Cortical Representation of Food-Related Odors in Gustatory Areas Differs According to Their Taste Association: An fMRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Olfactory and Gustatory Screening
2.3. Odors Evaluation in the MRI Scanner
2.4. fMRI Data Acquisition and Preprocessing
2.5. Statistical Analysis
3. Results
3.1. Psychophysical Responses
3.2. Brain Responses to Food Odors
3.2.1. Whole Brain
3.2.2. ROI Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.J.; Mielby, L.A.; Junge, J.Y.; Bertelsen, A.S.; Kidmose, U.; Spence, C.; Byrne, D.V. The Role of Intrinsic and Extrinsic Sensory Factors in Sweetness Perception of Food and Beverages: A Review. Foods 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.S.; Jansen, A.; Roefs, A.; Nederkoorn, C. Eating behavior in response to food-cue exposure: Examining the cue-reactivity and counteractive-control models. Psychol. Addict. Behav. 2009, 23, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Physiologic responses to sensory stimulation by food: Nutritional implications. J. Am. Diet. Assoc. 1997, 97, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Boesveldt, S.; Gort, G.; Lakemond, C.M.M.; van Boekel, M.A.J.S.; Luning, P.A. Sensory-Specific Appetite Is Affected by Actively Smelled Food Odors and Remains Stable Over Time in Normal-Weight Women. J. Nutr. 2014, 144, 1314–1319. [Google Scholar] [CrossRef]
- Zoon, H.F.; de Graaf, C.; Boesveldt, S. Food Odours Direct Specific Appetite. Foods 2016, 5, 12. [Google Scholar] [CrossRef]
- Proserpio, C.; de Graaf, C.; Laureati, M.; Pagliarini, E.; Boesveldt, S. Impact of ambient odors on food intake, saliva production and appetite ratings. Physiol. Behav. 2017, 174, 35–41. [Google Scholar] [CrossRef]
- Boesveldt, S.; Lundstrom, J.N. Detecting fat content of food from a distance: Olfactory-based fat discrimination in humans. PLoS ONE 2014, 9, e85977. [Google Scholar] [CrossRef]
- Small, D.M. Flavor and the Formation of Category-Specific Processing in Olfaction. Chemosens. Percept. 2008, 1, 136–146. [Google Scholar] [CrossRef]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, S.; Zhu, J.; Niu, Y.; Xiong, W.; Chen, F. Identification of key aromas of grapefruit juice and study of their contributions to the enhancement of sweetness perception. Eur. Food Res. Technol. 2023, 249, 537–551. [Google Scholar] [CrossRef]
- Boesveldt, S.; de Graaf, K. The Differential Role of Smell and Taste For Eating Behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sarafoleanu, C.; Mella, C.; Georgescu, M.; Perederco, C. The importance of the olfactory sense in the human behavior and evolution. J. Med. Life 2009, 2, 196–198. [Google Scholar] [PubMed]
- Kadohisa, M.; Rolls, E.T.; Verhagen, J.V. Neuronal representations of stimuli in the mouth: The primate insular taste cortex, orbitofrontal cortex and amygdala. Chem. Senses 2005, 30, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Grabenhorst, F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog. Neurobiol. 2008, 86, 216–244. [Google Scholar] [CrossRef]
- Rolls, E.T. The rules of formation of the olfactory representations found in the orbitofrontal cortex olfactory areas in primates. Chem. Senses 2001, 26, 595–604. [Google Scholar] [CrossRef]
- De Araujo, I.E.; Rolls, E.T.; Kringelbach, M.L.; McGlone, F.; Phillips, N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003, 18, 2059–2068. [Google Scholar] [CrossRef]
- Rolls, E.T.; Kringelbach, M.L.; de Araujo, I.E. Different representations of pleasant and unpleasant odours in the human brain. Eur. J. Neurosci. 2003, 18, 695–703. [Google Scholar] [CrossRef]
- Koeppel, C.J.; Ruser, P.; Kitzler, H.; Hummel, T.; Croy, I. Interoceptive accuracy and its impact on neuronal responses to olfactory stimulation in the insular cortex. Hum. Brain Mapp. 2020, 41, 2898–2908. [Google Scholar] [CrossRef]
- Bensafi, M.; Croy, I.; Phillips, N.; Rouby, C.; Sezille, C.; Gerber, J.; Small, D.M.; Hummel, T. The effect of verbal context on olfactory neural responses. Hum. Brain Mapp. 2014, 35, 810–818. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Nachtigal, D.; Teulings, L.; Gitelman, D.R.; Small, D.M. The insular taste cortex contributes to odor quality coding. Front. Hum. Neurosci. 2010, 4, 58. [Google Scholar] [CrossRef]
- Bragulat, V.; Dzemidzic, M.; Bruno, C.; Cox, C.A.; Talavage, T.; Considine, R.V.; Kareken, D.A. Food-related odor probes of brain reward circuits during hunger: A pilot FMRI study. Obesity 2010, 18, 1566–1571. [Google Scholar] [CrossRef]
- Frasnelli, J.; Hummel, C.; Bojanowski, V.; Warr, J.; Gerber, J.; Hummel, T. Food-Related Odors and the Reward Circuit: Functional MRI. Chemosens. Percept. 2015, 8, 192–200. [Google Scholar] [CrossRef]
- Han, P.; Mohebbi, M.; Seo, H.S.; Hummel, T. Sensitivity to sweetness correlates to elevated reward brain responses to sweet and high-fat food odors in young healthy volunteers. NeuroImage 2020, 208, 116413. [Google Scholar] [CrossRef]
- Han, P.; Roitzsch, C.; Horstmann, A.; Possel, M.; Hummel, T. Increased Brain Reward Responsivity to Food-Related Odors in Obesity. Obesity 2021, 29, 1138–1145. [Google Scholar] [CrossRef]
- Sorokowska, A.; Schoen, K.; Hummel, C.; Han, P.; Warr, J.; Hummel, T. Food-Related Odors Activate Dopaminergic Brain Areas. Front. Hum. Neurosci. 2017, 11, 625. [Google Scholar] [CrossRef]
- Cai, J.; Tong, Q. Anatomy and Function of Ventral Tegmental Area Glutamate Neurons. Front. Neural Circuits 2022, 16, 867053. [Google Scholar] [CrossRef]
- Darvas, M.; Fadok, J.P.; Palmiter, R.D. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn. Mem. 2011, 18, 136–143. [Google Scholar] [CrossRef]
- Kobal, G.; Hummel, T.; Sekinger, B.; Barz, S.; Roscher, S.; Wolf, S. “Sniffin’ sticks”: Screening of olfactory performance. Rhinology 1996, 34, 222–226. [Google Scholar]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hahner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef]
- Landis, B.N.; Welge-Luessen, A.; Bramerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef]
- Sommer, J.U.; Maboshe, W.; Griebe, M.; Heiser, C.; Hormann, K.; Stuck, B.A.; Hummel, T. A mobile olfactometer for fMRI-studies. J. Neurosci. Methods 2012, 209, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Hornstein, H.; Thaploo, D.; Faria, V.; Warr, J.; Hummel, T. Neural Processing of Odors with Different Well-Being Associations-Findings from Two Consecutive Neuroimaging Studies. Brain Sci. 2023, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Gu, H.; Salmeron, B.J.; Ross, T.J.; Geng, X.J.; Zhan, W.; Stein, E.A.; Yang, Y.H. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 2010, 53, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Ma, S.S.; Yip, S.W.; Wang, L.J.; Chen, C.; Yan, C.G.; Liu, L.; Liu, B.; Deng, L.Y.; Liu, Q.X.; et al. Decreased functional connectivity between ventral tegmental area and nucleus accumbens in Internet gaming disorder: Evidence from resting state functional magnetic resonance imaging. Behav. Brain Funct. 2015, 11, 37. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Prescott, J.; Boakes, R.A. The Acquisition of Taste Properties by Odors. Learn. Motiv. 1995, 26, 433–455. [Google Scholar] [CrossRef]
- Prescott, J.; Johnstone, V.; Francis, J. Odor-taste interactions: Effects of attentional strategies during exposure. Chem. Senses 2004, 29, 331–340. [Google Scholar] [CrossRef]
- Guichard, E.; Barba, C.; Thomas-Danguin, T.; Tromelin, A. Multivariate Statistical Analysis and Odor-Taste Network To Reveal Odor-Taste Associations. J. Agric. Food Chem. 2020, 68, 10318–10328. [Google Scholar] [CrossRef]
- Poessel, M.; Morys, F.; Breuer, N.; Villringer, A.; Hummel, T.; Horstmann, A. Brain response to food odors is not associated with body mass index and obesity-related metabolic health measures. Appetite 2022, 168, 105774. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Boakes, R.A.; Prescott, J. Changes in odor sweetness resulting from implicit learning of a simultaneous odor-sweetness association: An example of learned synesthesia. Learn. Motiv. 1998, 29, 113–132. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Mobini, S.; Elliman, T.D.; Walker, H.C.; Stevenson, R.J. Hedonic and sensory characteristics of odors conditioned by pairing with tastants in humans. J. Exp. Psychol. Anim. Behav. Process. 2006, 32, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Bender, G.; Veldhuizen, M.G.; Rudenga, K.; Nachtigal, D.; Felsted, J. The role of the human orbitofrontal cortex in taste and flavor processing. Ann. Acad. Sci. 2007, 1121, 136–151. [Google Scholar] [CrossRef]
- Scott, T.R.; Plata-Salaman, C.R. Taste in the monkey cortex. Physiol. Behav. 1999, 67, 489–511. [Google Scholar] [CrossRef]
- Rolls, E.T. Cerebral Cortex: Principles of Operation; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Sescousse, G.; Caldu, X.; Segura, B.; Dreher, J.C. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.P.; Hudry, J.; Zald, D.H.; Godinot, D.; Gregoire, M.C.; Lavenne, F.; Costes, N.; Holley, A. Functional neuroanatomy of different olfactory judgments. NeuroImage 2001, 13, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Shinohara, H.; Hori, O.; Ozaki, N.; Ueda, F.; Nakada, M.; Hamada, J.; Hayashi, Y. Association fibers connecting the Broca center and the lateral superior frontal gyrus: A microsurgical and tractographic anatomy. J. Neurosurg. 2012, 116, 323–330. [Google Scholar] [CrossRef]
- Li, W.; Qin, W.; Liu, H.G.; Fan, L.Z.; Wang, J.J.; Jiang, T.Z.; Yu, C.S. Subregions of the human superior frontal gyrus and their connections. NeuroImage 2013, 78, 46–58. [Google Scholar] [CrossRef]
- Pellegrino, R.; Han, P.; Reither, N.; Hummel, T. Effectiveness of olfactory training on different severities of posttraumatic loss of smell. Laryngoscope 2019, 129, 1737–1743. [Google Scholar] [CrossRef]
- Kjelvik, G.; Evensmoen, H.R.; Brezova, V.; Haberg, A.K. The human brain representation of odor identification. J. Neurophysiol. 2012, 108, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Silkis, I. The role of the hippocampus in the perception and recall of odors. A hypothetical neural mechanism. Neurosci. Behav. Physiol. 2023, 53, 1255–1268. [Google Scholar] [CrossRef]
- Shrager, Y.; Kirwan, C.B.; Squire, L.R. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron 2008, 59, 547–553. [Google Scholar] [CrossRef]
- Reske, M.; Kellermann, T.; Shah, N.J.; Schneider, F.; Habel, U. Impact of valence and age on olfactory induced brain activation in healthy women. Behav. Neurosci. 2010, 124, 414–422. [Google Scholar] [CrossRef]
- Rosenstein, D.; Oster, H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988, 59, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Hopfer, H.; Moulinier, V.; Prescott, J.; Monteleone, E.; Hayes, J.E. Distinct Sensory Hedonic Functions for Sourness in Adults. Food Qual. Prefer. 2024, 116, 105152. [Google Scholar] [CrossRef]
- Robin, O.; Rousmans, S.; Dittmar, A.; Vernet-Maury, E. Gender influence on emotional responses to primary tastes. Physiol. Behav. 2003, 78, 385–393. [Google Scholar] [CrossRef]
- Kim, B.; Im, H.I. The role of the dorsal striatum in choice impulsivity. Ann. Acad. Sci. 2019, 1451, 92–111. [Google Scholar] [CrossRef]
- Volman, S.F.; Lammel, S.; Margolis, E.B.; Kim, Y.; Richard, J.M.; Roitman, M.F.; Lobo, M.K. New Insights into the Specificity and Plasticity of Reward and Aversion Encoding in the Mesolimbic System. J. Neurosci. 2013, 33, 17569–17576. [Google Scholar] [CrossRef]
- Gottfried, J.A.; Deichmann, R.; Winston, J.S.; Dolan, R.J. Functional heterogeneity in human olfactory cortex: An event-related functional magnetic resonance imaging study. J. Neurosci. 2002, 22, 10819–10828. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine reward prediction error coding. Dialogues Clin. Neurosci. 2016, 18, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Hinton, E.C.; Parkinson, J.A.; Lawrence, A.D. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage 2012, 63, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Ster. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Nie, H.; Hu, X.; Gao, Y.; Ma, Y.; Han, P. Altered neural representation of olfactory food reward in the nucleus accumbens after acute stress. J. Affect. Disord. 2024, 354, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Robbins, T.W. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Cheregi, M.C.; Ciucu, A.A. Rapid voltammetric method for quinine determination in soft drinks. Food Chem. 2018, 253, 1–4. [Google Scholar] [CrossRef]
- Jin, J.; Zelano, C.; Gottfried, J.A.; Mohanty, A. Human Amygdala Represents the Complete Spectrum of Subjective Valence. J. Neurosci. 2015, 35, 15145–15156. [Google Scholar] [CrossRef]
- Luo, L.G.; Han, P.F. Assessing food-evoked emotions using functional magnetic resonance imaging: A systematic review. Food Qual. Prefer. 2023, 108, 104877. [Google Scholar] [CrossRef]
- Chao, A.M.; Loughead, J.; Bakizada, Z.M.; Hopkins, C.M.; Geliebter, A.; Gur, R.C.; Wadden, T.A. Sex/gender differences in neural correlates of food stimuli: A systematic review of functional neuroimaging studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 687–699. [Google Scholar] [CrossRef]
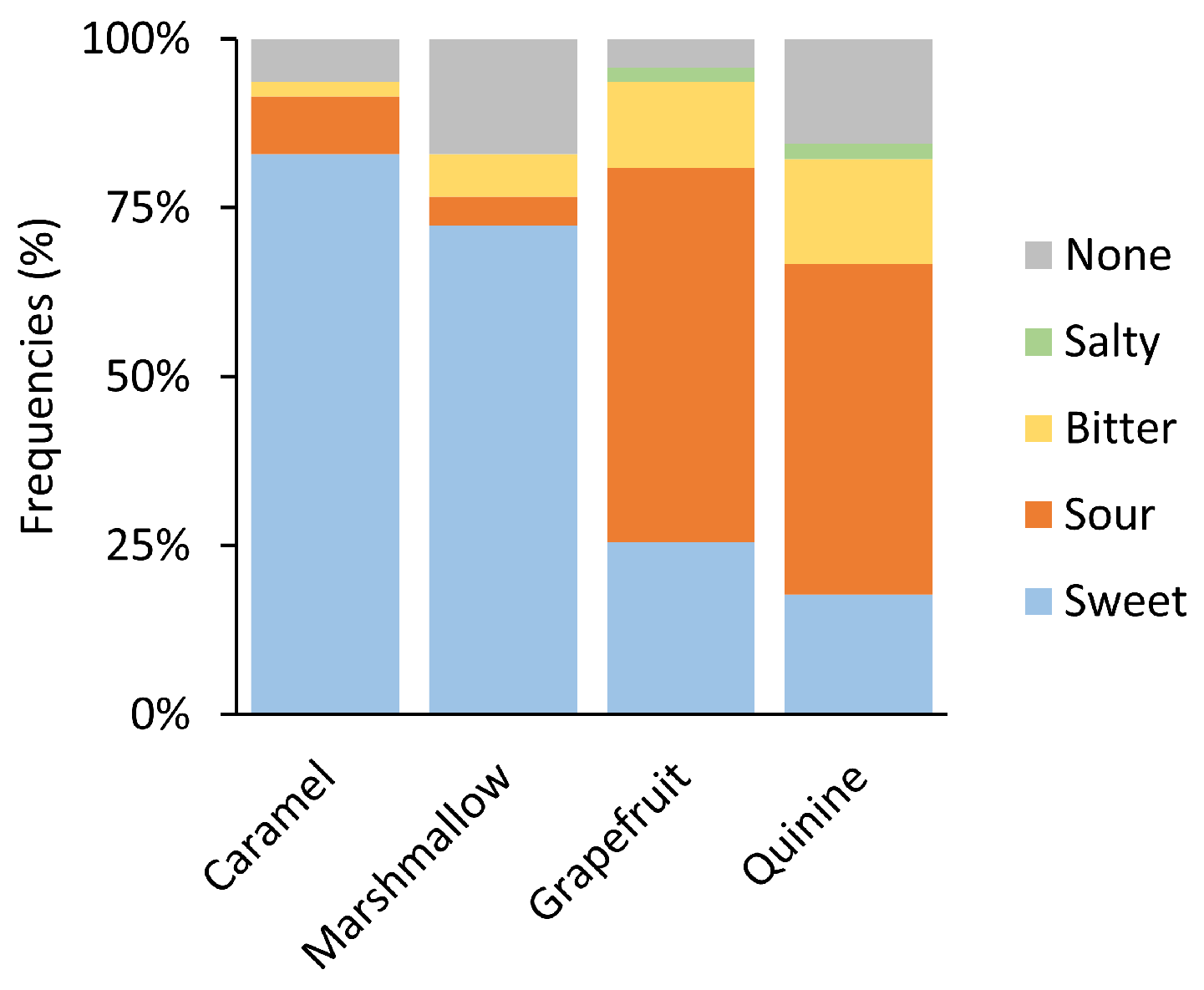


| Name | Dilution | Reference Code | |
|---|---|---|---|
| Sweet odors | Caramel | pure | ABX231360W |
| Marshmallow | 50% | IMP345590A/IMP | |
| Sour odors | Grapefruit | 25% | IMP344986A |
| Quinine | 10% | IMP181766DT |
| SOUR > SWEET | |||||
|---|---|---|---|---|---|
| Coordinates | |||||
| x | y | z | k | T | Region |
| 2 | 59 | 24 | 7535 | 4.75 | FP, PCG |
| −33 | −57 | 25 | 2759 | 4.54 | L, AG |
| 44 | −65 | 30 | 2123 | 4.12 | R, AG |
| 14 | 17 | −4 | 1013 | 4.29 | R, CAU, PUT, NAc, OFC |
| −31 | 18 | −23 | 65 | 3.58 | L, OFC |
| −32 | 9 | −15 | 32 | 3.32 | L, INS |
| All Odors | Sweet | Sour | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Coordinates | Coordinates | ||||||||||||||
| L/R | x | y | z | k | T | x | y | z | k | T | x | y | z | k | T | |
| Primary gustatory cortex | ||||||||||||||||
| FO | L | −43 | 22 | 2 | 10,350 | 7.48 | −44 | 23 | 0 | 4978 | 5.26 | −43 | 23 | 0 | 7461 | 6.25 |
| R | 43 | 25 | 1 | 6493 | 6.36 | 44 | 24 | −8 | 405 | 4.25 | 41 | 26 | 0 | 3139 | 4.96 | |
| INS | L | −37 | 14 | −6 | 1316 | 6.77 | −36 | 18 | −4 | 328 | 4.69 | −36 | 16 | −7 | 599 | 5.21 |
| R | 38 | 12 | −9 | 699 | 5.23 | 36 | 21 | −5 | 38 | 4.03 | 40 | 4 | −11 | 112 | 4.09 | |
| Primary olfactory cortex | ||||||||||||||||
| EnC | L | −25 | 0 | −19 | 3 | 3.94 | - | - | ||||||||
| R | 25 | 0 | −19 | 4 | 4.06 | - | - | |||||||||
| AMG | L | −26 | −3 | −19 | 445 | 5.39 | - | −26 | −8 | −17 | 73 | 4.06 | ||||
| R | 25 | −2 | −21 | 736 | 5.88 | - | 25 | 0 | −22 | 325 | 4.67 | |||||
| Secondary olfactory cortex | ||||||||||||||||
| OFC | L | −25 | 30 | −13 | 1510 | 6.92 | −27 | 28 | −13 | 446 | 4.93 | −25 | 20 | −13 | 1339 | 5.31 |
| R | 26 | 35 | −14 | 1379 | 5.30 | - | 26 | 34 | −15 | 725 | 4.49 | |||||
| THA | L | −7 | −9 | 9 | 979 | 6.38 | - | −7 | −9 | 8 | 950 | 5.56 | ||||
| R | 6 | −6 | 7 | 501 | 6.30 | - | 6 | −7 | 7 | 589 | 5.68 | |||||
| HIP | L | −30 | −20 | −11 | 2129 | 5.39 | - | −30 | −26 | −8 | 1633 | 5.16 | ||||
| R | 27 | −19 | −9 | 1772 | 5.88 | - | 23 | −28 | −2 | 919 | 5.04 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastinu, M.; Thaploo, D.; Warr, J.; Hummel, T. Cortical Representation of Food-Related Odors in Gustatory Areas Differs According to Their Taste Association: An fMRI Study. Brain Sci. 2025, 15, 418. https://doi.org/10.3390/brainsci15040418
Mastinu M, Thaploo D, Warr J, Hummel T. Cortical Representation of Food-Related Odors in Gustatory Areas Differs According to Their Taste Association: An fMRI Study. Brain Sciences. 2025; 15(4):418. https://doi.org/10.3390/brainsci15040418
Chicago/Turabian StyleMastinu, Mariano, Divesh Thaploo, Jonathan Warr, and Thomas Hummel. 2025. "Cortical Representation of Food-Related Odors in Gustatory Areas Differs According to Their Taste Association: An fMRI Study" Brain Sciences 15, no. 4: 418. https://doi.org/10.3390/brainsci15040418
APA StyleMastinu, M., Thaploo, D., Warr, J., & Hummel, T. (2025). Cortical Representation of Food-Related Odors in Gustatory Areas Differs According to Their Taste Association: An fMRI Study. Brain Sciences, 15(4), 418. https://doi.org/10.3390/brainsci15040418






