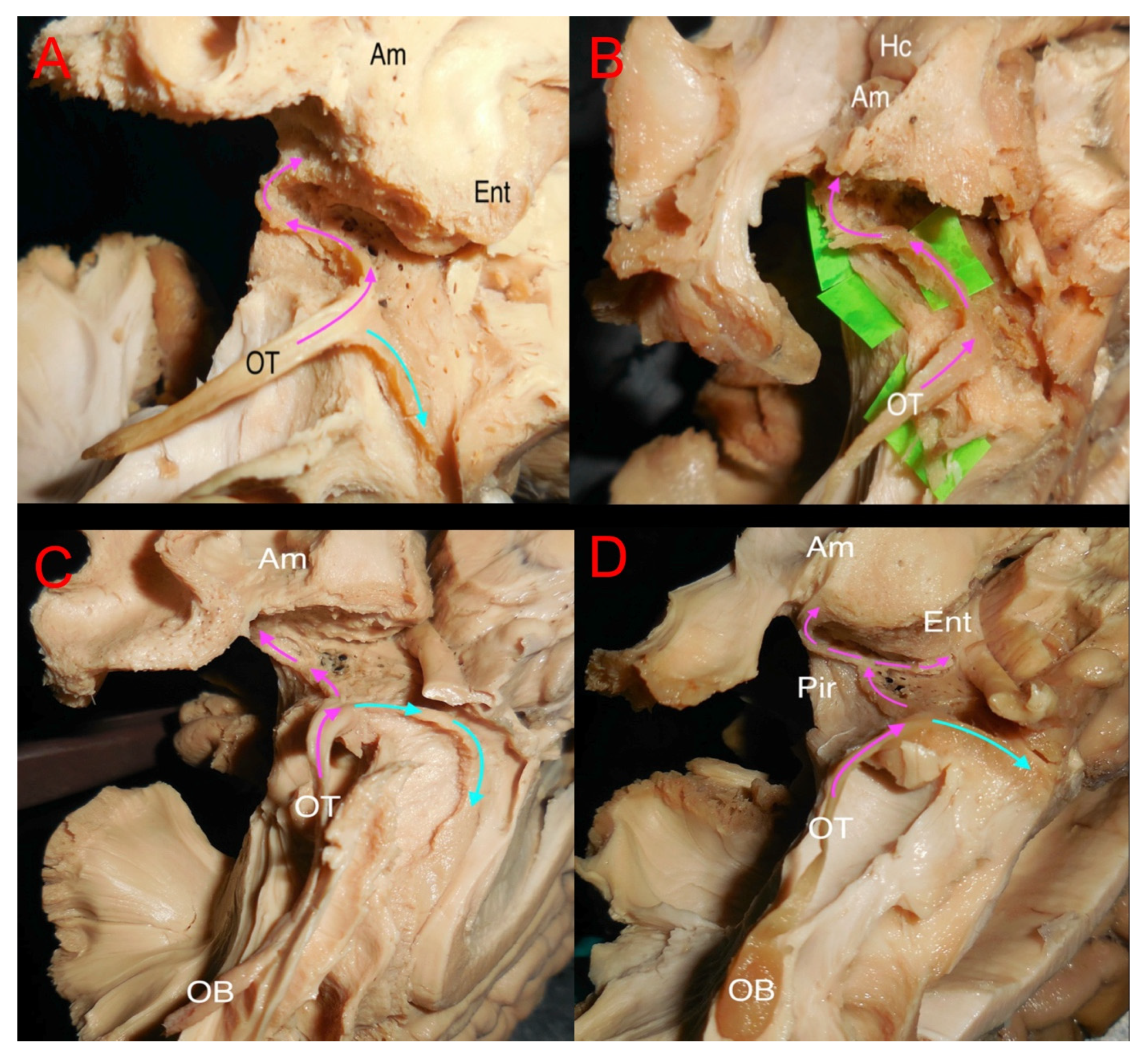
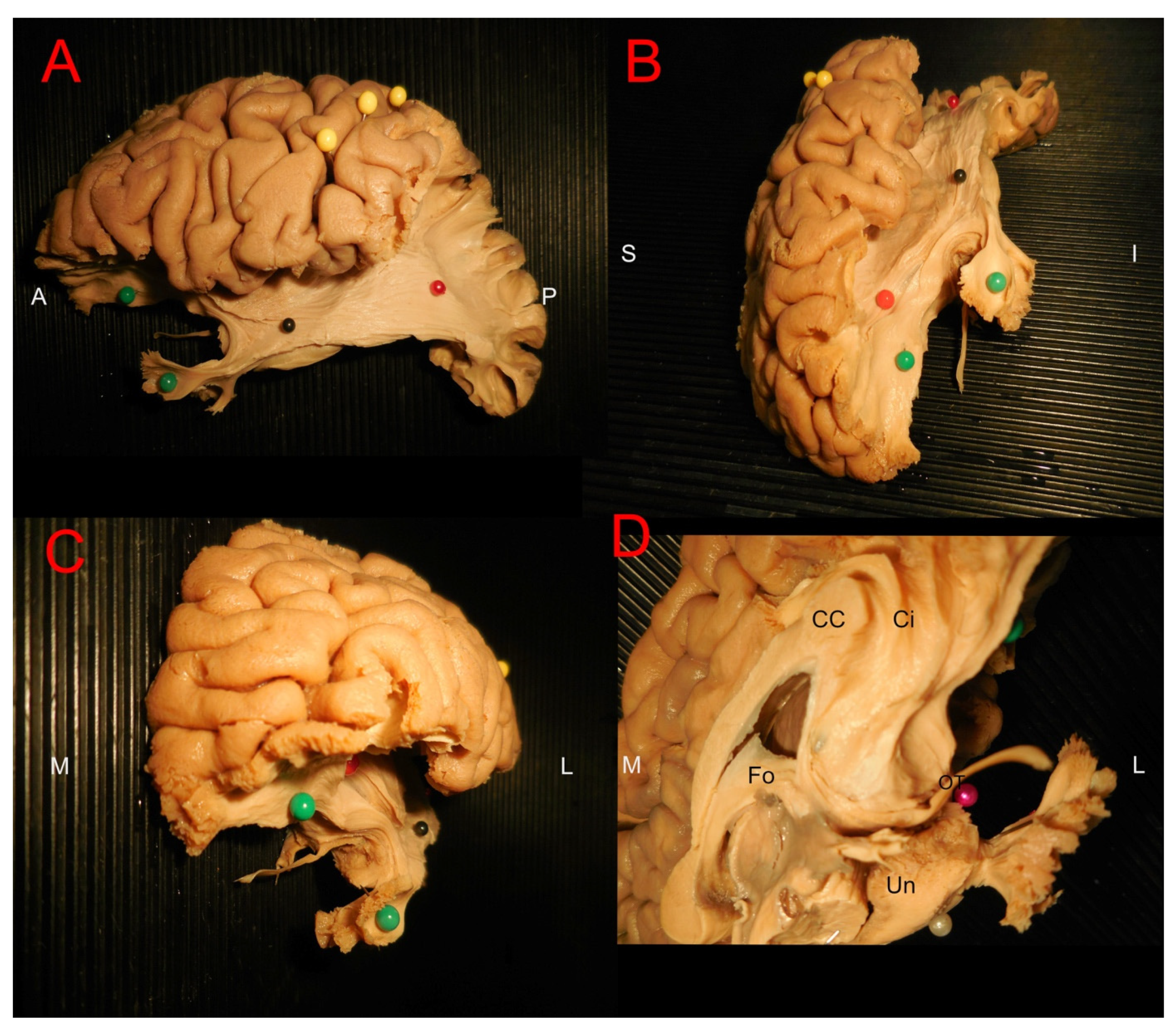
3.2. Ex Vivo White Matter Dissection and Analysis
3.3. Tractographic White Matter Analysis
3.4. Sensory and Cognitive Correlations (Cohort 2)
4. Discussion
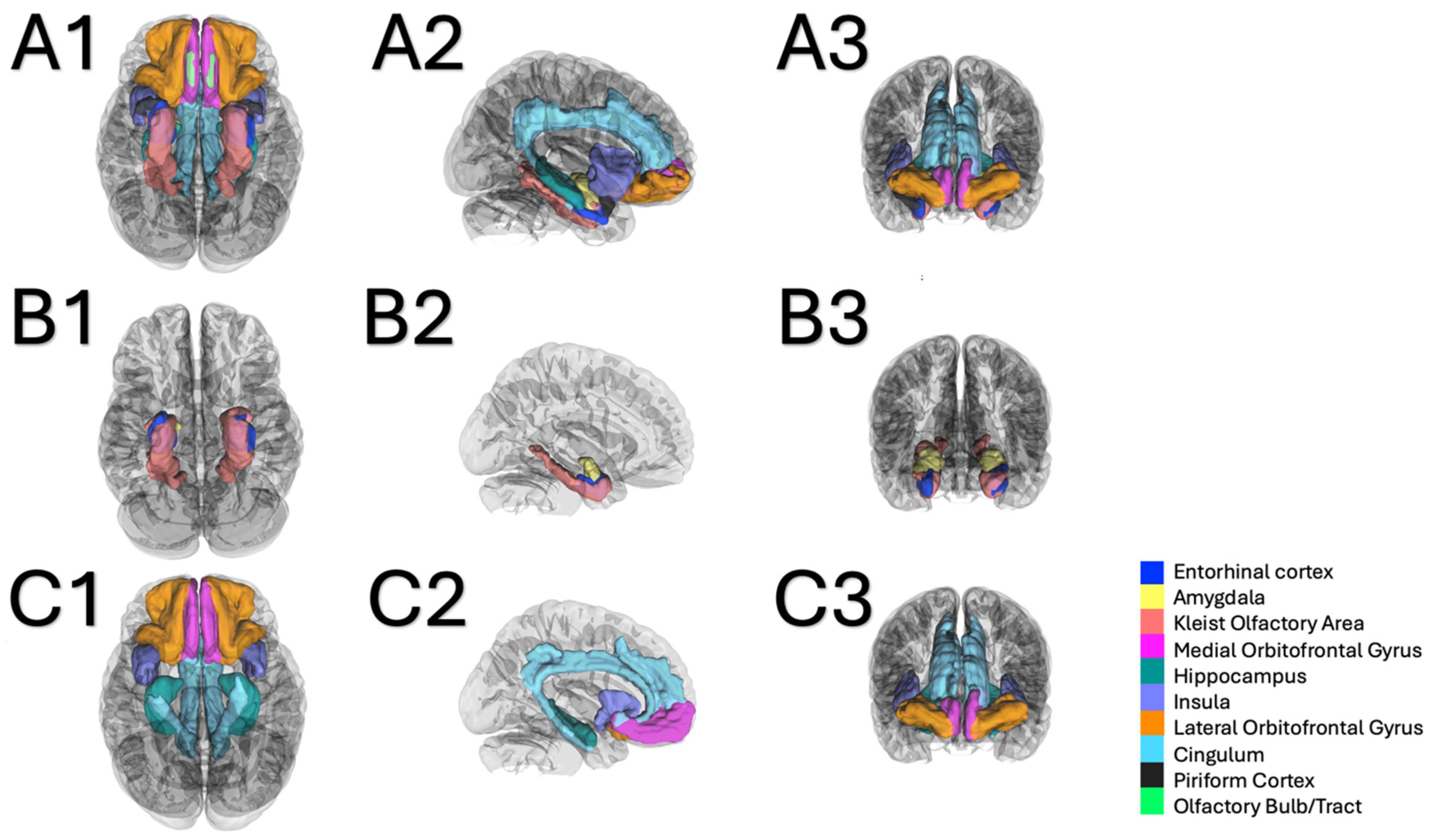
4.1. Olfactory System Connectivity: Primary Olfactory Network
4.2. Olfactory System Connectivity: Secondary Olfactory Network
4.3. Sensory and Cognitive Implications
4.4. Clinical Implications and Future Perspectives
4.5. Limitations and Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Corpus callosum |
| UF | Uncinate fasciculus |
| IFOF | Occipitofrontal fasciculus |
| AC | Anterior commissure |
| CING | Cingulum |
References
- Fjældstad, A. Testing olfactory function and mapping the structural olfactory networks in the brain. Dan. Med. J. 2018, 65, B5428. [Google Scholar] [PubMed]
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life—An updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Dikeçligil, G.N.; Gottfried, J.A. What Does the Human Olfactory System Do, and How Does It Do It? Annu. Rev. Psychol. 2024, 75, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Bratman, G.N.; Bembibre, C.; Daily, G.C.; Doty, R.L.; Hummel, T.; Jacobs, L.F.; Kahn, P.H.; Lashus, C.; Majid, A.; Miller, J.D.; et al. Nature and human well-being: The olfactory pathway. Sci. Adv. 2024, 10, eadn3028. [Google Scholar] [CrossRef]
- MacDonald, S.W.S.; Keller, C.J.C.; Brewster, P.W.H.; Dixon, R.A. Contrasting olfaction, vision, and audition as predictors of cognitive change and impairment in non-demented older adults. Neuropsychology 2018, 32, 450–460. [Google Scholar] [CrossRef]
- Croy, I.; Springborn, M.; Lötsch, J.; Johnston, A.N.B.; Hummel, T. Agreeable smellers and sensitive neurotics--correlations among personality traits and sensory thresholds. PLoS ONE 2011, 6, e18701. [Google Scholar] [CrossRef]
- Mori, K.; Sakano, H. Olfactory Circuitry and Behavioral Decisions. Annu. Rev. Physiol. 2021, 83, 231–256. [Google Scholar] [CrossRef]
- McCrae, R.R.; John, O.P. An introduction to the five-factor model and its applications. J. Pers. 1992, 60, 175–215. [Google Scholar] [CrossRef]
- Rochet, M.; El-Hage, W.; Richa, S.; Kazour, F.; Atanasova, B. Depression, Olfaction, and Quality of Life: A Mutual Relationship. Brain Sci. 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Del Bene, V.A.; Kamath, V.; Frank, J.S.; Billings, R.; Cho, D.-Y.; Byun, J.Y.; Jacob, A.; Anderson, J.N.; Visscher, K.; et al. Does Olfactory Training Improve Brain Function and Cognition? A Systematic Review. Neuropsychol. Rev. 2024, 34, 155–191. [Google Scholar] [CrossRef]
- Rezaeyan, A.; Asadi, S.; Kamrava, S.K.; Khoei, S.; Zare-Sadeghi, A. Reorganizing brain structure through olfactory training in post-traumatic smell impairment: An MRI study. J. Neuroradiol. 2022, 49, 333–342. [Google Scholar] [CrossRef]
- Milardi, D.; Cacciola, A.; Calamuneri, A.; Ghilardi, M.F.; Caminiti, F.; Cascio, F.; Andronaco, V.; Anastasi, G.; Mormina, E.; Arrigo, A.; et al. The Olfactory System Revealed: Non-Invasive Mapping by using Constrained Spherical Deconvolution Tractography in Healthy Humans. Front. Neuroanat. 2017, 11, 32. [Google Scholar] [CrossRef]
- Linster, C.; Cleland, T.A. Glomerular microcircuits in the olfactory bulb. Neural Netw. 2009, 22, 1169–1173. [Google Scholar] [CrossRef]
- Cleland, T.A.; Linster, C. Central olfactory structures. Handb. Clin. Neurol. 2019, 164, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Pinto, J.M. Olfaction: Anatomy, physiology, and disease. Clin. Anat. N. Y. 2014, 27, 54–60. [Google Scholar] [CrossRef]
- Allison, A.C. The secondary olfactory areas in the human brain. J. Anat. 1954, 88, 481–488. [Google Scholar] [PubMed]
- Kavoi, B.M.; Jameela, H. Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int. J. Morphol. 2011, 29, 939–946. [Google Scholar] [CrossRef]
- López--Elizalde, R.; Campero, A.; Sánchez--Delgadillo, T.; Lemus--Rodríguez, Y.; López--González, M.; Godínez--Rubí, M. Anatomy of the olfactory nerve: A comprehensive review with cadaveric dissection. Clin. Anat. 2018, 31, 109–117. [Google Scholar] [CrossRef]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 2022, 43, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Latini, F.; Hjortberg, M.; Aldskogius, H.; Ryttlefors, M. The use of a cerebral perfusion and immersion-fixation process for subsequent white matter dissection. J. Neurosci. Methods 2015, 253, 161–169. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A data acquisition perspective. NeuroImage 2012, 62, 2222–2231. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Skare, S.; Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Sotiropoulos, S.N.; Moeller, S.; Jbabdi, S.; Xu, J.; Andersson, J.L.; Auerbach, E.J.; Yacoub, E.; Feinberg, D.; Setsompop, K.; Wald, L.L.; et al. Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: Reducing the noise floor using SENSE. Magn. Reson. Med. 2013, 70, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef]
- Panesar, S.S.; Yeh, F.-C.; Jacquesson, T.; Hula, W.; Fernandez-Miranda, J.C. A Quantitative Tractography Study Into the Connectivity, Segmentation and Laterality of the Human Inferior Longitudinal Fasciculus. Front. Neuroanat. 2018, 12, 47. [Google Scholar] [CrossRef]
- Brett, M.; Johnsrude, I.S.; Owen, A.M. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 2002, 3, 243–249. [Google Scholar] [CrossRef]
- Pijnenburg, R.; Scholtens, L.H.; Ardesch, D.J.; de Lange, S.C.; Wei, Y.; van den Heuvel, M.P. Myelo- and cytoarchitectonic microstructural and functional human cortical atlases reconstructed in common MRI space. NeuroImage 2021, 239, 118274. [Google Scholar] [CrossRef]
- Latini, F.; Fahlström, M.; Berntsson, S.G.; Larsson, E.-M.; Smits, A.; Ryttlefors, M. A novel radiological classification system for cerebral gliomas: The Brain-Grid. PLoS ONE 2019, 14, e0211243. [Google Scholar] [CrossRef]
- Latini, F.; Mårtensson, J.; Larsson, E.-M.; Fredrikson, M.; Åhs, F.; Hjortberg, M.; Aldskogius, H.; Ryttlefors, M. Segmentation of the inferior longitudinal fasciculus in the human brain: A white matter dissection and diffusion tensor tractography study. Brain Res. 2017, 1675, 102–115. [Google Scholar] [CrossRef]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Rennaker, R.L. Cortical Activity Evoked by Odors. In The Neurobiology of Olfaction; Menini, A., Ed.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-7197-9. [Google Scholar]
- Van Hartevelt, T.J.; Kringelbach, M.L. The Olfactory System. In The Human Nervous System; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1219–1238. ISBN 978-0-12-374236-0. [Google Scholar]
- Sakamoto, N.; Pearson, J.; Shinoda, K.; Alheid, G.F.; Olmos, J.S.D.; Heimer, L. The human basal forebrain. Part I. An overview. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 15, pp. 1–55. ISBN 978-0-444-50043-4. [Google Scholar]
- Price, J.L. Olfactory Higher Centers Anatomy. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 129–136. ISBN 978-0-08-045046-9. [Google Scholar]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain J. Neurol. 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Otsubo, H.; Uchikawa, H.; Yamada, K.; Kuratsu, J.-I. Olfactory auras caused by a very focal isolated epileptic network in the amygdala. Epilepsy Behav. Case Rep. 2014, 2, 142–144. [Google Scholar] [CrossRef]
- Dahmani, L.; Courcot, B.; Near, J.; Patel, R.; Amaral, R.S.C.; Chakravarty, M.M.; Bohbot, V.D. Fimbria-Fornix Volume Is Associated With Spatial Memory and Olfactory Identification in Humans. Front. Syst. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Sobhani, S.; Rahmani, F.; Aarabi, M.H.; Sadr, A.V. Exploring white matter microstructure and olfaction dysfunction in early parkinson disease: Diffusion MRI reveals new insight. Brain Imaging Behav. 2019, 13, 210–219. [Google Scholar] [CrossRef]
- Segura, B.; Baggio, H.C.; Solana, E.; Palacios, E.M.; Vendrell, P.; Bargalló, N.; Junqué, C. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav. Brain Res. 2013, 246, 148–153. [Google Scholar] [CrossRef]
- De Benedictis, A.; Marras, C.E.; Petit, L.; Sarubbo, S. The inferior fronto-occipital fascicle: A century of controversies from anatomy theaters to operative neurosurgery. J. Neurosurg. Sci. 2021, 65, 605–615. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Jones-Gotman, M.; Evans, A.C.; Meyer, E. Functional localization and lateralization of human olfactory cortex. Nature 1992, 360, 339–340. [Google Scholar] [CrossRef]
- Courtiol, E.; Wilson, D.A. The olfactory thalamus: Unanswered questions about the role of the mediodorsal thalamic nucleus in olfaction. Front. Neural Circuits 2015, 9, 49. [Google Scholar] [CrossRef]
- Woo, C.C.; Miranda, B.; Sathishkumar, M.; Dehkordi-Vakil, F.; Yassa, M.A.; Leon, M. Overnight olfactory enrichment using an odorant diffuser improves memory and modifies the uncinate fasciculus in older adults. Front. Neurosci. 2023, 17, 1200448. [Google Scholar] [CrossRef]
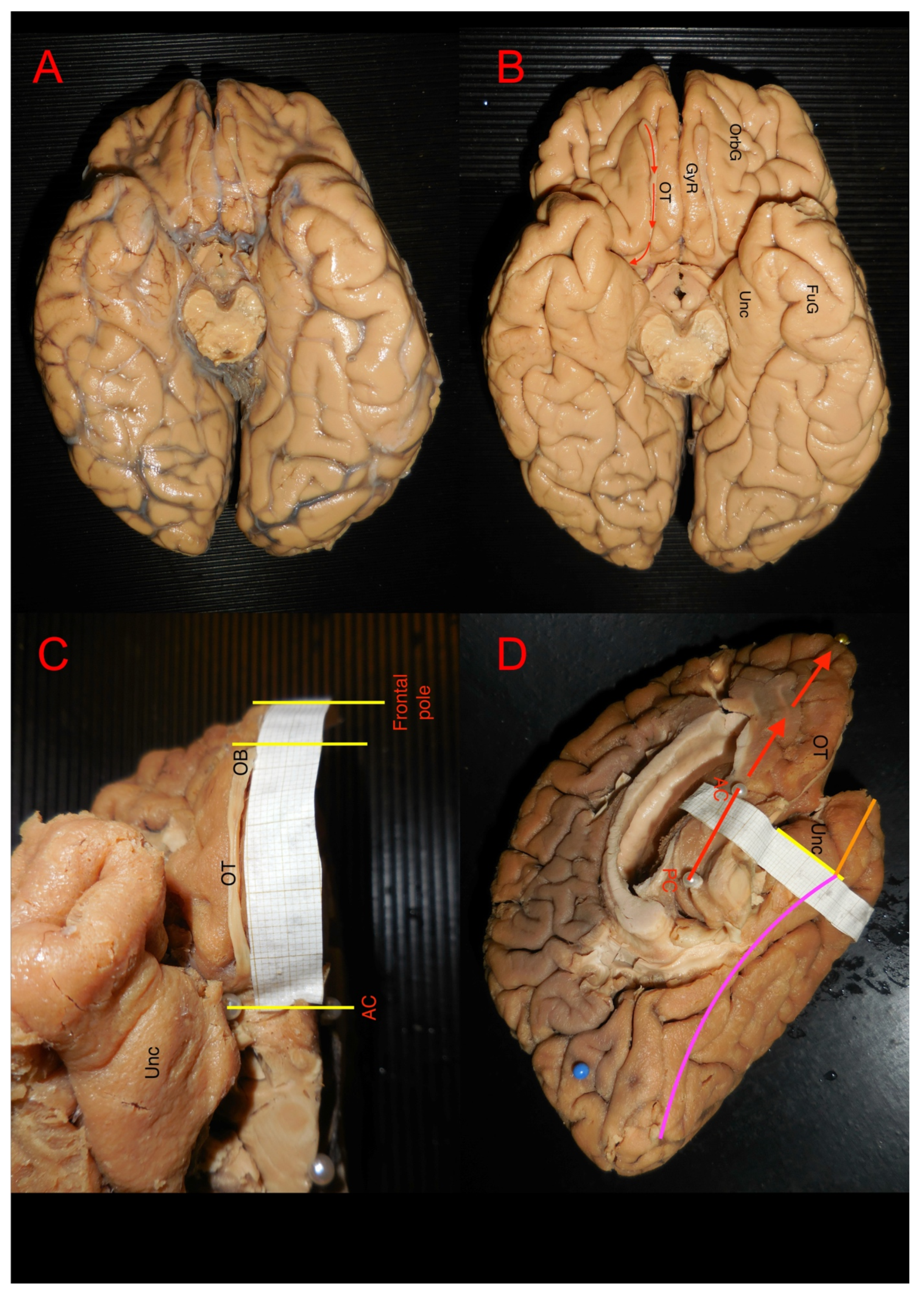


| Measured Area | Mean Values | Range Values | |||||
|---|---|---|---|---|---|---|---|
| Length | Width | Height | Length | Width | Height | ||
| Uncus + parahippocampal gyrus | 4.6 (0.4) | 2.2 (0.4) | 1.6 | 1.1 | |||
| Fusiform gyrus | 7.5 (0.7) | 1.7 (0.5) | 2.1 | 1.6 | |||
| Frontal lobe | 7.5 (0.3) | 3.4 (0.3) | 1.0 | 1.0 | |||
| Olfactory tract | Both | 4.2 (0.7) | 0.3 (0.1) | 1.8 | 0.2 | ||
| Left | 4.2 (0.7) | 0.3 (0.1) | 1.6 | 0.2 | |||
| Right | 4.3 (0.7) | 0.3 (0.1) | 1.6 | 0.1 | |||
| Temporal lobe | 2.9 (0.3) | 1.1 | |||||
| AC-PC line | 2.5 (0.1) | 0.4 | |||||
| Gyrus rectus | 0.6 (0.1) | 0.4 | |||||
| Medial orbital gyrus | 1.4 (0.3) | 0.7 | |||||
| Medial gyrus rectus + rostral gyrus | 2.1 (0.2) | 0.7 | |||||
| Lateral stria | Both | 2.8 (0.7) | 2.4 | ||||
| Left | 3.3 (0.3) | 0.8 | |||||
| Right | 2.3 (0.6) | 1.6 | |||||
| Medial stria | Both | 1.5 (0.7) | 2.2 | ||||
| Left | 1.4 (0.9) | 2.2 | |||||
| Right | 1.5 (0.6) | 1.5 | |||||
| Comparison of Left and Right Side | p-Value | Left Mean Value (cm) | Right Mean Value (cm) |
|---|---|---|---|
| Olfactory tract length | 1 | 4.2 | 0.3 |
| Olfactory tract width | 0.2 | 4.3 | 0.3 |
| Lateral stria length | 0.01 | 3.3 | 2.3 |
| Medial stria length | 0.8 | 1.4 | 1.5 |
| Tract | Olfactory Bulb/Tract to Primary OC, Mean % | Olfactory Bulb/Tract to Secondary OC, Mean % | Primary to Secondary OC, Mean % | Mean FA | Mean RD |
|---|---|---|---|---|---|
| Right UF | 71.4 | 53.8 | 42.6 | 0.18898 | 0.13288 |
| Left UF | 70.5 | 66.9 | 44.1 | 0.16306 | 0.1588 |
| AC | 56.4 | 57.3 | 23.2 | 0.3420 | 0.2238 |
| Right IFOF | 31.6 | 30.6 | 25.3 | 0.3709 | 0.2160 |
| Left IFOF | 28.9 | 21.5 | 25.1 | 0.2648 | 0.2016 |
| Right Cingulum | 66.5 | 29.8 | 0.3294 | 0.2762 | |
| Left Cingulum | 65.4 | 27.6 | 0.5620 | 0.1739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenwall, A.; Uggla, A.-L.; Weibust, D.; Fahlström, M.; Ryttlefors, M.; Latini, F. The Bulb, the Brain and the Being: New Insights into Olfactory System Anatomy, Organization and Connectivity. Brain Sci. 2025, 15, 368. https://doi.org/10.3390/brainsci15040368
Stenwall A, Uggla A-L, Weibust D, Fahlström M, Ryttlefors M, Latini F. The Bulb, the Brain and the Being: New Insights into Olfactory System Anatomy, Organization and Connectivity. Brain Sciences. 2025; 15(4):368. https://doi.org/10.3390/brainsci15040368
Chicago/Turabian StyleStenwall, Anton, Aino-Linnea Uggla, David Weibust, Markus Fahlström, Mats Ryttlefors, and Francesco Latini. 2025. "The Bulb, the Brain and the Being: New Insights into Olfactory System Anatomy, Organization and Connectivity" Brain Sciences 15, no. 4: 368. https://doi.org/10.3390/brainsci15040368
APA StyleStenwall, A., Uggla, A.-L., Weibust, D., Fahlström, M., Ryttlefors, M., & Latini, F. (2025). The Bulb, the Brain and the Being: New Insights into Olfactory System Anatomy, Organization and Connectivity. Brain Sciences, 15(4), 368. https://doi.org/10.3390/brainsci15040368






