Abstract
Inherited neuropathies are a heterogeneous group of disorders that affect the peripheral nervous system, leading to motor, sensory, and autonomic dysfunction. These disorders are classified into various subgroups, including hereditary sensory and motor neuropathies, distal hereditary motor neuropathies, hereditary sensory and autonomic neuropathies, and more complex forms. Advances in genetic testing, particularly next-generation sequencing (NGS), have significantly improved the identification of these disorders. Emerging therapies, such as gene therapy, small molecule therapies, and antisense oligonucleotides, offer promising treatment options. However, current treatments remain limited, and their clinical benefits in humans are not yet fully established. This review provides a comprehensive overview of recent developments and evolving therapeutic options for hereditary neuropathies, focusing on gene therapy, small molecule therapies, and antisense oligonucleotides. It also highlights the current state of inherited neuropathies in Saudi Arabia, emphasizing the need for national guidelines, patient registries, and collaborative research efforts. By integrating advanced genomic technologies and fostering international collaboration, we can improve the diagnosis, management, and treatment outcomes for patients with inherited neuropathies.
1. Introduction
Hereditary neuropathies encompass a diverse group of genetically inherited disorders that affect the peripheral motor, sensory, and autonomic nerves [1]. These disorders are categorized into four major subgroups: hereditary sensory and motor neuropathies, distal hereditary motor neuropathies, hereditary sensory and autonomic neuropathies, and more complex hereditary neuropathies [2]. The advent of advanced genetic testing, particularly next-generation sequencing (NGS), has significantly enhanced the identification of these disorders [3,4]. These tests enable the detection of genetic mutations that compromise the integrity of either the axon or myelin of peripheral nerves, leading to the development of neuropathies [5].
In response to the increasing identification of hereditary neuropathies, there has been a corresponding surge in therapeutic options [6]. Among the most promising treatments is gene therapy, which has advanced due to improvements in gene manipulation technologies [7]. Gene therapy aims to correct or replace defective genes responsible for neuropathies, offering a potential long-term solution. Another emerging approach is the use of small molecule therapies (SMTs), which target specific pathways involved in the pathology of hereditary neuropathies [8]. SMTs modulate disease processes at the molecular level, providing targeted treatment options that may alter the disease course.
Antisense oligonucleotides (ASOs) represent another innovative therapeutic strategy. ASOs are short deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) molecules designed to bind to specific mRNA sequences, thereby modulating gene expression [9,10]. ASOs are relatively easy to deliver, as they do not need to integrate into the genome and are simple to synthesize. They have shown potential in treating various genetic disorders, including hereditary neuropathies, by altering the expression of disease-causing genes [10].
Despite these advancements, symptomatic treatment remains essential for managing many hereditary neuropathies. Current treatment options are limited, and while several therapies have shown efficacy in animal studies, their clinical benefits in humans are not yet fully established [6]. This review explores recent updates and evolving therapeutic options for major inherited neuropathies, focusing on the latest advancements in gene therapy, small molecule therapies, and antisense oligonucleotides. Our goal is to provide an overview of the current state of treatment and future directions in managing hereditary peripheral neuropathies.
2. Review
2.1. Overview on Major Inherited Neuropathies
Inherited neuropathies affect approximately 1 in 5000 children, with variations in prevalence depending on the specific disorder [11]. These disorders are a diverse group of genetic conditions that impact the peripheral nervous system, which transmits signals between the central nervous system (the brain and spinal cord) and the rest of the body (Table 1). Clinical manifestations of these neuropathies can vary significantly, even among individuals with the same genetic mutation, complicating diagnosis and treatment [12]. Despite significant progress in understanding the genetic and molecular mechanisms underlying inherited neuropathies, effective treatments remain elusive [1,2,12].

Table 1.
Classification, subtypes, and genetic involvement of inherited neuropathies.
Current management strategies are primarily supportive, aiming to relieve symptoms, improve mobility, and enhance the overall quality of life for affected individuals. Targeted therapies that address the underlying causes of these disorders are increasingly being researched, with promising avenues including gene therapy, molecular chaperones, and high-throughput drug screening [6].
2.2. Charcot-Marie-Tooth Disease (CMT)
CMT is the most prevalent inherited neuropathy, affecting approximately 1 in 2500 people. It was first described in the late 19th century by British neurologist Howard Henry Tooth and French neurologists Jean Martin Charcot and Pierre Marie [13]. CMT can be subdivided into demyelinating and axonal forms based on their pathogenesis. Axonal forms primarily involve axon degeneration, while demyelinating forms involve myelin sheath degeneration preceding axon damage [14].
Despite extensive research efforts, there are currently no effective pharmacological treatments available for patients with Charcot–Marie–Tooth disease (CMT). The development of effective therapies faces several significant challenges: (1) the extensive genetic heterogeneity of CMT, with over 1500 identified mutations, including the notable 1.4 Mb duplication in PMP22 associated with CMT1A, which leads to overlapping and variable disease phenotypes; (2) the rarity of individuals with specific genotypes, which limits both research interest and pharmaceutical investment; and (3) the inherent complexity of translating findings from preclinical studies in rodent and cellular models to successful human clinical trials [15].
Among the promising treatments explored, ascorbic acid (vitamin C) was one of the first therapies evaluated for CMT1A. Although widely studied, its efficacy remains unproven. Preclinical in vivo studies on C22 mice demonstrated that ascorbic acid reduced the expression of PMP22, a key protein implicated in CMT1A pathogenesis, and improved motor function [16,17]. Despite these encouraging preclinical results and the favorable safety profile of ascorbic acid, Phase III clinical trials in humans, which tested doses ranging from 1 to 4 g/day over two years, failed to demonstrate clinical efficacy [18].
Another investigational therapy, PXT3003, has shown promise in clinical trials for immune-mediated peripheral neuropathies, with significant improvements observed compared to placebo [8,19,20,21]. However, other therapeutic approaches, such as progesterone receptor antagonists and ACE-083, faced trial termination due to various issues, including lack of efficacy or safety concerns [19,22]. In contrast, therapies targeting P2X7 purinoreceptors and lipid supplementation have demonstrated satisfactory safety and tolerability in early trials, though their efficacy remains under investigation [19,23]. Looking ahead, a Phase IIa randomized, double-blind trial is set to evaluate the efficacy, safety, and tolerability of NMD670, administered twice daily for 21 days, in ambulatory adult patients with CMT1A.
In addition to pharmacological approaches, emerging technologies such as cellular reprogramming and high-throughput drug screening hold significant potential for advancing CMT research. Cellular reprogramming allows for the generation of patient-specific cell types, including stem cells, neurons, and glia, from somatic cells like fibroblasts or lymphocytes [24,25]. This technique enables the creation of disease-specific models for studying pathogenesis and screening potential therapies. However, challenges remain, such as developing subtype-specific differentiation protocols and co-culture systems to accurately model myelination and neuromuscular junction pathology in vitro [26,27] (Table 2).

Table 2.
Recent studies on possible treatments for CMT disease.
2.3. Hereditary Neuropathy with Liability to Pressure Palsies (HNPP)
Hereditary neuropathy with liability to pressure palsies (HNPP) is a genetic disorder linked to deletions in the PMP22 gene, which contrast with PMP22 duplications that cause Charcot–Marie–Tooth disease type 1A (CMT1A). CMT1A is a progressive neuropathy characterized by muscle weakness, atrophy, sensory deficits, and reduced reflexes [28,29].
HNPP manifests clinically as painless recurrent pressure palsies that target specific nerves or nerve plexuses, including the brachial plexus, and lead to focal motor and sensory symptoms. Preventive management includes both symptom control and lifestyle adjustments to avoid nerve compression and injuries. Some treatments and interventions have the potential to worsen the medical condition. The use of vincristine in chemotherapy treatments has led to increased HNPP symptoms [30], and surgical procedures must be avoided because they can cause serious nerve trauma [31].
Healthcare providers currently emphasize preventive approaches, including nerve pressure avoidance and ergonomic adjustments in routine activities [32]. While there is no definitive cure for the condition, some treatment methods have demonstrated potential benefits. The therapeutic use of IVIG has shown symptom improvement during acute disease episodes. Liew and Lo’s 2017 case study demonstrated that IVIG treatment produced significant symptom relief in an HNPP patient [33], which aligns with previous research supporting IVIG as a potential immunomodulatory therapy [34] (Table 3).

Table 3.
A recent study on a possible treatment for HNPP disease.
2.4. Hereditary Sensory and Autonomic Neuropathies (HSAN)
Hereditary Sensory and Autonomic Neuropathies (HSAN) encompass a group of rare inherited disorders characterized by sensory dysfunction and varying degrees of autonomic dysfunction [35]. These disorders primarily affect the peripheral nervous system, leading to symptoms such as loss of pain and temperature sensation, ulcerations, and autonomic disturbances, including cardiovascular and gastrointestinal dysfunction. The classification of HSAN remains unresolved and contentious, with ongoing debates over the delineation of subtypes. Initially, Dyck and Ohta proposed a classification system based on four numerical subtypes (HSAN I-IV) [36]. However, subsequent discoveries of additional genetic mutations and clinical phenotypes have introduced complexity into the classification, leading to semantic controversies and the recognition of further subtypes [37].
Each HSAN subtype is genetically distinct, with mutations in specific genes implicated in the pathogenesis of the disorder. For example, HSAN I is often associated with mutations in the SPTLC1 gene, while HSAN III (also known as Familial Dysautonomia) is linked to mutations in the IKBKAP gene [38,39]. Understanding these genetic distinctions is critical for developing targeted therapies and improving diagnostic accuracy. Currently, management options for HSAN are largely supportive, focusing on alleviating symptoms and improving patients’ quality of life [40].
Recent research has shed light on the molecular mechanisms underlying HSAN1, a subtype caused by mutations in the SPTLC1 gene. Studies have identified the accumulation of two neurotoxic sphingolipids—deoxysphingolipids—in patients with HSAN1 [41]. This accumulation results from a mutant enzyme’s reduced affinity for its normal substrate, L-serine, leading to the production of toxic metabolites [42]. This discovery prompted a two-year randomized, double-blind, placebo-controlled trial to evaluate the effectiveness of L-serine supplementation in correcting biochemical and neurological abnormalities in HSAN1 patients. The trial demonstrated that L-serine supplementation significantly reduced levels of neurotoxic deoxysphingolipids, suggesting it as a promising first treatment option for HSAN1 [43] (Table 4).

Table 4.
Recent studies on possible treatments for HSAN disease.
2.5. Familial Amyloid Polyneuropathy (FAP)
Familial amyloid polyneuropathies (FAPs) are fatal multisystem conditions inherited as autosomal dominant traits that cause nerve damage through amyloid fibril deposits resulting from mutated transthyretin [44]. A particular mutation in the transthyretin gene leads to a hereditary polyneuropathy that emerges in adulthood and stands as the most severe form of its type [45]. According to the scientific literature, the management strategy for this disorder includes three essential steps [46]. The main strategy of disease-modifying targeted therapy aims to halt both production and accumulation of amyloid fibrils [47,48]. Management of this disorder involves liver transplantation (LT) because it replaces the mutant transthyretin (TTR) protein source or the application of transthyretin kinetic stabilizers such as tafamidis and diflunisal [49,50,51] (Table 5).

Table 5.
Recent studies on possible treatments for FAP.
2.6. Refsum Disease
Refsum disease is a genetic disorder that produces peripheral neuropathy symptoms and occurs in both adult and infantile forms [52]. A deficiency in phytanoyl-CoA hydroxylase causes the condition because this enzyme is essential for metabolizing phytanic acid [53]. When phytanic acid breakdown is impaired by enzyme deficiency, it builds up in tissues and produces multiple clinical signs [54]. Refsum disease treatment relies on dietary changes and plasmapheresis as primary management approaches [55]. Patients must limit their consumption of foods rich in phytanic acid to manage their condition [54,55]. Although some symptoms improve with treatment, conditions such as retinitis pigmentosa and sensorineural hearing loss show lesser improvement [56,57,58,59].
2.7. Giant Axonal Neuropathy (GAN)
GAN is a rare inherited disorder impacting both the central and peripheral nervous systems, marked by the presence of abnormally large axons [60]. The clinical presentation of GAN can vary significantly, which often leads to the consideration of other conditions, such as classic infantile neuroaxonal dystrophy, Charcot–Marie–Tooth hereditary neuropathy type 4 (CMT4), and leukodystrophies, including arylsulfatase-A deficiency [61,62]. Despite this, the characteristics of GAN have been extensively documented in the medical literature [63,64,65,66].
Regarding treatment, a groundbreaking in-human trial began in 2015 to evaluate the efficacy of intrathecal administration of scAAV9/JeT-GAN, a gene therapy. This trial has shown promise in slowing motor decline in GAN patients but also highlighted several associated adverse events [67]. Detailing the adverse events in this phase 1 trial evaluating intrathecal scAAV9/JeT-GAN gene therapy for giant axonal neuropathy, safety analysis revealed 682 adverse events (AEs) over a median observation period of 68.7 months, with 129 (18.9%) deemed possibly treatment-related. Among 48 serious adverse events (SAEs), only one (fever with emesis) was linked to therapy. Common SAEs included scoliosis (9 participants), urinary tract infections (6), and upper respiratory infections (5). Two fatalities occurred due to disease progression, unrelated to treatment. Treatment-related AEs included cerebrospinal fluid (CSF) pleocytosis (13 participants), elevated CSF IgG index (13), leukocytosis (8), thrombocytosis (7), and headaches (7). Mild, transient hepatic transaminase elevations (grade 1–3) were observed in three participants. Immune responses, such as persistent AAV9 neutralizing antibodies and CSF pleocytosis, were frequent but asymptomatic. No dose-limiting toxicities or neuroinflammatory pathologies were identified, even at the highest dose (3.5 × 1014 vg) [67]. The safety profile of scAAV9/JeT-GAN underscores both the expected and novel challenges in intrathecal gene therapy. While most AEs reflected underlying disease progression or immunosuppressive regimens (e.g., glucocorticoid-related infections), immune activation—evidenced by CSF pleocytosis, elevated neutralizing antibodies, and T-cell responses to AAV9 capsid—highlight the inherent hurdles of AAV-mediated delivery. Persistent humoral immunity suggests potential barriers to redosing, a critical consideration for future trials. Notably, the absence of severe neuroinflammation or dose-dependent toxicity, despite widespread biodistribution, supports the feasibility of higher doses. However, the transient, manageable nature of hepatic and hematologic abnormalities suggests these are unlikely to preclude clinical use. The single SAE directly linked to therapy (fever) resolved rapidly, reinforcing the tolerability of the approach. These findings align with broader gene therapy safety trends, where immune responses dominate AE profiles, yet emphasize the need for optimized immunosuppression strategies to mitigate anticapsid immunity and enhance therapeutic durability [67]. Nonetheless, given the recent positive outcomes, ongoing studies are necessary to further assess the safety and efficacy of this gene transfer therapy (Table 6).

Table 6.
Recent studies on possible treatments for GAN.
2.8. Congenital Hypomyelinating Neuropathy (CHN)
CHN represents a rare inherited peripheral neuropathy disorder [68]. Patients with this condition show nonprogressive muscle weakness together with diminished reflexes, low muscle tone, substantially reduced nerve conduction velocities, and incomplete development of nerve myelin sheaths [69]. There does not yet exist any targeted or definitive cure to treat this disorder. The treatment strategy utilizes symptom management together with complete supportive care to improve patients’ overall health and quality of life [70,71] (Table 7).

Table 7.
A recent study on a possible treatment for CHN.
2.9. Tangier Disease
Tangier disease is a rare genetic condition where affected individuals exhibit very low levels of high-density lipoprotein in their blood serum [72]. Specific manifestations of Tangier disease dictate the treatment strategies that guide its management. Peripheral neuropathy represents an additional possible complication for patients with Tangier disease. While specific clinical treatments for this condition remain unproven, patients can benefit from supportive measures like transient bracing and personalized exercise programs [73] (Table 8).

Table 8.
A recent study on a possible treatment for Tangier disease.
3. Saudi Perspective and Existing Consensus on Inherited Neuropathies
Saudi Arabia exhibits one of the highest global incidences of inherited neuropathies, resulting in a substantial and growing population of affected individuals spanning pediatric and adult demographics [74]. These disorders, which are hereditary and predominantly affect the peripheral nervous system, are particularly prevalent in Saudi Arabia due to genetic factors and the high rate of consanguineous marriages [75,76]. Given the significant burden of these conditions, this review explores the evolving landscape of inherited neuropathies and their management within the Saudi context.
To address the complexity of these disorders, the Saudi Board of Pediatric Neurology curriculum emphasizes recognizing the pathological characteristics and clinical features of inherited neuropathies encountered in pediatric practice [77]. This educational framework ensures pediatric neurologists are equipped to diagnose and manage these conditions effectively. The curriculum covers a wide spectrum of inherited neuropathies, detailing their genetic basis, disease mechanisms, and clinical presentations. By integrating this knowledge into training, clinicians are better prepared to differentiate neuropathies, understand disease progression, and anticipate complications, improving diagnostic accuracy and enabling tailored treatments [78].
The medical community in Saudi Arabia acknowledges the challenges of diagnosing and managing inherited neuropathies, particularly late-onset forms in adults. Consensus has been reached on key issues: the rising incidence of these disorders [79], their genetic heterogeneity [79], and primary clinical features such as muscle weakness, sensory abnormalities, and impaired motor function [80]. A multidisciplinary approach and genetic counseling are increasingly emphasized as essential components of care [81,82].
Despite growing awareness, Saudi Arabia lacks national clinical guidelines for inherited neuropathies. Current management relies on broad evidence-based principles without standardized protocols [81]. This underscores the urgent need for stakeholders—neurologists, geneticists, researchers, and patient advocates—to collaborate on comprehensive national guidelines. A recent Spanish multidisciplinary initiative developing CMT guidelines serves as a model [82]. Standardized protocols would improve diagnostic criteria, treatment consistency, and data collection, driving innovation in care and research [82].
Inherited neuropathies pose significant challenges due to genetic diversity and clinical complexity [79]. Effective management requires comprehensive genetic and phenotypic data. Saudi Arabia’s lack of a national neuropathies registry highlights the need for a centralized repository to support research and clinical trials [80,81,82]. The Global Registry for Inherited Neuropathies (GRIN) offers a collaborative platform for data sharing [83].
A recent Saudi initiative systematically collects data on inherited neuropathy cases nationwide, aiming to identify novel genetic mutations and correlate them with clinical phenotypes. This effort will enhance understanding of disease mechanisms, improve diagnostic accuracy, and facilitate personalized therapies. By cataloging mutations unique to the Saudi population, this initiative lays the groundwork for targeted research and tailored treatments.
The rarity and genetic heterogeneity of these disorders necessitate data sharing. International databases like RD-CONNECT, DECIPHER, and GENESIS integrate NGS data with clinical phenotyping, providing invaluable resources for clinicians and researchers [84]. In Saudi Arabia, where inherited neuropathies affect up to six generations in single families, a national registry would offer insights into genetic risks, improve counseling, and connect families for shared learning [85].
Patient advocacy groups, including the Hereditary Neuropathy Foundation (HNF) and the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM), play a vital role in education and support. These organizations enhance knowledge dissemination and foster community networks critical for patient care.
In Figure 1, a summary of efforts and gaps in addressing inherited neuropathies in Saudi Arabia is presented, highlighting the need for continued investment in research, registry development, and multidisciplinary collaboration to improve patient outcomes.
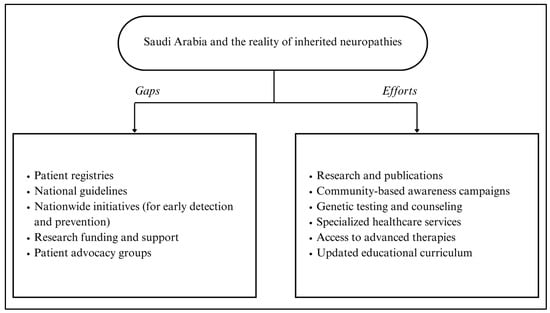
Figure 1.
Summary of efforts and gaps to address inherited neuropathies in Saudi Arabia.
4. Future Directions
While recent advances in disease-modifying therapies for inherited neuropathies—driven by insights into pathogenic mechanisms—offer transformative potential, future research must prioritize standardized, objective assessment tools to rigorously evaluate therapeutic efficacy, address heterogeneity in trial design, and ensure clinically meaningful outcomes across diverse genetic subtypes [12]. New studies should focus on developing gene therapy and precision medicine solutions targeting the specific genetic mutations responsible for CMT disease. Investigating the molecular mechanisms of CMT will help researchers identify new potential therapeutic targets. Establishing detailed patient registries and biobanks will enable longitudinal studies and the design of personalized treatments. Research efforts should expand to explore the pathophysiological mechanisms underlying HNPP. Advancements in genomic technologies, such as next-generation sequencing (NGS), will help identify additional genetic factors contributing to HNPP. Research should prioritize therapies targeting HNPP’s molecular pathways and establishing clinical guidelines to improve patient care.
Research priorities for hereditary neuropathies must align with the unique pathomechanisms and clinical challenges of each disorder. For HSAN, elucidating genetic drivers and biochemical pathways is foundational; such insights could inform targeted therapies, including neuroprotective agents or gene-editing approaches to correct mutations. Collaborative efforts to build international patient registries will strengthen genotype-phenotype correlations, enabling stratified therapeutic development. Similarly, in FAP, disease-modifying strategies should prioritize RNA interference or antisense oligonucleotides to suppress amyloidogenic transthyretin production, complemented by advances in biomarker discovery to facilitate early diagnosis and treatment monitoring. For Refsum disease, biomarker development is critical not only for early detection but also for tracking disease progression, which could optimize the timing of interventions such as gene therapy or enzyme replacement. In parallel, refining dietary protocols to restrict phytanic acid intake remains essential for mitigating acute exacerbations. In GAN, therapeutic development should center on gene therapies to restore gigaxonin function, supported by mechanistic studies exploring its role in neuronal integrity. Natural history studies and patient registries are indispensable for accurately assessing treatment efficacy and identifying novel therapeutic targets. Furthermore, CMT research must clarify molecular regulators of myelination to advance stem cell-based or myelin-repair therapies, while standardizing diagnostic criteria to reduce heterogeneity in clinical trials. For Tangier disease, therapies targeting cholesterol metabolism pathways—coupled with investigations into genetic and environmental modifiers—could enable personalized approaches. Global collaborative registries will enhance data quality across all these disorders, accelerating translational research and trial design. By integrating mechanistic discovery, therapeutic innovation, and collaborative data infrastructure, the field can address both shared and unique challenges in hereditary neuropathies.
Moreover, Saudi Arabia must establish a national inherited neuropathy registry to advance research and care. Developing specialized clinical guidelines requires collaborative networks of local and international experts. Successful management also demands public health initiatives focused on awareness and genetic counseling.
5. Conclusions
The landscape of inherited neuropathies is rapidly evolving, driven by advances in genetic research and therapeutics. The high prevalence of these conditions in Saudi Arabia underscores the need for focused research, comprehensive registries, and collaborative guideline development. By integrating advanced genomic technologies, robust patient support networks, and international partnerships, we can improve diagnosis, management, and outcomes. Personalized therapies hold significant promise for enhancing quality of life for affected individuals and families.
A dedicated multidisciplinary rehabilitation team is critical across nearly all disorders. Exploring therapeutic pathways specific to each condition remains essential. Establishing a national neuropathies registry in Saudi Arabia is not only beneficial but imperative. Such a registry would transform diagnosis, treatment, and research while advancing the country’s understanding of these disorders. By addressing challenges and leveraging global best practices, Saudi Arabia can build a system that benefits patients, families, and the medical community. Investing in this resource now will ensure better health outcomes for future generations.
Author Contributions
Conceptualization was conducted by A.K.B. and A.S.A.; methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, and project administration were handled by A.K.B.; validation contributions also included A.S.A. and F.K.A.; funding acquisition was managed by A.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
- The following abbreviations are used in this manuscript:
| NGS | Next-Generation Sequencing |
| SMTs | Small Molecule Therapies |
| ASOs | Antisense Oligonucleotides |
| CMT | Charcot-Marie-Tooth Disease |
| HSAN | Hereditary Sensory and Autonomic Neuropathies |
| HNPP | Hereditary Neuropathy with Liability to Pressure Palsies |
| PMP22 | Peripheral Myelin Protein 22 |
| IVIG | Intravenous Immunoglobulin |
| SPTLC1 | Serine Palmitoyltransferase Long Chain Base Subunit 1 |
| TTR | Transthyretin |
| LT | Liver Transplantation |
| GAN | Giant Axonal Neuropathy |
| scAAV9 | Self-Complementary Adeno-Associated Virus 9 |
| IF | Intermediate Filaments |
| GRIN | Global Registry for Inherited Neuropathies |
| HNF | Hereditary Neuropathy Foundation |
| AANEM | American Association of Neuromuscular and Electrodiagnostic Medicine |
| ABCA1 | ATP-Binding Cassette Transporter A1 |
| CHN | Congenital Hypomyelinating Neuropathy |
| FAP | Familial Amyloid Polyneuropathy |
| HDL | High-Density Lipoprotein |
| IT | Intrathecal |
| KO | Knockout |
References
- Eggermann, K.; Gess, B.; Häusler, M.; Weis, J.; Hahn, A.; Kurth, I. Hereditary Neuropathies. Dtsch. Arztebl. Int. 2018, 115, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.J.; Lochmüller, A.; Atalaia, A.; Horvath, R. Targeted therapies for hereditary peripheral neuropathies: Systematic review and steps towards a ‘treatabolome’. J. Neuromuscul. Dis. 2021, 8, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Baets, J.; De Jonghe, P.; Timmerman, V. Recent advances in Charcot-Marie-Tooth disease. Curr. Opin. Neurol. 2014, 27, 532–540. [Google Scholar] [CrossRef]
- Timmerman, V.; Strickland, A.V.; Züchner, S. Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the Human Genome Project success. Genes 2014, 5, 13–32. [Google Scholar] [CrossRef]
- Harding, A.E.; Thomas, P.K. Genetic aspects of hereditary motor and sensory neuropathy (types I and II). J. Med. Genet. 1980, 17, 329–336. [Google Scholar] [CrossRef]
- Thenmozhi, R.; Lee, J.S.; Park, N.Y.; Choi, B.O.; Hong, Y.B. Gene therapy options as new treatment for inherited peripheral neuropathy. Exp. Neurobiol. 2020, 29, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, G. State-of-the-art human gene therapy: Part II. Gene therapy strategies and clinical applications. Discov. Med. 2014, 18, 151–161. [Google Scholar]
- Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Kagiava, A.; Kleopa, K.A. Emerging therapies for Charcot-Marie-Tooth inherited neuropathies. Int. J. Mol. Sci. 2021, 22, 6048. [Google Scholar] [CrossRef]
- Amanat, M.; Nemeth, C.L.; Fine, A.S.; Leung, D.G.; Fatemi, A. Antisense oligonucleotide therapy for the nervous system: From bench to bedside with emphasis on pediatric neurology. Pharmaceutics 2022, 14, 2389. [Google Scholar] [CrossRef]
- Askari, F.K.; McDonnell, W.M. Antisense-oligonucleotide therapy. N. Engl. J. Med. 1996, 334, 316–318. [Google Scholar] [CrossRef]
- Saporta, M.A.; Shy, M.E. Inherited peripheral neuropathies. Neurol. Clin. 2013, 31, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Hustinx, M.; Shorrocks, A.M.; Servais, L. Novel Therapeutic Approaches in Inherited Neuropathies: A Systematic Review. Pharmaceutics 2023, 15, 1626. [Google Scholar] [CrossRef] [PubMed]
- Skre, H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin. Genet. 1974, 6, 98–118. [Google Scholar] [CrossRef]
- Magy, L.; Mathis, S.; Le Masson, G.; Goizet, C.; Tazir, M.; Vallat, J.M. Updating the classification of inherited neuropathies: Results of an international survey. Neurology 2018, 90, e870–e876. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Burns, J.; Saporta, M.A.; Timmerman, V. Challenges in modelling the Charcot-Marie-Tooth neuropathies for therapy development. J. Neurol. Neurosurg. Psychiatry 2019, 90, 58–67. [Google Scholar] [CrossRef]
- Passage, E.; Norreel, J.C.; Noack-Fraissignes, P.; Sanguedolce, V.; Pizant, J.; Thirion, X.; Robaglia-Schlupp, A.; Pellissier, J.F.; Fontés, M. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat. Med. 2004, 10, 396–401. [Google Scholar] [CrossRef]
- Kiepura, A.J.; Kochański, A. Charcot Marie Tooth type 1A drug therapies: Role of adenylyl cyclase activity and G protein coupled receptors in disease pathomechanism. Acta Neurobiol. Exp. 2018, 78, 198–209. [Google Scholar] [CrossRef]
- Pareyson, D.; Reilly, M.M.; Schenone, A.; Fabrizi, G.M.; Cavallaro, T.; Santoro, L.; Vita, G.; Quattrone, A.; Padua, L.; Gemignani, F.; et al. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): A double-blind randomised trial. Lancet Neurol. 2011, 10, 320–328. [Google Scholar] [CrossRef]
- Pisciotta, C.; Saveri, P.; Pareyson, D. Updated review of therapeutic strategies for Charcot-Marie-Tooth disease and related neuropathies. Expert. Rev. Neurother. 2021, 21, 701–713. [Google Scholar] [CrossRef]
- Attarian, S.; Young, P.; Brannagan, T.H.; Adams, D.; Van Damme, P.; Thomas, F.P.; Casanovas, C.; Kafaie, J.; Tard, C.; Walter, M.C.; et al. A double-blind, placebo-controlled, randomized trial of PXT3003 for the treatment of Charcot-Marie-Tooth type 1A. Orphanet J. Rare Dis. 2021, 16, 433. [Google Scholar] [CrossRef]
- Chumakov, I.; Milet, A.; Cholet, N.; Primas, G.; Boucard, A.; Pereira, Y.; Graudens, E.; Mandel, J.; Laffaire, J.; Foucquier, J.; et al. Polytherapy with a combination of three repurposed drugs (PXT3003) down-regulates Pmp22 over-expression and improves myelination, axonal and functional parameters in models of CMT1A neuropathy. Orphanet J. Rare Dis. 2014, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.P.; Brannagan, T.H., 3rd; Butterfield, R.J.; Desai, U.; Habib, A.A.; Herrmann, D.N.; Eichinger, K.J.; Johnson, N.E.; Karam, C.; Pestronk, A.; et al. Randomized Phase 2 Study of ACE-083 in Patients With Charcot-Marie-Tooth Disease. Neurology 2022, 98, e2356–e2367. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef]
- Saporta, M.A.; Grskovic, M.; Dimos, J.T. Induced pluripotent stem cells in the study of neurological diseases. Stem Cell Res. Ther. 2011, 2, 37. [Google Scholar] [CrossRef]
- Saporta, M.A. Cellular reprogramming and inherited peripheral neuropathies: Perspectives and challenges. Neural Regen. Res. 2015, 10, 894–896. [Google Scholar] [CrossRef]
- van Paassen, B.W.; van der Kooi, A.J.; van Spaendonck-Zwarts, K.Y.; Verhamme, C.; Baas, F.; de Visser, M. PMP22 related neuropathies: Charcot-Marie-Tooth disease type 1A and Hereditary Neuropathy with liability to Pressure Palsies. Orphanet J. Rare Dis. 2014, 9, 38. [Google Scholar] [CrossRef]
- Reilly, M.M. Sorting out the inherited neuropathies. Pract. Neurol. 2007, 7, 93–105. [Google Scholar]
- Kalfakis, N.; Panas, M.; Karadima, G.; Floroskufi, P.; Kokolakis, N.; Vassilopoulos, D. Hereditary neuropathy with liability to pressure palsies emerging during vincristine treatment. Neurology 2002, 59, 1470–1471. [Google Scholar] [CrossRef]
- Chance, P.F. Inherited focal, episodic neuropathies: Hereditary neuropathy with liability to pressure palsies and hereditary neuralgic amyotrophy. Neuromolecular Med. 2006, 8, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Chrestian, N. Hereditary Neuropathy with Liability to Pressure Palsy. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Stevenson, R.E., et al., Eds.; University of Washington: Seattle, DC, USA, 2020; pp. 1–21. [Google Scholar]
- Liew, Z.H.; Lo, Y.L. Intravenous Immunoglobulin in Treatment of Hereditary Neuropathy With Liability to Pressure Palsy. J. Clin. Neuromuscul. Dis. 2017, 18, 160–161. [Google Scholar] [CrossRef]
- Vrinten, C.; Gu, X.; Weinreich, S.S.; Schipper, M.H.; Wessels, J.; Ferrari, M.D.; Hoijtink, H.; Verschuuren, J.J. An n-of-one RCT for intravenous immunoglobulin G for inflammation in hereditary neuropathy with liability to pressure palsy (HNPP). J. Neurol. Neurosurg. Psychiatry 2016, 87, 790–791. [Google Scholar] [CrossRef]
- Axelrod, F.B.; Gold-von Simson, G. Hereditary sensory and autonomic neuropathies: Types II, III, and IV. Orphanet J. Rare Dis. 2007, 2, 39. [Google Scholar] [CrossRef]
- Dyck, P.J. The causes, classification, and treatment of peripheral neuropathy. N. Engl. J. Med. 1982, 307, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, F.B.; Pearson, J. Congenital sensory neuropathies. Diagnostic distinction from familial dysautonomia. Am. J. Dis. Child. 1984, 138, 947–954. [Google Scholar] [CrossRef]
- Houlden, H.; King, R.H.; Hashemi-Nejad, A.; Wood, N.W.; Mathias, C.J.; Reilly, M.; Thomas, P.K. A novel TRK A (NTRK1) mutation associated with hereditary sensory and autonomic neuropathy type V. Ann. Neurol. 2001, 49, 521–525. [Google Scholar] [CrossRef]
- Bejaoui, K.; Uchida, Y.; Yasuda, S.; Ho, M.; Nishijima, M.; Brown, R.H., Jr.; Holleran, W.M.; Hanada, K. Hereditary sensory neuropathy type 1 mutations confer dominant negative effects on serine palmitoyltransferase, critical for sphingolipid synthesis. J. Clin. Investig. 2002, 110, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, F.B. Familial dysautonomia: A review of the current pharmacological treatments. Expert. Opin. Pharmacother. 2005, 6, 561–567. [Google Scholar] [CrossRef]
- Penno, A.; Reilly, M.M.; Houlden, H.; Laurá, M.; Rentsch, K.; Niederkofler, V.; Stoeckli, E.T.; Nicholson, G.; Eichler, F.; Brown, R.H., Jr.; et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 2010, 285, 11178–11187. [Google Scholar] [CrossRef]
- Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 2003, 1632, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, K.; Penno, A.; Schmidt, B.P.; Lee, H.J.; Frosch, M.P.; von Eckardstein, A.; Brown, R.H.; Hornemann, T.; Eichler, F.S. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Investig. 2011, 121, 4735–4745. [Google Scholar] [CrossRef]
- Planté-Bordeneuve, V.; Said, G. Familial amyloid polyneuropathy. Lancet Neurol. 2011, 10, 1086–1097. [Google Scholar] [CrossRef]
- Rohatgi, S.; Nirhale, S.; Manohar, P.; Rao, P.; Naphade, P.; Khan, F.M.A.; Dave, D.; Kotaru, V.V.S.; Gupta, S.; Gitay, A.; et al. Novel transthyretin gene mutation in familial amyloid neuropathy in India: Case. Ann. Afr. Med. 2022, 21, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Suhr, O.B.; Hund, E.; Obici, L.; Tournev, I.; Campistol, J.M.; Slama, M.S.; Hazenberg, B.P.; Coelho, T.; European Network for TTR-FAP (ATTReuNET). First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr. Opin. Neurol. 2016, 29 (Suppl. S1), S14–S26. [Google Scholar] [CrossRef]
- Ando, Y.; Coelho, T.; Berk, J.L.; Cruz, M.W.; Ericzon, B.G.; Ikeda, S.; Lewis, W.D.; Obici, L.; Planté-Bordeneuve, V.; Rapezzi, C.; et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J. Rare Dis. 2013, 8, 31. [Google Scholar] [CrossRef]
- Adams, D.; Théaudin, M.; Cauquil, C.; Algalarrondo, V.; Slama, M. FAP neuropathy and emerging treatments. Curr. Neurol. Neurosci. Rep. 2014, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Adams, D. Recent advances in the treatment of familial amyloid polyneuropathy. Ther. Adv. Neurol. Disord. 2013, 6, 129–139. [Google Scholar] [CrossRef]
- Schilling, M. Gentherapieoptionen der hereditären Transthyretinamyloidose. Nervenarzt 2022, 93, 557–565. [Google Scholar] [CrossRef]
- Habib, M.H.; Tiger, Y.K.R.; Dima, D.; Schlögl, M.; McDonald, A.; Mazzoni, S.; Khouri, J.; Williams, L.; Anwer, F.; Raza, S. Role of palliative care in the supportive management of AL amyloidosis—A review. J. Clin. Med. 2024, 13, 1991. [Google Scholar] [CrossRef]
- Baldwin, E.J.; Gibberd, F.B.; Harley, C.; Sidey, M.C.; Feher, M.D.; Wierzbicki, A.S. The effectiveness of long-term dietary therapy in the treatment of adult Refsum disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 954–957. [Google Scholar] [CrossRef]
- Baldwin, E.J.; Harrington, D.J.; Sampson, B.; Feher, M.D.; Wierzbicki, A.S. Safety of long-term restrictive diets for peroxisomal disorders: Vitamin and trace element status of patients treated for Adult Refsum Disease. Int. J. Clin. Pract. 2016, 70, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.; Waterham, H.R. Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta 2006, 1763, 1707–1720. [Google Scholar] [CrossRef]
- Rizzo, W.B.; Jenkens, S.M.; Boucher, P. Recognition and diagnosis of neuro-ichthyotic syndromes. Semin. Neurol. 2012, 32, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rüether, K.; Baldwin, E.; Casteels, M.; Feher, M.D.; Horn, M.; Kuranoff, S.; Leroy, B.P.; Wanders, R.J.; Wierzbicki, A.S. Adult Refsum disease: A form of tapetoretinal dystrophy accessible to therapy. Surv. Ophthalmol. 2010, 55, 531–538. [Google Scholar] [CrossRef]
- Zolotov, D.; Wagner, S.; Kalb, K.; Bunia, J.; Heibges, A.; Klingel, R. Long-term strategies for the treatment of Refsum’s disease using therapeutic apheresis. J. Clin. Apher. 2012, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, H.U.; Siegmund, J.B.; Hoppmann, I. Lipapheresis: An immunoglobulin-sparing treatment for Refsum’s disease. Acta Neurol. Scand. 1996, 94, 190–193. [Google Scholar] [CrossRef]
- Harari, D.; Gibberd, F.B.; Dick, J.P.; Sidey, M.C. Plasma exchange in the treatment of Refsum’s disease (heredopathia atactica polyneuritiformis). J. Neurol. Neurosurg. Psychiatry 1991, 54, 614–617. [Google Scholar] [CrossRef]
- Demir, E.; Bomont, P.; Erdem, S.; Cavalier, L.; Demirci, M.; Kose, G.; Muftuoglu, S.; Cakar, A.N.; Tan, E.; Aysun, S.; et al. Giant axonal neuropathy: Clinical and genetic study in six cases. J. Neurol. Neurosurg. Psychiatry 2005, 76, 825–832. [Google Scholar] [CrossRef]
- Johnson-Kerner, B.L.; Roth, L.; Greene, J.P.; Wichterle, H.; Sproule, D.M. Giant axonal neuropathy: An updated perspective on its pathology and pathogenesis. Muscle Nerve 2014, 50, 467–476. [Google Scholar] [CrossRef]
- Edem, P.; Karakaya, M.; Wirth, B.; Okur, T.D.; Yiş, U. Giant axonal neuropathy: A differential diagnosis of consideration. Turk. J. Pediatr. 2019, 61, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Su, Q.; Zhu, X.; Wang, J.; Lou, Y.; Miao, P.; Wang, Y.; Zhang, B.; Jin, Y.; Gao, L.; et al. Giant axonal neuropathy (GAN) in an 8-year-old girl caused by a homozygous pathogenic splicing variant in GAN gene. Am. J. Med. Genet. A 2022, 188, 836–846. [Google Scholar] [CrossRef]
- Ashrafi, M.R.; Dehnavi, A.Z.; Tavasoli, A.R.; Heidari, M.; Ghahvechi Akbari, M.; Ronagh, A.R.; Ghafouri, M.; Mahdieh, N.; Mohammadi, P.; Rezaei, Z. Expanding the genetic spectrum of giant axonal neuropathy: Two novel variants in Iranian families. Mol. Genet. Genom. Med. 2023, 11, e2159. [Google Scholar] [CrossRef] [PubMed]
- Koichihara, R.; Saito, T.; Ishiyama, A.; Komaki, H.; Yuasa, S.; Saito, Y.; Nakagawa, E.; Sugai, K.; Shiihara, T.; Shioya, A.; et al. A mild case of giant axonal neuropathy without central nervous system manifestation. Brain Dev. 2016, 38, 350–353. [Google Scholar] [CrossRef]
- Akagi, M.; Mohri, I.; Iwatani, Y.; Kagitani-Shimono, K.; Okinaga, T.; Sakai, N.; Ozono, K.; Taniike, M. Clinicogenetical features of a Japanese patient with giant axonal neuropathy. Brain Dev. 2012, 34, 156–162. [Google Scholar] [CrossRef]
- Bharucha-Goebel, D.X.; Todd, J.J.; Saade, D.; Norato, G.; Jain, M.; Lehky, T.; Bailey, R.M.; Chichester, J.A.; Calcedo, R.; Armao, D.; et al. Intrathecal gene therapy for giant axonal neuropathy. N. Engl. J. Med. 2024, 390, 1092–1104. [Google Scholar] [CrossRef]
- Lesmana, H.; Vawter Lee, M.; Hosseini, S.A.; Burrow, T.A.; Hallinan, B.; Bove, K.; Schapiro, M.; Hopkin, R.J. CNTNAP1-Related Congenital Hypomyelinating Neuropathy. Pediatr. Neurol. 2019, 93, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, T.; Lupo, V.; Sivera, R.; Marco-Marín, C.; Martínez-Rubio, D.; Rivas, E.; Hernández, A.; Palau, F.; Espinós, C. Congenital hypomyelinating neuropathy due to a novel MPZ mutation. J. Peripher. Nerv. Syst. 2011, 16, 347–352. [Google Scholar] [CrossRef]
- Smit, L.S.; Roofthooft, D.; van Ruissen, F.; Baas, F.; van Doorn, P.A. Congenital hypomyelinating neuropathy, a long term follow-up study in an affected family. Neuromuscul. Disord. 2008, 18, 59–62. [Google Scholar] [CrossRef]
- Hahn, J.S.; Henry, M.; Hudgins, L.; Madan, A. Congenital hypomyelination neuropathy in a newborn infant: Unusual cause of diaphragmatic and vocal cord paralyses. Pediatrics 2001, 108, E95. [Google Scholar] [CrossRef]
- Utech, M.; Höbbel, G.; Rust, S.; Reinecke, H.; Assmann, G.; Walter, M. Accumulation of RhoA, RhoB, RhoG, and Rac1 in fibroblasts from Tangier disease subjects suggests a regulatory role of Rho family proteins in cholesterol efflux. Biochem. Biophys. Res. Commun. 2001, 280, 229–236. [Google Scholar] [CrossRef]
- Burnett, J.R.; Hooper, A.J.; McCormick, S.P.A.; Hegele, R.A. Tangier Disease. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, DC, USA, 2019; pp. 1–18. [Google Scholar]
- Sulaiman, R.A.; Al-Owain, M. Inherited Metabolic Disorders in Adults: A view from Saudi Arabia. Eur. J. Med. Genet. 2019, 62, 103562. [Google Scholar] [CrossRef] [PubMed]
- Khayat, A.M.; Alshareef, B.G.; Alharbi, S.F.; AlZahrani, M.M.; Alshangity, B.A.; Tashkandi, N.F. Consanguineous Marriage and Its Association with Genetic Disorders in Saudi Arabia: A Review. Cureus 2024, 16, e53888. [Google Scholar] [CrossRef] [PubMed]
- Albanghali, M.A. Prevalence of Consanguineous Marriage among Saudi Citizens of Albaha, a Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 3767. [Google Scholar] [CrossRef]
- Bissar-Tadmouri, N.; Al Homssi, M.; Nair, P. Hereditary Diseases of the Nervous System in Arabs. In Genomics and Health in the Developing World; Kumar, D., Ed.; Oxford Academic: Oxford, UK, 2012; pp. 450–467. [Google Scholar]
- Monies, D.; Abouelhoda, M.; AlSayed, M.; Alhassnan, Z.; Alotaibi, M.; Kayyali, H.; Al-Owain, M.; Shah, A.; Rahbeeni, Z.; Al-Muhaizea, M.A.; et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum. Genet. 2017, 136, 921–939. [Google Scholar] [CrossRef] [PubMed]
- Balobaid, A.; Qari, A.; Al-Zaidan, H. Genetic counselors’ scope of practice and challenges in genetic counseling services in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Bamaga, A.K.; Muthaffar, O.Y.; Alyazidi, A.S.; Abu Alqam, R. Giant Axonal Neuropathy: A Case Report of Subclinical Childhood Manifestations. Cureus 2024, 16, e54368. [Google Scholar] [CrossRef]
- Avan, A.; Hachinski, V. Brain health: Key to health, productivity, and well-being. Alzheimers Demen. 2022, 18, 1396–1407. [Google Scholar] [CrossRef]
- Sivera Mascaró, R.; García Sobrino, T.; Horga Hernández, A.; Pelayo Negro, A.L.; Alonso Jiménez, A.; Antelo Pose, A.; Calabria Gallego, M.D.; Casasnovas, C.; Cemillán Fernández, C.A.; Esteban Pérez, J.; et al. Clinical practice guidelines for the diagnosis and management of Charcot-Marie-Tooth disease. In Neurologia; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Courtney. Charcot-Marie-Tooth Disease—JOIN GRIN Patient Registry [Internet]. Charcot-Marie-Tooth Disease. Available online: https://www.hnf-cure.org/cmt/cmt-research/grin-patient-registry/ (accessed on 12 January 2025).
- Laurie, S.; Piscia, D.; Matalonga, L.; Corvó, A.; Fernández-Callejo, M.; Garcia-Linares, C.; Hernandez-Ferrer, C.; Luengo, C.; Martínez, I.; Papakonstantinou, A.; et al. The RD-Connect Genome-Phenome Analysis Platform: Accelerating diagnosis, research, and gene discovery for rare diseases. Hum. Mutat. 2022, 43, 717–733. [Google Scholar] [CrossRef]
- Salih, M.A.; Maisonobe, T.; Kabiraj, M.; al Rayess, M.; al-Turaiki, M.H.; Akbar, M.; Tahan, A.; Urtizberea, J.A.; Grid, D.; Hamadouche, T.; et al. Autosomal recessive hereditary neuropathy with focally folded myelin sheaths and linked to chromosome 11q23: A distinct and homogeneous entity. Neuromuscul. Disord. 2000, 10, 10–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).