Long-Term Fish Oil Supplementation Attenuates Spike Wave Discharges in the Amygdala of Adult Rats with Early-Life Febrile Seizures
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutritional Supplements
2.2. Subjects and Treatments
2.3. Induction of Hyperthermia
2.4. Surgical Implantation of the Electrodes
2.5. EEG Recording
2.6. Behavioral Evaluation During EEG Recording
2.7. EEG Capture and Analysis
2.8. Histology
2.9. Statistical Analysis
3. Results
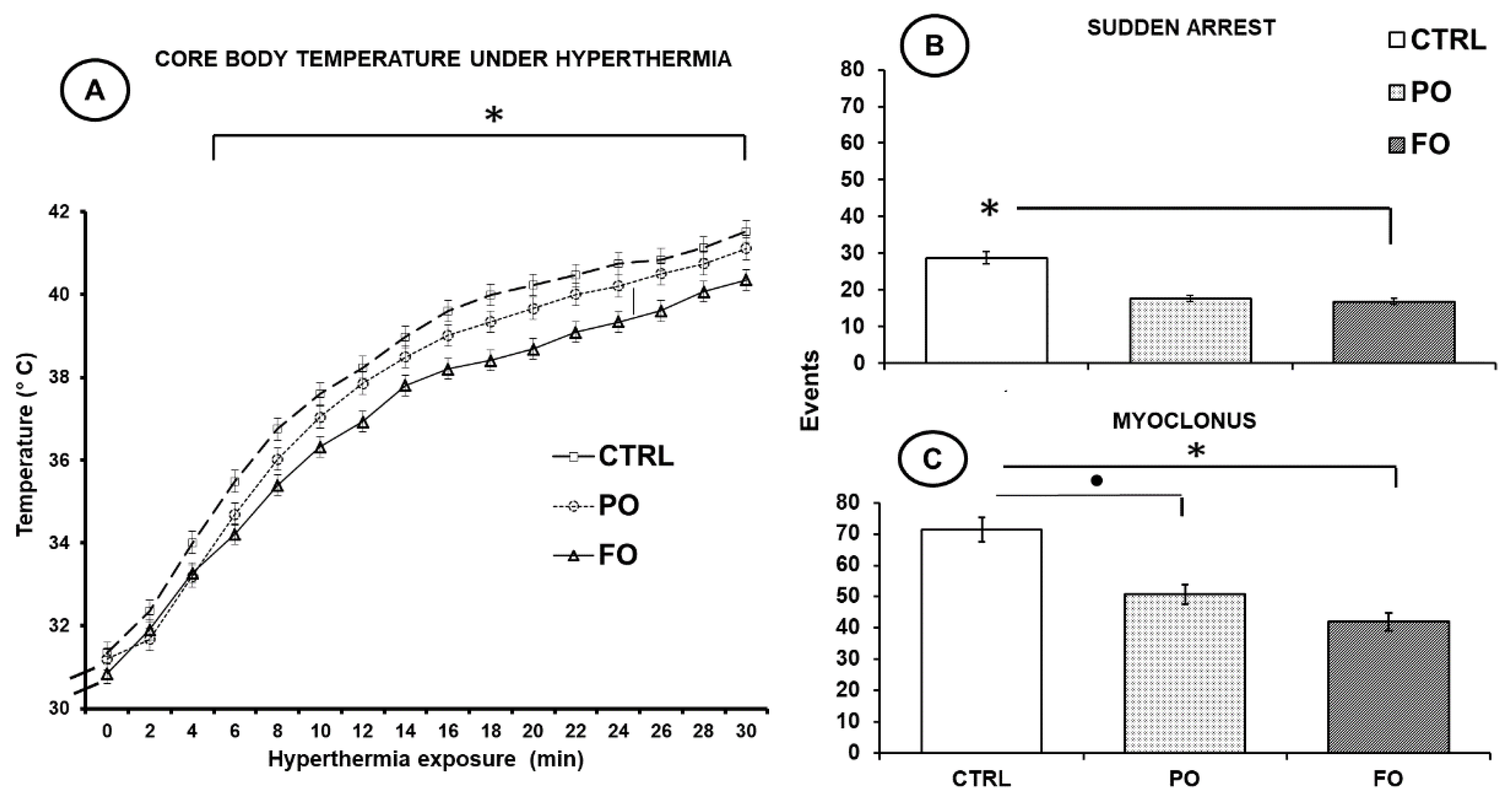
3.1. Effects of Exposure to Hyperthermia on Core Temperature
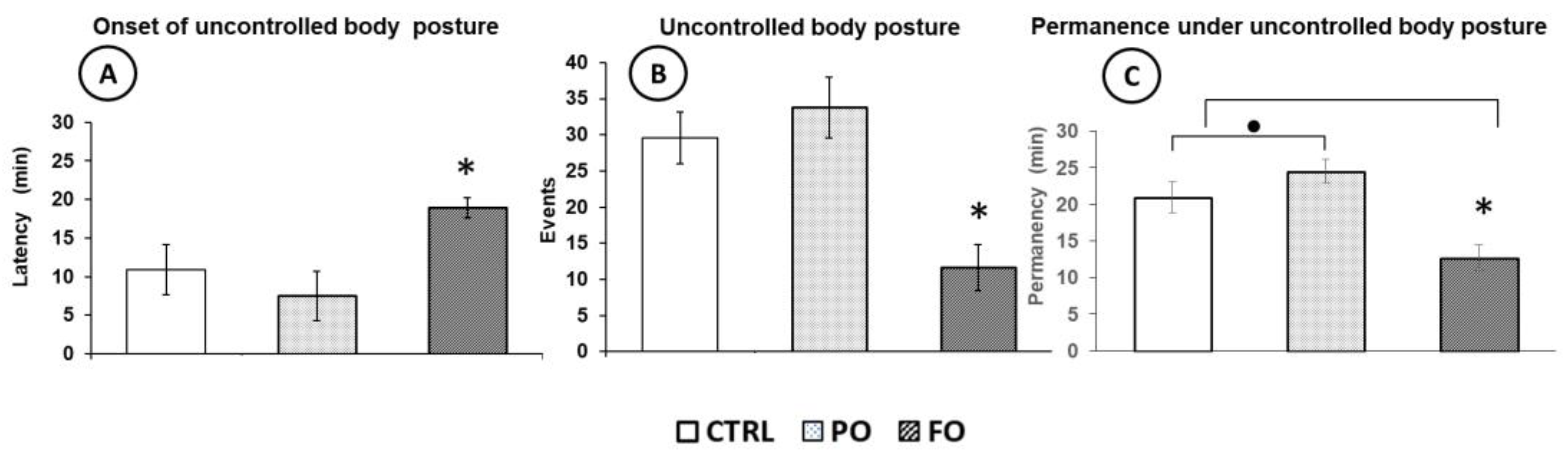
3.2. Effects of Exposure to Hyperthermia on the Pups’ Behavior
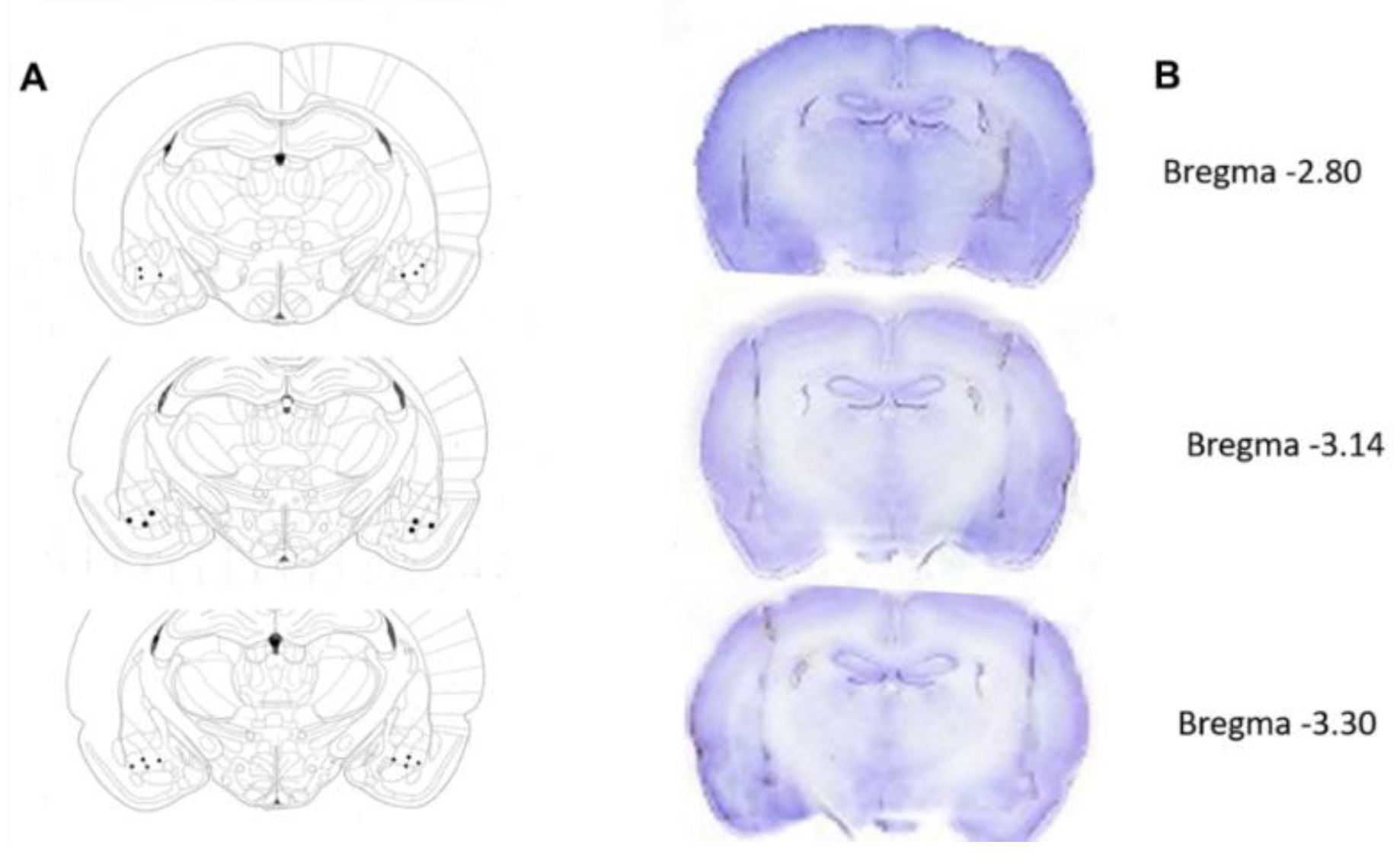
3.3. Histological Verification of the Electrode Tips
3.4. Body Weight and Food and Water Intake of the Groups with and Without a History of HP up to Adulthood
3.5. Behavioral Evaluation of the Normothermic Groups
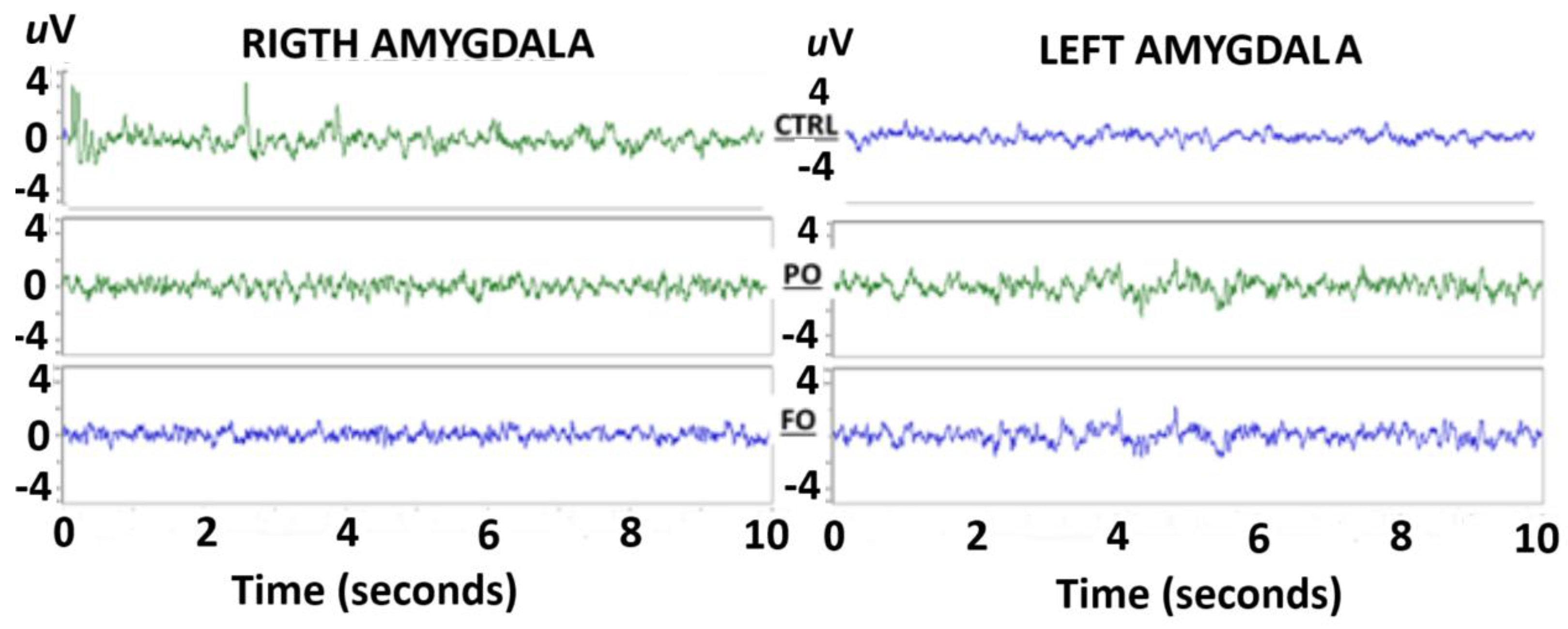
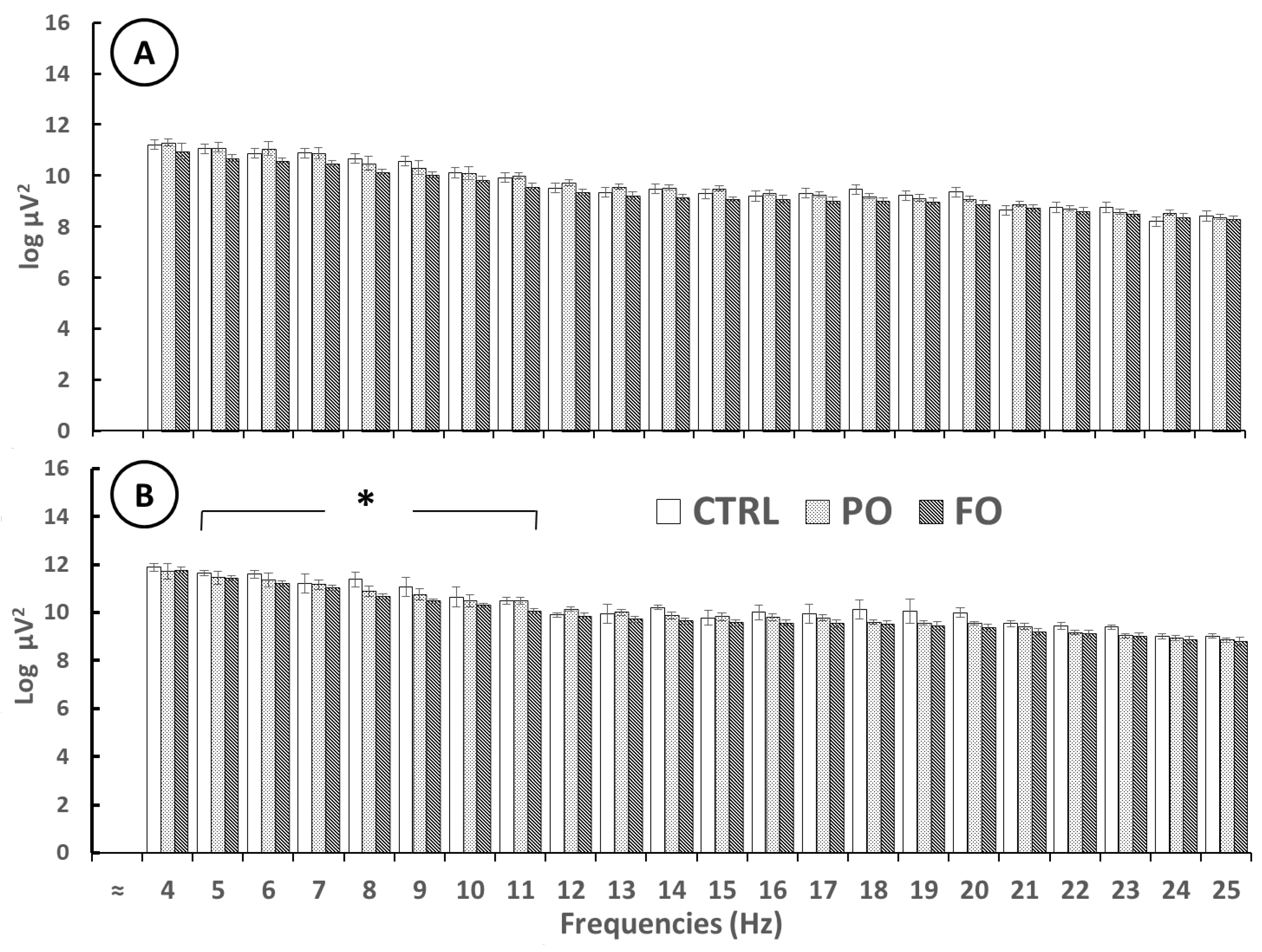
3.6. Evaluation of the EEG from the Amygdala of the Normothermic Groups
3.7. Behavioral Evaluation of the Adult Rats with Early-Life History of HP
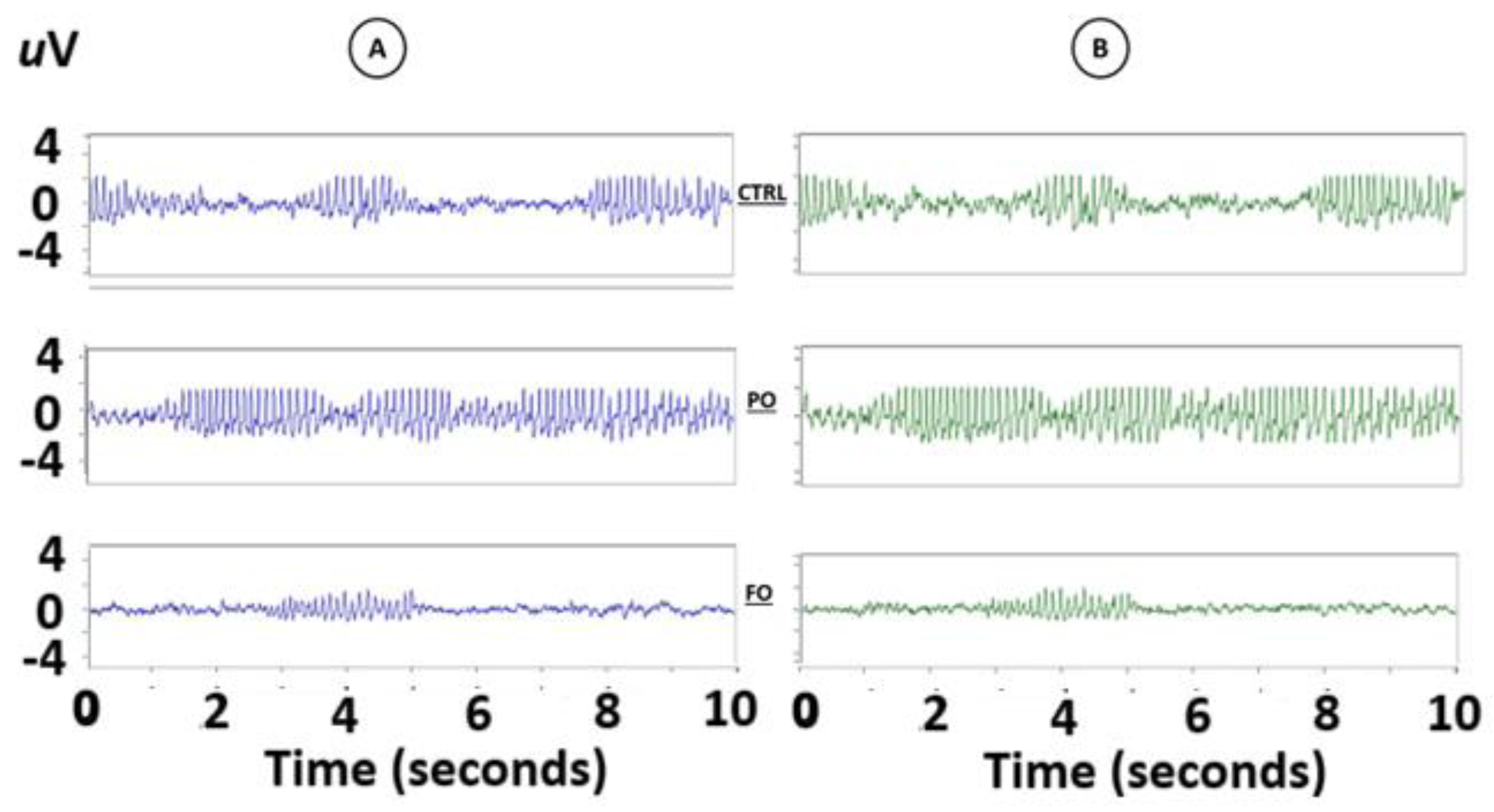
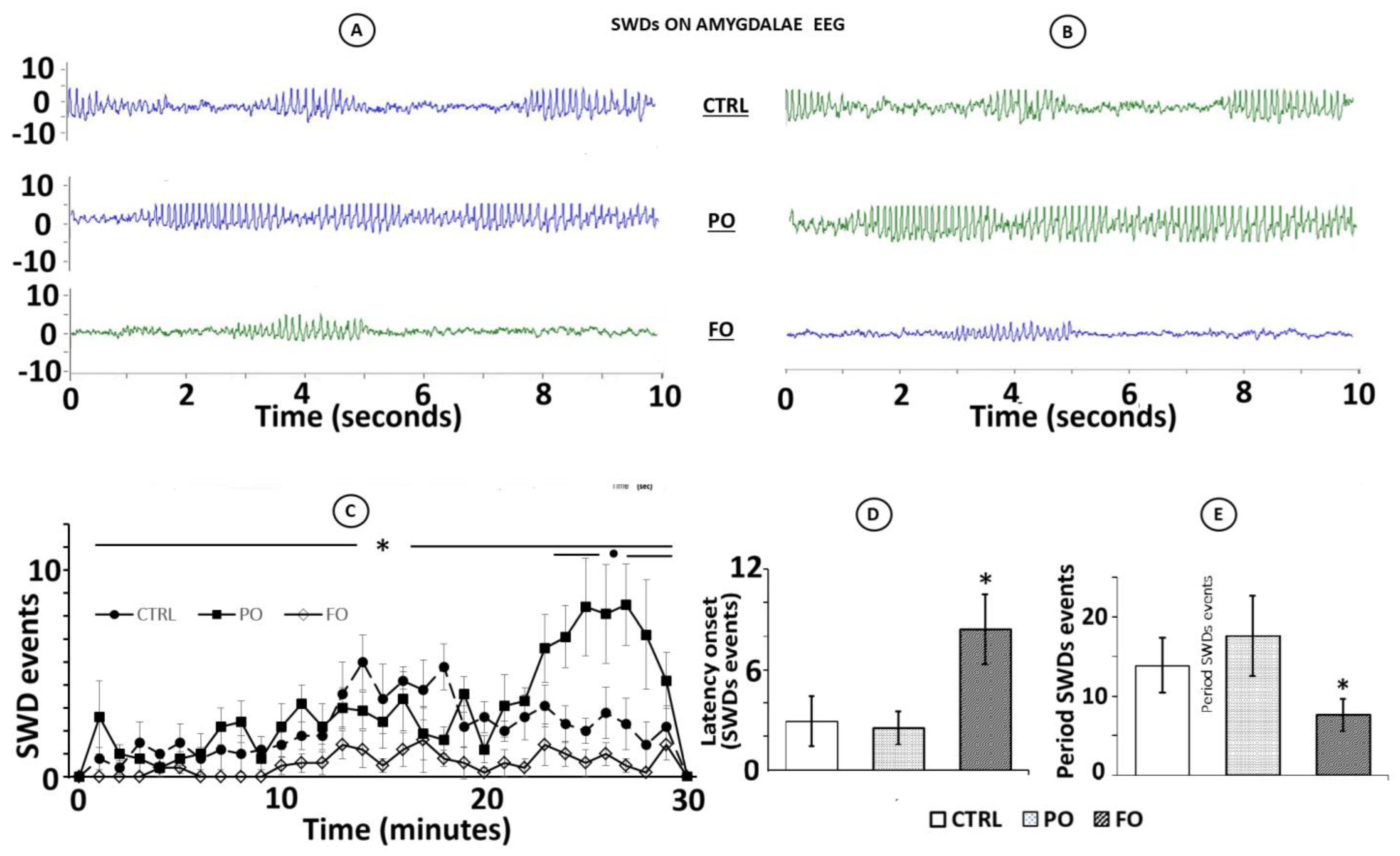
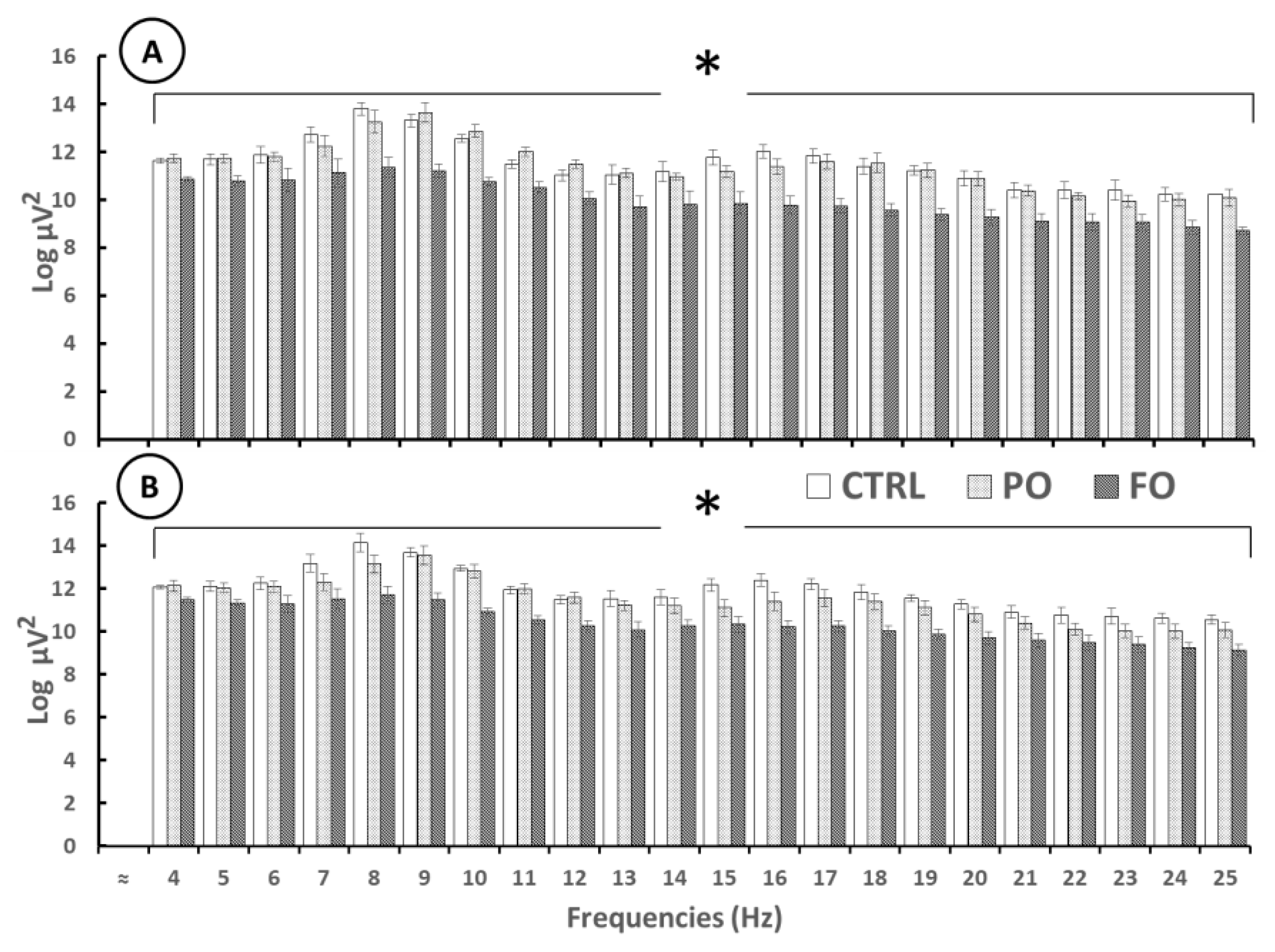
3.8. Analysis of the EEG of Amygdala Prior to SWDs in Adult Rats with Early-Life History of HP
3.9. Analysis of the EEG from the Amygdala During SWDs in Rats with Early-Life History of HP
3.10. Analysis of the EEG from the Amygdala After the SWDs in Rats with Early-Life History of HP
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| CT | core temperature |
| CTRL | deionized water |
| DHA | docosahexaenoic acid |
| EEG | electroencephalographic activity |
| EPA | eicosapentaenoic acid |
| FO | fish oil |
| FS | febrile seizures |
| GAD | glutamate decarboxylase |
| HP | hyperthermia |
| PO | palm oil |
| SWDs | spike-wave discharges |
| TLE | temporal lobe epilepsy |
| θ-3 | omega-3 fatty acids |
References
- Whitney, R.; Samanta, D.; Sharma, S.; Jain, P. Complex Febrile Seizures: Usual and the Unusual. Indian J. Pediatr. 2025, 92, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sawires, R.; Buttery, J.; Fahey, M.A. Review of Febrile Seizures: Recent Advances in Understanding of Febrile Seizure Pathophysiology and Commonly Implicated Viral Triggers. Front. Pediat. 2022, 13, 801321. [Google Scholar] [CrossRef] [PubMed]
- Eilbert, W.; Chan, C. Febrile seizures: A review. J. Am. Coll. Emerg. Phys. Open. 2022, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Biltz, S.; Speltz, L. Febrile Seizures. Pediatr. Ann. 2023, 52, e388–e393. [Google Scholar] [CrossRef]
- Corsello, A.; Marangoni, M.B.; Macchi, M.; Cozzi, L.; Agostoni, C.; Milanim, G.P.; Dilena, R. Febrile Seizures: A Systematic Review of Different Guidelines. Pediatr. Neurol. 2024, 155, 141–148. [Google Scholar] [CrossRef]
- Mewasingh, L.D.; Chin, R.F.M.; Scott, R.C. Current understanding of febrile seizures and their long-term outcomes. Dev. Med. Child. Neurol. 2020, 62, 1245–1249. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, C.; Tian, M.; Kang, J.Q.; Shen, W.; Verdier, K.; Pimenta, A.; MacDonald, R.L. Overexpressing wild-type γ2 subunits rescued the seizure phenotype in Gabrg2+/Q390X Dravet syndrome mice. Epilepsia 2017, 58, 1451–1461. [Google Scholar] [CrossRef]
- Raijmakers, M.; Clynen, E.; Smisdom, N.; Nelissen, S.; Brône, B.; Rigo, J.M.; Hoogland, G.; Swijsen, A. Experimental febrile seizures increase dendritic complexity of newborn dentate granule cells. Epilepsia 2016, 57, 717–726. [Google Scholar] [CrossRef]
- Yi, Y.; Zhong, C.; Wei-Wei, H. The long-term neurodevelopmental outcomes of febrile seizures and underlying mechanisms. Front. Cell Dev. Biol. 2023, 25, 1186050. [Google Scholar] [CrossRef]
- Cendes, F. Febrile seizures and mesial temporal sclerosis. Curr. Opin. Neurol. 2004, 17, 161–164. [Google Scholar] [CrossRef]
- Smith, D.K.; Sadler, K.P.; Benedum, M. Febrile Seizures: Risks, Evaluation, and Prognosis. Am. Fam. Physician 2019, 99, 445–450. [Google Scholar]
- Asadi-Pooya, A.A.; Nei, M.; Rostami, C.; Sperling, M.R. Mesial temporal lobe epilepsy with childhood febrile seizure. Acta Neurol. Scand. 2017, 135, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.W.; Li, J.; Sun, Y.; Christensen, J. Evaluation of Long-term Risk of Epilepsy, Psychiatric Disorders, and Mortality among Children with Recurrent Febrile Seizures: A National Cohort Study in Denmark. JAMA Pediatr. 2019, 173, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Santoni, J.R.; Santoni-Williams, C. Abnormal theta response to nociceptive stimuli during sleep EEG in children with febrile convulsions and control children. Neurophysiol. Clin. 1996, 26, 164–169. [Google Scholar] [CrossRef]
- Okumura, A.; Mizuguchi, M.; Kidokoro, H.; Tanaka, M.; Abe, S.; Hosoya, M.; Aiba, H.; Maegaki, Y.; Yamamoto, H.; Tanabe, T.; et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009, 31, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pearce, P.S.; Friedman, D.; Lafrancois, J.J.; Iyengar, S.S.; Fenton, A.; Maclusky, N.J.; Scharfman, H.E. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 2014, 32, 121–131. [Google Scholar] [CrossRef]
- Li, R.; Millist, L.; Foster, E.; Yuan, X.; Guvenc, U.; Radfar, M.; Marendy, P.; Ni, W.; O’Brien, T.J.; Casillas-Espinosa, P.M. Spike and wave discharges detection in genetic absence epilepsy rat from Strasbourg and patients with genetic generalized epilepsy. Epilepsy Res. 2023, 194, 107181. [Google Scholar] [CrossRef]
- Coenen, A.M.; Van Luijtelaar, E.L. Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Manandhar, B.P.; Adhikari, S.; Bhattarai, T.; Shrestha, N.J.; Bhattarai, S. Study of electroencephalogram characteristics in neurological conditions in pediatric tertiary care hospital. Intl. J. Contemp. Pediatr. 2023, 10, 447–452. [Google Scholar] [CrossRef]
- Miyahara, H.; Akiyama, T.; Waki, K.; Arakaki, Y. Differential diagnosis of nonepileptic twilight state with convulsive manifestations after febrile seizures. Brain Dev. 2018, 40, 781–785. [Google Scholar] [CrossRef]
- Kasahara, Y.; Igata, H.; Sasaki, T.; Ikegaya, Y.; Koyama, R. The Pharmacological Assessment of GABAA Receptor Activation in Experimental Febrile Seizures in Mice. eNeuro 2019, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.M.; Ravizza, T.; Hamamura, M.; Zha, Q.; Keebaugh, A.; Fok, K.; Andres, A.L.; Nalcioglu, O.; Obenaus, A.; Vezzani, A.; et al. Epileptogenesis provoked by prolonged experimental febrile seizures: Mechanisms and biomarkers. J. Neurosci. 2010, 30, 7484–7494. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.W.; Im, H.; Song, Y.; Kim, S.J.; Lee, Y.R.; Kim, G.W.; Hwang, C.; Park, D.K.; Kim, D.S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells 2022, 11, 3228. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wu, D.C.; Feng, B.; Chen, B.; Tang, Y.S. Prolonged febrile seizures induce inheritable memory deficits in rats through DNA methylation. CNS Neurosci. Ther. 2019, 25, 601–611. [Google Scholar] [CrossRef]
- Ramírez Rentería, M.L. Effect of the Sexual Behavior on Febrile Seizures and Dopamine Levels in Male Rats. Ph.D. Thesis, University of Guadalajara, Guadalajara, Mexico, 2020. [Google Scholar]
- Arias, C.; Valero, H.; Tapia, R. Inhibition of brain glutamate decarboxylase activity is related to febrile seizures in rat pups. J. Neurochem. 1992, 58, 369–373. [Google Scholar] [CrossRef]
- Kubová, H.; Mikulecká, A.; Mareš, P. The outcome of early life status epilepticus-lessons from laboratory animals. Epilepsia Open 2023, 8 (Suppl. S1), S90–S109. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Yuen, A.W.; Sander, J.W.; Fluegel, D.; Patsalos, P.N.; Bell, G.S.; Johnson, T.; Koepp, M.J. Omega-3 fatty acid supplementation in patients with chronic epilepsy: A randomized trial. Epilepsy Behav. 2005, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Vosoughi, N.; Derakhshandeh-Rishehri, S.M.; Assarroudi, M.; Heidari-Beni, M. Association of Omega-3 Fatty Acid and Epileptic Seizure in Epileptic Patients: A Systematic Review. Int. J. Prev. Med. 2018, 9, 36. [Google Scholar] [CrossRef]
- Yehuda, S.; Carasso, R.L.; Mostofsky, D.I. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur. J. Pharmacol. 1994, 254, 193–198. [Google Scholar] [CrossRef]
- Ferrari, D.; Cysneiros, R.M.; Scorza, C.A.; Arida, R.M.; Cavalheiro, E.A.; de Almeida, A.C.; Scorza, F.A. Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: Quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. 2008, 13, 36–42. [Google Scholar] [CrossRef]
- Stillwell, W.; Shaikh, S.R.; Zerouga, M.; Siddiqui, R.; Wassal, S.R. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005, 45, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Itoh, K.; Ishihara, Y. Maternal intake of docosahexaenoic acid decreased febrile seizure sensitivity by increasing estrogen synthesis in offspring. Epilepsy Behav. 2021, 121 Pt A, 108038. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Itoh, K.; Ishihara, Y. Suppressive Effects of Docosahexaenoic Acid Intake on Increased Seizure Susceptibility after Growth Due to Febrile Seizures in Infancy. Biol. Pharm. Bull. 2023, 46, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.A.; Abd-El-Fatah, N.; Omar, T.-S.I.; Darwish, O.H. Fish oil intake and seizure control in children with medically resistant epilepsy. N. Am. J. Med. Sci. 2015, 7, 317. [Google Scholar] [CrossRef]
- Taha, A.Y.; Trepanier, M.O.; Ciobanu, F.A.; Taha, N.M.; Ahmed, M.; Zeng, Q.; Cheuk, W.I.; Ip, B.; Filo, E.; Scott, B.W.; et al. A minimum of 3 months of dietary fish oil supplementation is required to raise amygdaloid after discharge seizure thresholds in rats-implications for treating complex partial seizures. Epilepsy Behav. 2013, 27, 49–58. [Google Scholar] [CrossRef]
- Pessoa, D.T.; da Silva, E.L.A.; Costa, E.V.L.; Nogueira, R.A. Effect of diet with omega-3 in basal brain electrical activity and during status epilepticus in rats. Epilepsy Res. 2017, 137, 33–38. [Google Scholar] [CrossRef]
- Flores-Mancilla, L.E.; Hernández-González, M.; Guevara, M.A.; Benavides-Haro, D.E.; Martínez-Arteaga, P. Long-term fish oil supplementation attenuates seizure activity in the amygdala induced by 3-mercaptopropionic acid in adult male rats. Epilepsy Behav. 2014, 33, 126–134. [Google Scholar] [CrossRef]
- Kafadar, İ.H.; Yalçın, Y.; Çakar, B.L. Vitamin D3 and omega-3 polyunsaturated fatty acids have beneficial effects on fracture union in an experimental rat model. Jt. Dis. Relat. Surg. 2024, 35, 121–129. [Google Scholar] [CrossRef]
- Hoogland, G.; Raijmakers, M.; Clynen, E.; Brône, B.; Rigo, J.M.; Swijsen, A. Experimental early-life febrile seizures cause a sustained increase in excitatory neurotransmission in newborn dentate granule cells. Brain Behav. 2022, 12, e2505. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain, in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Pérez, M.A.G.; Loyo, J.R.; Hernández-González, M.; Madera-Carrillo, H.; Corsi-Cabrera, M. CAPTUSEN: Un Sistema para la Adquisición Computarizada del EEG y los Potenciales Relacionados a Eventos. Rev. Mex. Psicol. 2000, 17, 77–88. [Google Scholar]
- Ferreira, B.L.C.; Valle, A.C.; Cavalheiro, E.A.; Timo-Iaria, C. Absence-like seizures in adult rats following pilocarpine-induced status epilepticus early in life. Braz. J. Med. Biol. Res. 2003, 36, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Guevara Pérez, M.A.; Hernández-González, M. EEGmagic: Programa para analizar señales electroencefalográficas. Rev. Mex. Ing. Biom. 2009, 30, 41–53. [Google Scholar]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child. Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Dietary Implications of Polyunsaturated Fatty Acids during Pregnancy and in Neonates. Life 2023, 13, 1656. [Google Scholar] [CrossRef]
- Fukuda, M.; Morimoto, T.; Nagao, H.; Kida, K. The effect of GABAergic system activity on hyperthermia-induced seizures in rats. Develop. Brain Res. 1997, 104, 197–199. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Ezz, H.S.A.; Sayed, H.M.; Ali, M.A. Electroencephalographic and biochemical long–lasting abnormalities in animal model of febrile seizure. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 2120–2125. [Google Scholar] [CrossRef]
- Yu, Y.H.; Kim, S.-W.; Im, H.; Lee, Y.R.; Kim, G.W.; Ryu, S.; Park, D.-K.; Kim, D.-S. Febrile Seizure Causes Deficit in Social Novelty, Gliosis, and Proinflammatory Cytokine Response in the Hippocampal CA2 Region in Rats. Cells 2023, 12, 2446. [Google Scholar] [CrossRef]
- Feng, B.; Tang, Y.S.; Chen, B.; Dai, Y.J.; Xu, C.L.; Xu, Z.H.; Zhang, X.N.; Zhang, S.H.; Hu, W.W.; Chen, Z. Dysfunction of thermoregulation contributes to the generation of hyperthermia-induced seizures. Neurosci. Lett. 2014, 581, 129–134. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Prolonged Febrile Seizures Impair Synaptic Plasticity and Alter Developmental Pattern of Glial Fibrillary Acidic Protein (GFAP)-Immunoreactive Astrocytes in the Hippocampus of Young Rats. Int. J. Mol. Sci. 2022, 23, 12224. [Google Scholar] [CrossRef]
- Cristina-Silva, C.; Martins, V.; Gargaglioni, L.H.; Bícego, K.C. Mu and kappa opioid receptors of the periaqueductal gray stimulate and inhibit thermogenesis, respectively, during psychological stress in rats. Pflugers Arch. 2017, 469, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J. Endogenous opiates and behavior: 2023. Peptides 2024, 179, 171268. [Google Scholar] [CrossRef]
- Skyers, P.S.; Einheber, S.; Pierce, J.P.; Milner, T.A. Increased mu-opioid receptor labeling is found on inner molecular layer terminals of the dentate gyrus following seizures. Exp. Neurol. 2003, 179, 200–209. [Google Scholar] [CrossRef]
- Jones, L.S.; Grooms, S.Y.; Salvadori, S.; Lazarus, L.H. Dermorphin-induced hyperexcitability in hippocampal CA3 and CA1 in vitro. Eur. J. Pharmacol. 1994, 264, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hakimian, J.K.; Dong, T.S.; Barahona, J.A.; Lagishetty, V.; Tiwari, S.; Azani, D.; Barrera, M.; Lee, S.; Severino, A.L.; Mittal, N.; et al. Dietary Supplementation with Omega-3 Polyunsaturated Fatty Acids Reduces Opioid-Seeking Behaviors and Alters the Gut Microbiome. Nutrients 2019, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Qin, J.; Chang, X.Z.; Yang, Z.X. Effect of naloxone on remote seizure susceptibility. Beijing Da Xue Xue Bao Yi Xue Ban. 2004, 36, 57–60. (In Chinese) [Google Scholar]
- Sokol, D.K.; Demyer, W.E.; Edwards-Brown, M.; Sanders, S.; Garg, B. From swelling to sclerosis: Acute change in mesial hippocampus after prolonged febrile seizure. Seizure 2003, 12, 237–240. [Google Scholar] [CrossRef]
- Scott, R.C.; King, M.D.; Gadian, D.G.; Neville, B.G.R.; Connelly, A. Prolonged febrile seizures are associated with hippocampal vasogenic edema and developmental changes. Epilepsia 2006, 47, 1493–1498. [Google Scholar] [CrossRef]
- Zhong, L.; Wan, J.; Wu, J.; He, S.; Zhong, X.; Huang, Z.; Li, Z. Temporal and spatial dynamic propagation of electroencephalogram by combining power spectral and synchronization in childhood absence epilepsy. Front. Neuroinform. 2022, 16, 962466. [Google Scholar] [CrossRef]
- Hsu, D.A.; Rayer, K.; Jackson, D.C.; Stafstrom, C.E.; Hsu, M.; Ferrazzano, P.A.; Dabbs, K.; Worrell, G.A.; Jones, J.E.; Hermann, B.P. Correlation of EEG with neuropsychological status in children with epilepsy. Clin. Neurophysiol. 2016, 127, 1196–1205. [Google Scholar] [CrossRef]
- Börjesson, S.I.; Hammarström, S.; Elinder, F. Lipoelectric Modification of Ion Channel Voltage Gating by Polyunsaturated Fatty Acids. Biophys. J. 2008, 95, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Rico-Barrio, I.; Grandes, P. The effect of omega-3 fatty acids on alcohol-induced damage. Front. Nutr. 2023, 10, 1068343. [Google Scholar] [CrossRef]
- Timofeev, I.; Grenier, Ã.F.; Steriade, M. The role of chloride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave electrographic seizures. Neuroscience 2002, 114, 1115–1132. [Google Scholar] [CrossRef]
- Timofeev, I.; Steriade, M. Neocortical seizures: Initiation, development and cessation. Neuroscience 2004, 123, 299–336. [Google Scholar] [CrossRef]
- Erichsen, D.; Xiu, X.P.; Elinder, F. Docosahexanoic acid inducing opening of voltage of voltage-gated K channels prevents repetitive firing in neurons. Brain Dev. 2002, 24, 399. [Google Scholar]
- Xu, X.P.; Erichsen, D.; Börjesson, S.I.; Dahlin, M.; Amark, P.; Elinder, F. Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: A putative mechanism of antiseizure action. Epilepsy Res. 2008, 80, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef]
- Song, J. Amygdala activity and amygdala-hippocampus connectivity: Metabolic diseases, dementia, and neuropsychiatric issues. Biomed. Pharmacother. 2023, 162, 114647. [Google Scholar] [CrossRef]
- Hrebień-Filisińska, A.M. Application of natural antioxidants in the oxidative stabilization of fish oils: A mini-review. J. Food Process Preser. 2021, 45, e15342. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Mancilla, L.E.; Hernández-González, M.; Guevara-Pérez, M.Á.; Bonilla-Jaime, H.; Gaytán-Pacheco, N.; Reyes-Estrada, C.A.; Pacheco-Moisés, F.P. Long-Term Fish Oil Supplementation Attenuates Spike Wave Discharges in the Amygdala of Adult Rats with Early-Life Febrile Seizures. Brain Sci. 2025, 15, 395. https://doi.org/10.3390/brainsci15040395
Flores-Mancilla LE, Hernández-González M, Guevara-Pérez MÁ, Bonilla-Jaime H, Gaytán-Pacheco N, Reyes-Estrada CA, Pacheco-Moisés FP. Long-Term Fish Oil Supplementation Attenuates Spike Wave Discharges in the Amygdala of Adult Rats with Early-Life Febrile Seizures. Brain Sciences. 2025; 15(4):395. https://doi.org/10.3390/brainsci15040395
Chicago/Turabian StyleFlores-Mancilla, Leopoldo Eduardo, Marisela Hernández-González, Miguel Ángel Guevara-Pérez, Herlinda Bonilla-Jaime, Noemí Gaytán-Pacheco, Claudia Araceli Reyes-Estrada, and Fermín Paul Pacheco-Moisés. 2025. "Long-Term Fish Oil Supplementation Attenuates Spike Wave Discharges in the Amygdala of Adult Rats with Early-Life Febrile Seizures" Brain Sciences 15, no. 4: 395. https://doi.org/10.3390/brainsci15040395
APA StyleFlores-Mancilla, L. E., Hernández-González, M., Guevara-Pérez, M. Á., Bonilla-Jaime, H., Gaytán-Pacheco, N., Reyes-Estrada, C. A., & Pacheco-Moisés, F. P. (2025). Long-Term Fish Oil Supplementation Attenuates Spike Wave Discharges in the Amygdala of Adult Rats with Early-Life Febrile Seizures. Brain Sciences, 15(4), 395. https://doi.org/10.3390/brainsci15040395





