Sex-Specific Behavioral Features of the Prenatal Valproic Acid Rat Model of Autism Spectrum Disorder
Abstract
:1. Introduction
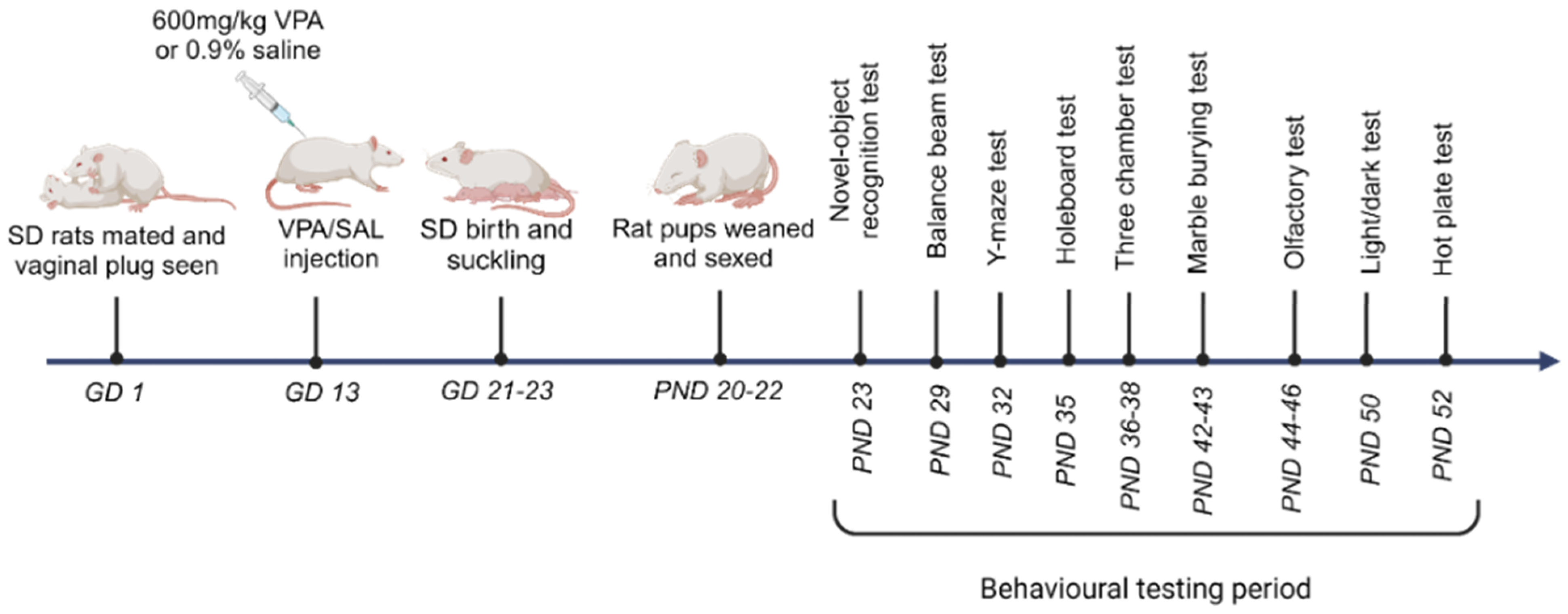
2. Materials and Methods
2.1. Animals
2.2. Behavioral Tests
- Response to social stimulus/sociability: three-chamber sociability test
+ time in nonsocial chamber).
- 2.
- Non-verbal communication: cotton-tip based olfactory test
- 3.
- Repetitive/restrictive behavior, lack of interest, and anxiety-like behaviors: hole board, marble burying, and light/dark test
- (a)
- Hole Board Test
- (b)
- Marble Burying Test
- (c)
- Light/Dark Test
- 4.
- Motor and sensory stimuli: balance beam and hot plate thermal test
- (a)
- Balance Beam Test
- (b)
- Hot Plate Thermal Test
- 5.
- Cognition: Novel object recognition and Y-maze tests
- (a)
- Novel Object Recognition Test
- (b)
- Y-Maze Test
2.3. Data Analysis
3. Results
3.1. Valproic Acid Effects on Sociability
Three-Chamber Sociability Test
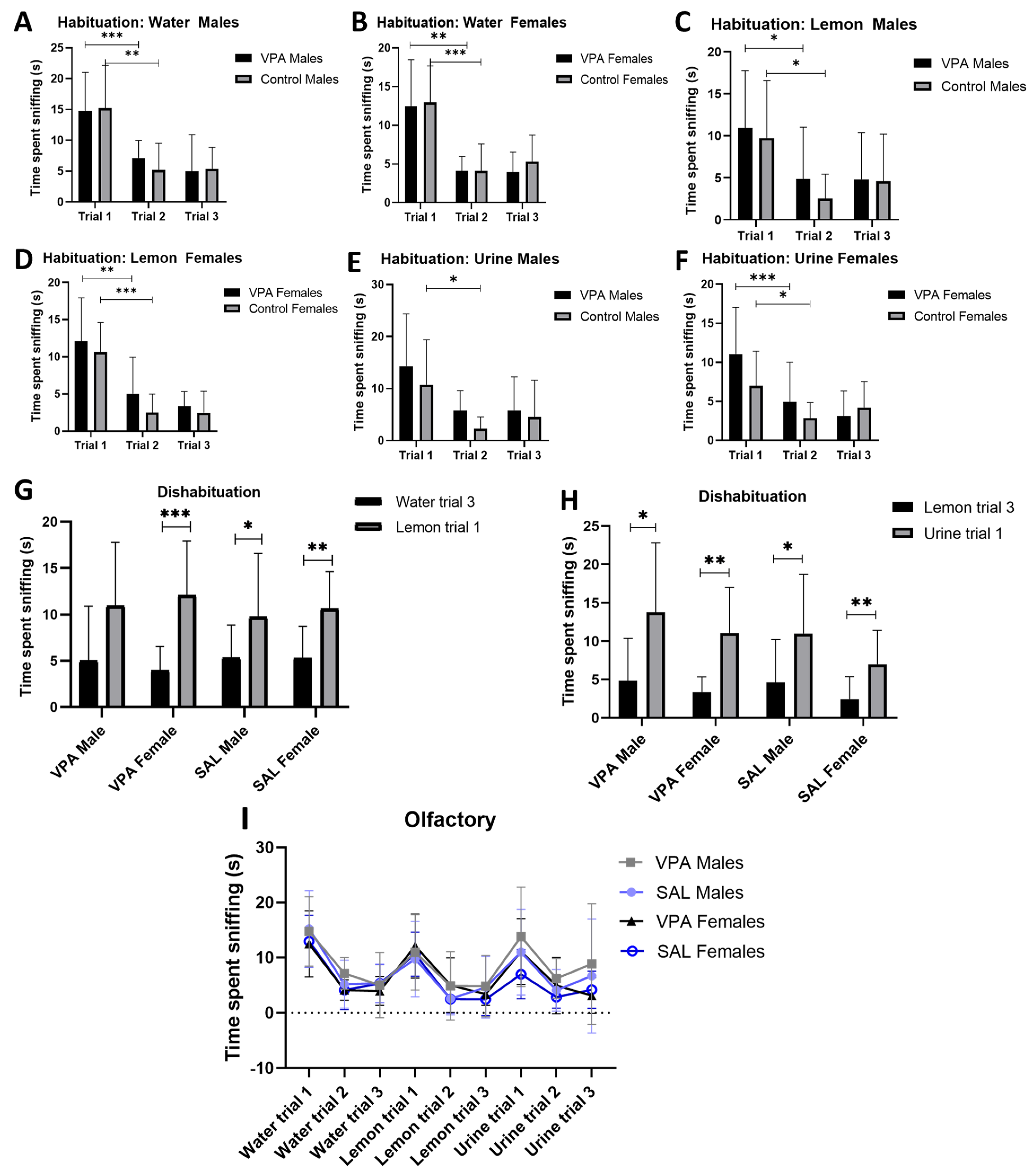
3.2. Valproic Acid Effects on Non-Verbal Communication
Cotton-Tip Based Olfactory Test
3.3. Valproic Acid Effects on Exploratory, Repetitive, and Anxiety-like Behaviors
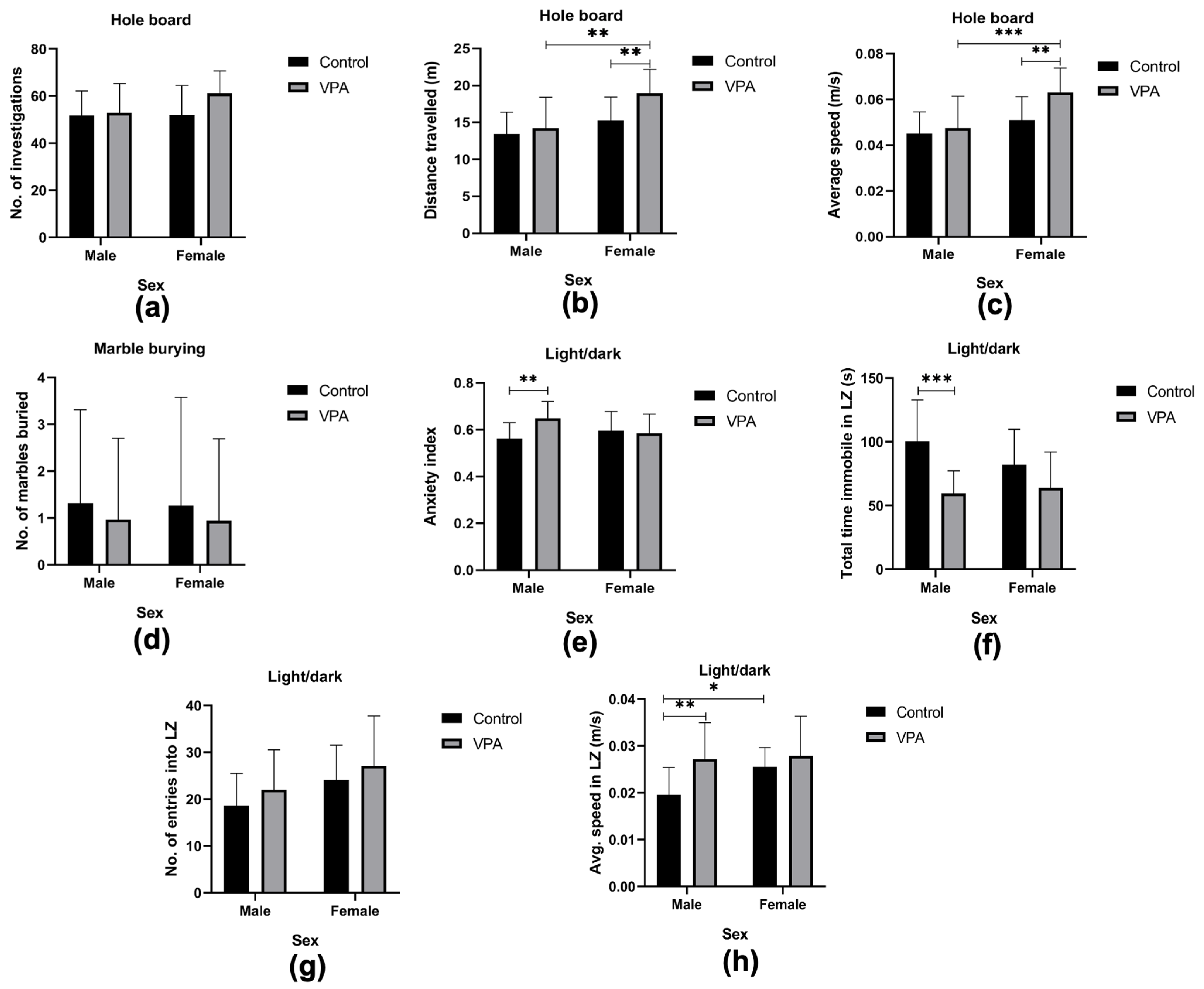
3.3.1. Assessing Exploratory Behavior
3.3.2. Assessing Repetitive Behavior
3.3.3. Assessing Anxiety-like Behavior
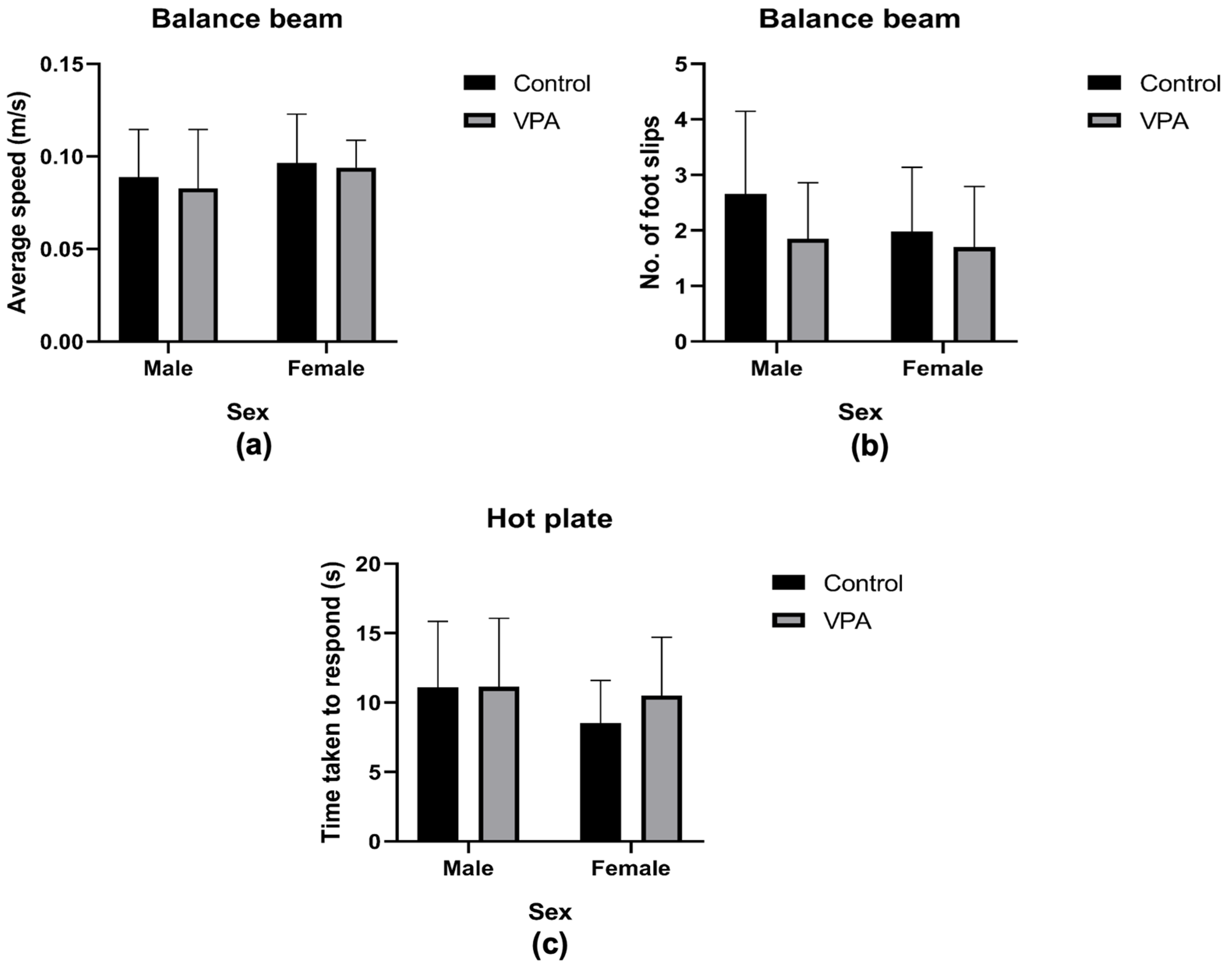
3.3.4. Assessment of Motor Abilities
3.3.5. Assessing Nociception
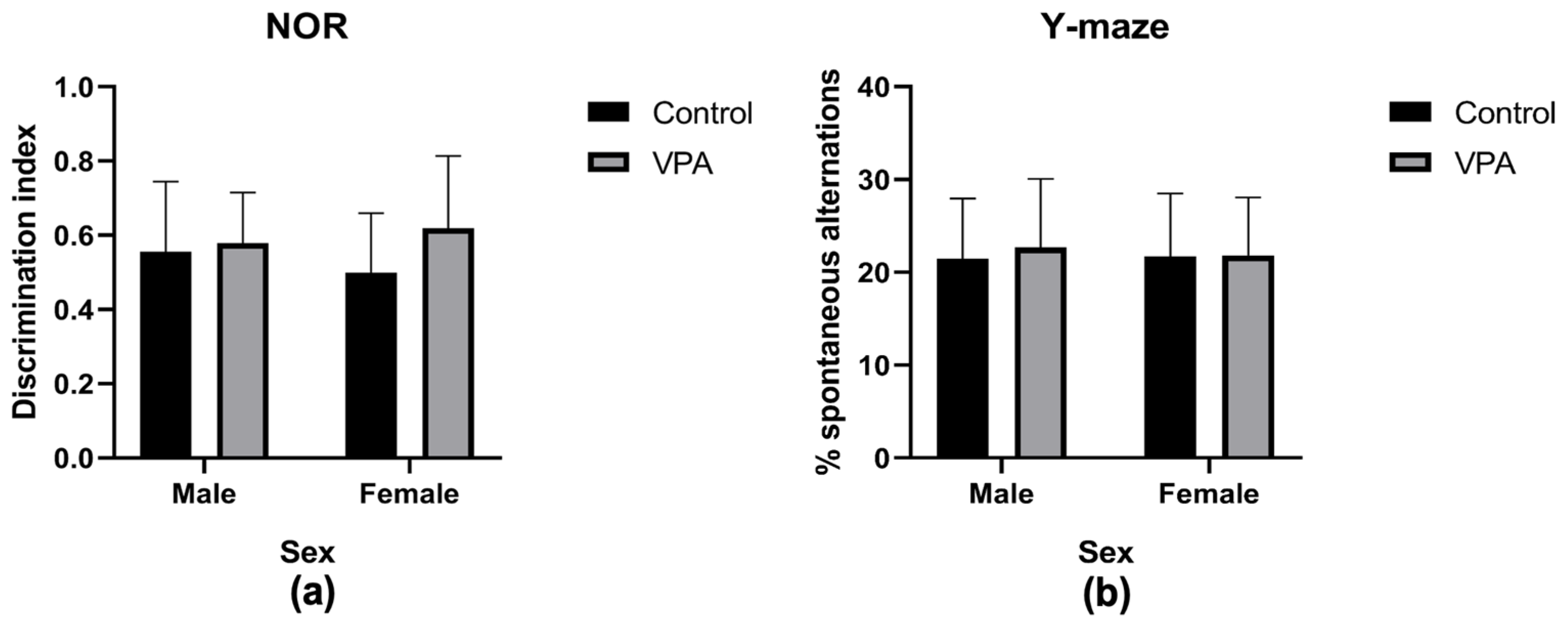
3.3.6. Assessing Cognitive Abilities
3.3.7. Assessing Spatial Memory and Repetitive Behavior
4. Discussion
- Prenatal valproic acid exposure leads to impaired sociability
- Prenatal valproic acid exposure leads to hyperactivity but not impaired exploration
- Motor, sensory, cognitive, and repetitive behavior impairments were not apparent after valproic acid exposure
- Prenatal valproic acid exposure increased anxiety-like behaviors
- Sex differences in behavioral outcomes following valproic acid exposure
- The validity of the valproic acid model of autism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Won, H.; Mah, W.; Kim, E. Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Front. Mol. Neurosci. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Hong, J.Y.; Bae, S.N. Microglia and autism spectrum disorder: Overview of current evidence and novel immunomodulatory treatment. Clin. Psychopharmacol. Neurosci. 2018, 16, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. The prevalence of autism. JAMA 2003, 289, 87–89. [Google Scholar] [CrossRef]
- Ashwood, P.; Wills, S.; Van de Water, J. The immune response in autism: A new frontier for autism research. J. Leukoc. Biol. 2006, 80, 1–15. [Google Scholar] [CrossRef]
- Grosvenor, L.P.; Croen, L.A.; Lynch, F.L.; Marafino, B.J.; Maye, M.; Penfold, R.B.; Simon, G.E.; Ames, J.L. Autism Diagnosis Among US Children and Adults, 2011–2022. JAMA Netw. Open 2024, 7, e2442218. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Brentani, H.; Paula, C.S.; Bordini, D.; Rolim, D.; Sato, F.; Portolese, J.; Pacifico, M.C.; McCracken, J.T. Autism spectrum disorders: An overview on diagnosis and treatment. Braz. J. Psychiatry 2013, 35 (Suppl. 1), S62–S72. [Google Scholar] [CrossRef]
- Geier, D.A.G.; Geier, M.R. Clinical and laboratory evaluation of methionine cycle-transsulfuration and androgen pathway markers. Horm. Res. 2006, 66, 182–188. [Google Scholar]
- Blaylock, R.L. A possible central mechanism in autism spectrum disorders, part 1. Altern. Ther. Health Med. 2008, 14, 46–53. [Google Scholar]
- Salari, N.; Rasoulpoor, S.; Rasoulpoor, S.; Shohaimi, S.; Jafarpour, S.; Abdoli, N.; Khaledi-Paveh, B.; Mohammadi, M. The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 2022, 48, 112. [Google Scholar] [CrossRef]
- Nemechek, P.M.; Nemechek, J.R. The Nemechek Protocol for Autism and Developmental Disorders: A How-to Guide for Restoring Neurological Function; Autonomic Recovery, LLC: Buckeye, AZ, USA, 2017. [Google Scholar]
- Santos, S.; Ferreira, H.; Martins, J.; Gonçalves, J.; Castelo-Branco, M. Male sex bias in early and late onset neurodevelopmental disorders: Shared aspects and differences in Autism Spectrum Disorder, Attention Deficit/hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. (In English) [Google Scholar] [CrossRef]
- Chen, C.; Van Horn, J.D. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 2017, 11, 38–61. (In English) [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Lin, W.; Xu, X.; Zhang, G.; Zhou, Q.G.; Naveed, M.; Meng, F.; Fukunaga, K.; Han, F. Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment. Biomed. Pharmacother. 2021, 137, 111322. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Dietert, J.M.; Dewitt, J.C. Environmental risk factors for autism. Emerg. Health Threat. J. 2011, 4, 7111. (In English) [Google Scholar] [CrossRef]
- Williams, P.G.; Hersh, J.H. A male with fetal valproate syndrome and autism. Dev. Med. Child. Neurol. 1997, 39, 632–634. [Google Scholar] [CrossRef]
- Williams, G.; King, J.; Cunningham, M.; Stephan, M.; Kerr, B.; Hersch, J.H. Fetal valproate syndrome and autism: Additional evidence of an association. Dev. Med. Child. Neurol. 2001, 43, 202–206. [Google Scholar] [CrossRef]
- Schneider, T.; Przewlocki, R. Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Vorhees, C.V. Behavioral teratogenicity of valproic acid: Selective effects on behavior after prenatal exposure to rats. Psychopharmacology 1987, 92, 173–179. [Google Scholar] [CrossRef]
- Christensen, J.; Gronborg, T.K.; Sorensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Hirsch, M.M.; Deckmann, I.; Santos-Terra, J.; Staevie, G.Z.; Fontes-Dutra, M.; Carello-Collar, G.; Korbes-Rockenbach, M.; Brum Schwingel, G.; Bauer-Negrini, G.; Rabelo, B.; et al. Effects of single-dose antipurinergic therapy on behavioral and molecular alterations in the valproic acid-induced animal model of autism. Neuropharmacology 2020, 167, 107930. [Google Scholar] [CrossRef]
- Favre, M.R.; Barkat, T.R.; LaMendola, D.; Khazen, G.; Markram, H.; Markram, K. General developmental health in the VPA-rat model of autism. Front. Behav. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef]
- Ornoy, A.; Echefu, B.; Becker, M. Animal Models of Autistic-like Behavior in Rodents: A Scoping Review and Call for a Comprehensive Scoring System. Int. J. Mol. Sci. 2024, 25, 10469. [Google Scholar] [CrossRef] [PubMed]
- Rodier, P.M.; Ingram, J.L.; Tisdale, B.; Nelson, S.; Romano, J. Embryological origin of autism, developmental abnormalities of cranial motor nuclei. J. Comp. Neurol. 1996, 370, 247–261. [Google Scholar] [CrossRef]
- Chomiak, T.; Karnik, V.; Block, E.; Hu, B. Altering the trajectory of early postnatal cortical development can lead to behavioral features of autism. BMC Neurosci. 2010, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Dufour-Rainfray, D.; Vourc’h, P.; Le Guisquet, A.M.; Garreau, L.; Ternant, D.; Bodard, S.; Jaumain, E.; Gulhan, Z.; Belzung, C.; Andres, C.R.; et al. Behavior and serotonergic disorders in rats exposed prenatally to valproate: A model for autism. Neurosci. Lett. 2010, 470, 55–59. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Yang, S.I.; Cheong, J.H.; Shin, C.Y.; Ko, K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol. Lett. 2011, 201, 137–142. [Google Scholar] [CrossRef]
- Banerjee, A.; Engineer, C.T.; Sauls, B.L.; Morales, A.A.; Kilgard, M.P.; Ploski, J.E. Abnormal emotional learning in a rat model of autism exposed to valproic acid in utero. Front. Behav. Neurosci. 2014, 8, 387. [Google Scholar] [CrossRef]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Höcht, C.; Depino, A.M. Sociability deficits after prenatal exposure to valproic acid are rescued by early social enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef]
- Bringas, M.E.; Carvajal-Flores, F.N.; Lopez-Ramirez, T.A.; Atzori, M.; Flores, G. Rearrangement of the dendritic morphology in limbic regions and altered exploratory behavior in a rat model of autism spectrum disorder. Neuroscience 2013, 241, 170–187. [Google Scholar] [CrossRef]
- Chomiak, T.; Hung, J.; Cihal, A.; Dhaliwal, J.; Baghdadwala, M.I.; Dzwonek, A.; Podgorny, P.; Hu, B. Auditory-cued sensorimotor task reveals disengagement deficits in rats exposed to the autism-associated teratogen valproic acid. Neuroscience 2014, 268, 212–220. [Google Scholar] [CrossRef]
- Yang, E.J.; Ahn, S.; Lee, K.; Mahmood, U.; Kim, H.S. Early behavioral abnormalities and perinatal alterations of PTEN/AKT pathway in valproic acid autism model mice. PLoS ONE 2016, 11, e0153298. [Google Scholar]
- Crawley, J.N. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 2012, 14, 293–305. [Google Scholar] [CrossRef]
- Baronio, D.; Castro, K.; Gonchoroski, C.; Mueller de Melo, G.; Nunes, G.D.F.; Bambini-Junior, V.; Gottfried, C.; Riesgo, R. Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PLoS ONE 2015, 10, e0116363. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 8, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, W.; Pan, Y.W.; Lu, S.; Xia, Z. Methods to measure olfactory behavior in mice. Curr. Protoc. Toxicol. 2016, 63, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Coronas-Samano, G.; Ivanova, A.V.; Verhagen, J.V. The habituation/cross-habituation test revisited: Guidance from sniffing and video tracking. Neural Plast. 2016, 2016, 9131284. [Google Scholar] [CrossRef]
- Ferdenzi, C.; Poncelet, J.; Rouby, C.; Bensafi, M. Repeated exposure to odors induces affective habituation of perception and sniffing. Front. Behav. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Poe, M.D.; Nonneman, R.J.; Young, N.B.; Koller, B.H.; Crawley, J.N.; Duncan, G.E.; Bodfish, J.W. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav. Brain Res. 2008, 188, 178–194. [Google Scholar] [CrossRef]
- Angao-Perez, M.; Kane, M.J.; Briggs, D.I.; Farancescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- Pierce, K.; Haist, F.; Sedaghat, F.; Courchesne, E. The brain response to personally familiar faces in autism: Findings of fusiform activity and beyond. Brain 2004, 127, 2703–2716. [Google Scholar] [CrossRef]
- Crawley, J.N. Behavioral phenotyping strategies for mutant mice. ILAR J. 2012, 53, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Buccafusco, J.J.; Jackson, W.J.; Fox, G.B.; Curzon, P.; Decker, M.W.; Graham, J.H.; Levin, E.D. Methods of Behavior Analysis in Neuroscience; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Luong, T.N.; Carlisle, H.J.; Southwell, A.; Patterson, P.H. Assessment of motor balance and coordination in mice using the balance beam. J. Vis. Exp. 2011, 49, 2376. [Google Scholar]
- Mulder, G.B.; Pritchett, K. Rodent analgesiometry: The hot plate, tail flick and von frey hairs. Contemp. Top. Lab. Anim. Sci. 2004, 43, 54–55. [Google Scholar] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Mathiasen, J.R.; DiCamillo, A. Novel object recognition in the rat: A facile test for cognitive function. Curr. Protoc. Pharmacol. 2010, 5, 5–59. [Google Scholar]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, B. Minocycline ameliorates prenatal valproic acid induced autistic behavior, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016, 1630, 83–97. [Google Scholar] [CrossRef]
- Sandhya, T.; Sowjanya, J.; Veeresh, B. Bacopa monniera (L.) Wettst ameliorates behavioral alterations and oxidative markers in sodium valproate induced autism in rats. Neurochem. Res. 2012, 37, 1121–1131. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Rodrigues, L.; Behr, G.A.; Moreira, J.C.; Riesgo, R.; Gottfried, C. Animal model of autism induced by prenatal exposure to valproate: Behavioral changes and liver parameters. Brain Res. 2011, 1408, 8–16. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Park, J.H.; Kim, H.J.; Jeon, S.J.; Dela Pena, I.C.; Han, S.H.; Cheong, J.H.; et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neuroch. 2013, 124, 832–843. [Google Scholar] [CrossRef]
- Bambini-Junior, V.; Zanatta, G.; Della Flora Nunes, G.; Mueller de Melo, G.; Michels, M.; Fontes-Dutra, M.; Nogueira Freire, V.; Riesgo, R.; Gottfried, C. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci. Lett. 2014, 583, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Kulangara, K.; Antoniello, K.; Markram, H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. USA 2007, 104, 13501–13506. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A systematic review of the valproic acid-induced rodent model of autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Jabarin, R.; Netser, S.; Wagner, S. Beyond the three-chamber test: Toward a multimodal and objective assessment of social behavior in rodents. Mol. Autism 2022, 13, 41. [Google Scholar] [CrossRef]
- Markram, K.; Rinaldi, T.; La Mendola, D.; Sandi, C.; Markram, H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 2008, 33, 901–912. [Google Scholar] [CrossRef]
- Mabunga, D.F.; Gonzales, E.L.; Kim, J.W.; Kim, K.C.; Shin, C.Y. Exploring the validity of valproic acid animal model of autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Baum, M.J.; Cherry, J.A. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J. Neurosci. 2004, 24, 9451–9457. [Google Scholar] [CrossRef]
- Belzung, C.; Lemoine, M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef]
- Ayres, J. Sensory Integration and the Child: Understanding Hidden Sensory Challenges; Western Psychological Services: Los Angeles, CA, USA, 1979. [Google Scholar]
- Kilroy, E.; Aziz-Zadeh, L.; Cermak, S. Ayres theories of autism and sensory integration revisited: What contemporary neuroscience has to say. Brain Sci. 2019, 9, 68. [Google Scholar] [CrossRef]
- Russell, V.A. Overview of animal models of Attention Deficit Hyperactivity Disorder (ADHD). Curr. Protoc. Neurosci. 2011, 9, 1–25. [Google Scholar] [CrossRef]
- Sagvolden, T.; Pettersen, M.B.; Larsen, M.C. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol. Behav. 1993, 54, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, B.A.; Yager, R.D.; Klopper, J.H. Selective brain dopamine depletion in developing rats: An experimental model of minimal brain dysfunction. Science 1976, 191, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Hong, M.; Kim, K.C.; Kim, J.W.; Yang, S.M.; Seung, H.; Ko, M.J.; Choi, D.H.; You, J.S.; Shin, C.Y.; et al. Effects of atomoxetine on hyper-locomotive activity of the prenatally valproate-exposed rat offspring. Biomol. Ther. 2014, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Main, S.L.; Kulesza, R.J. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 2017, 340, 34–47. [Google Scholar] [CrossRef]
- Fereshetyan, K.; Chavushyan, V.; Danielyan, M.; Yenkoyan, K. Assessment of behavioral, morphological and electrophysiological changes in prenatal and postnatal valproate induced rat models of autism spectrum disorder. Sci. Rep. 2021, 11, 23471. [Google Scholar] [CrossRef]
- Kerr, D.M.; Downey, L.; Conboy, M.; Finn, D.P.; Roche, M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav. Brain Res. 2013, 249, 124–132. [Google Scholar] [CrossRef]
- Militerni, R.; Bravaccio, C.; Falco, C.; Puglisi-Allegra, S.; Pascucci, T.; Fico, C. Pain reactivity in children with autistic disorder. J. Headache Pain. 2000, 1, 53–56. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. J. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, D.K.; Go, H.S.; Kim, P.; Choi, C.S.; Kim, J.W.; Jeon, S.J.; Song, M.R.; Shin, C.Y. Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol. Neurobiol. 2014, 49, 512–528. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and white matter brain chemistry in young children with autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Karvat, G.; Kimchi, T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 2014, 39, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Rendall, A.R.; Truong, D.T.; Fitch, R.H. Learning delays in a mouse model of Autism Spectrum Disorder. Behav. Brain Res. 2016, 303, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, N.; Seiffe, A.; Campolongo, M.; Zappala, C.; Depino, A.M. Sex-specific effects of prenatal valproic acid exposure on sociability and neuroinflammation: Relevance for susceptibility and resilience in autism. Psychoneuroendocrinology 2019, 110, 104441. [Google Scholar] [CrossRef]
- Jeon, S.J.; Gonzales, E.L.; Mabunga, D.F.N.; Valencia, S.T.; Kim, D.G.; Kim, Y.; Adil, K.J.L.; Shin, D.; Park, D.; Shin, C.Y. Sex-specific behavioral features of rodent models of autism spectrum disorder. Exp. Neurobiol. 2018, 27, 321–343. [Google Scholar] [CrossRef]
- Auyeung, B.; Lombardo, M.; Knickmeyer, R.; Baron-Cohen, S. Extreme Male Brain (EMB) Theory. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Favre, M.R.; La Mendola, D.; Meystre, J.; Christodoulou, D.; Cochrane, M.J.; Markram, H.; Markram, K. Predictable enriched environment prevents development of hyper-emotionality in the VPA rat model of autism. Front. Neurosci. 2015, 9, 127. [Google Scholar] [CrossRef]
- Matsuo, K.; Yabuki, Y.; Fukunaga, K. Improvement of social interaction and cognition by oxytocin for autism-like behaviors in valproic acid-exposed rats. Biol. Psychiatry 2017, 81, S200–S201. [Google Scholar] [CrossRef]
- Hill, E.L.; Bird, C.M. Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia 2006, 44, 2822–2835. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, R.; Ma, X.; Gong, Z.; Xia, D.; Zhou, Q. Elevated level of PKM? underlies the excessive anxiety in an autism model. Front. Mol. Neurosci. 2019, 12, 291. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, Y.; Li, Y.; Chen, D.; Yang, F.; Wang, S. A developmental study of abnormal behaviors and altered gabaergic signaling in the vpa-treated rat model of autism. Front. Behav. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef]
- South, M.; Larson, M.J.; White, S.E.; Dana, J.; Crowley, M.J. Better fear conditioning is associated with reduced symptom severity in autism spectrum disorders. Autism Res. 2011, 4, 412–421. [Google Scholar] [CrossRef]
- Arenella, M.; Fanelli, G.; Kiemeney, L.A.; McAlonan, G.; Murphy, D.G.; Bralten, J. Genetic relationship between the immune system and autism. Brain Behav. Immun. Health Dec. 2023, 34, 100698. (In English) [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. (In English) [Google Scholar] [CrossRef] [PubMed]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. Feb 2017, 88, 95–105. (In English) [Google Scholar] [CrossRef]
- Ellenbroek, B.A.; Cools, A.R. Animal models with construct validity for schizophrenia. Behav. Pharmacol. 1990, 1, 469–490. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Kim, K.C.; Choi, C.S.; Kim, J.W.; Han, S.H.; Cheong, J.H.; Ryu, J.H.; Shin, C.Y. MeCP2 modulates sex differences in the postsynaptic development of the valproate animal model of autism. Mol. Neurobiol. 2014, 53, 40–56. [Google Scholar] [CrossRef]
- Kataoka, S.; Takuma, K.; Hara, Y.; Maeda, Y.; Ago, Y.; Matsuda, T. Autism-like behaviors with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamali, P.M.; Dignon, C.; Ngwenya, A.; Maseko, B.C. Sex-Specific Behavioral Features of the Prenatal Valproic Acid Rat Model of Autism Spectrum Disorder. Brain Sci. 2025, 15, 388. https://doi.org/10.3390/brainsci15040388
Mamali PM, Dignon C, Ngwenya A, Maseko BC. Sex-Specific Behavioral Features of the Prenatal Valproic Acid Rat Model of Autism Spectrum Disorder. Brain Sciences. 2025; 15(4):388. https://doi.org/10.3390/brainsci15040388
Chicago/Turabian StyleMamali, Patience Mulalo, Christine Dignon, Ayanda Ngwenya, and Busisiwe Constance Maseko. 2025. "Sex-Specific Behavioral Features of the Prenatal Valproic Acid Rat Model of Autism Spectrum Disorder" Brain Sciences 15, no. 4: 388. https://doi.org/10.3390/brainsci15040388
APA StyleMamali, P. M., Dignon, C., Ngwenya, A., & Maseko, B. C. (2025). Sex-Specific Behavioral Features of the Prenatal Valproic Acid Rat Model of Autism Spectrum Disorder. Brain Sciences, 15(4), 388. https://doi.org/10.3390/brainsci15040388





