A Study of the Central Motor Drives Interactions Between the Eyes, and an Index Finger, and a Little Finger
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
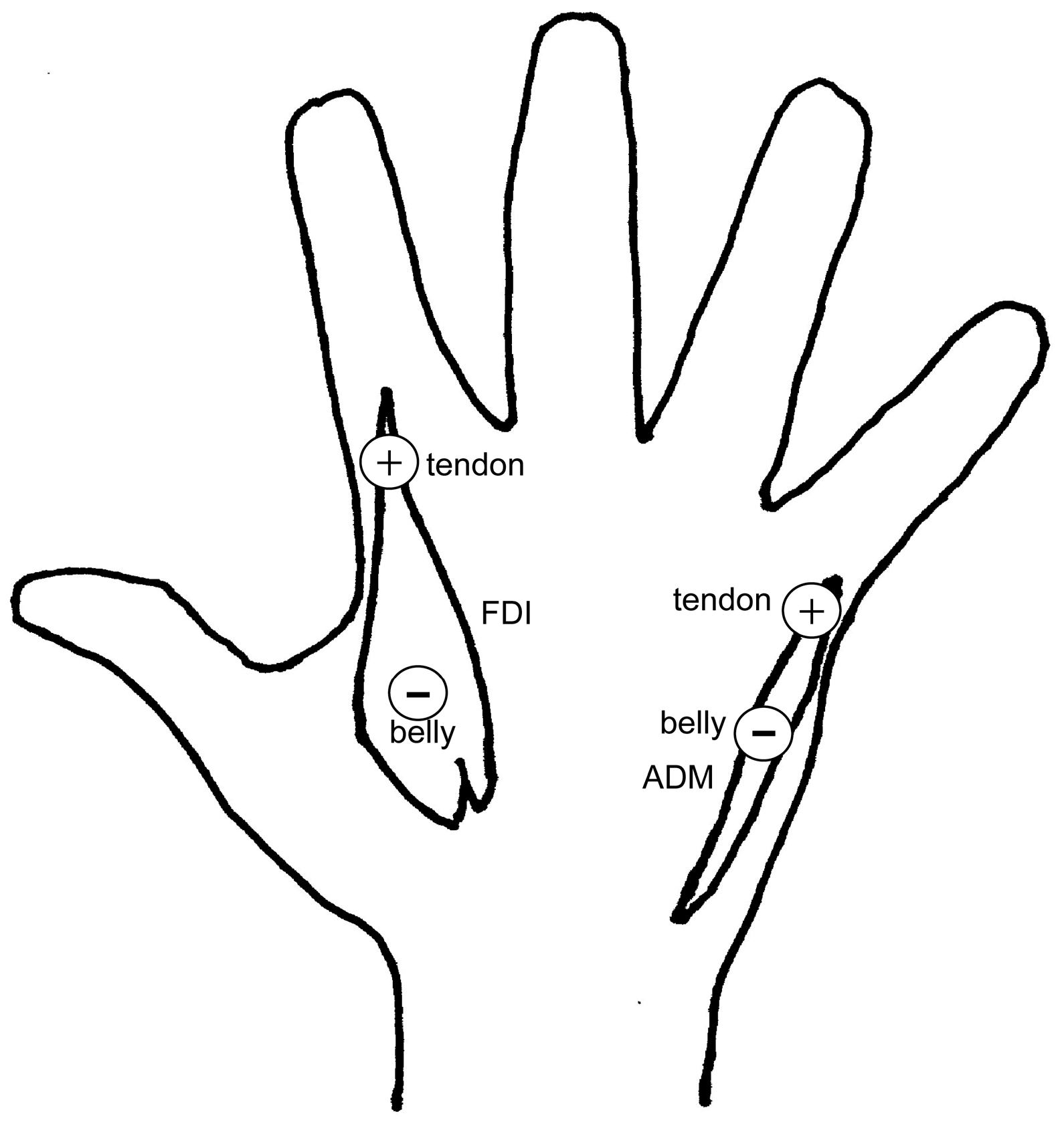
2.2. Apparatus
2.3. Procedure
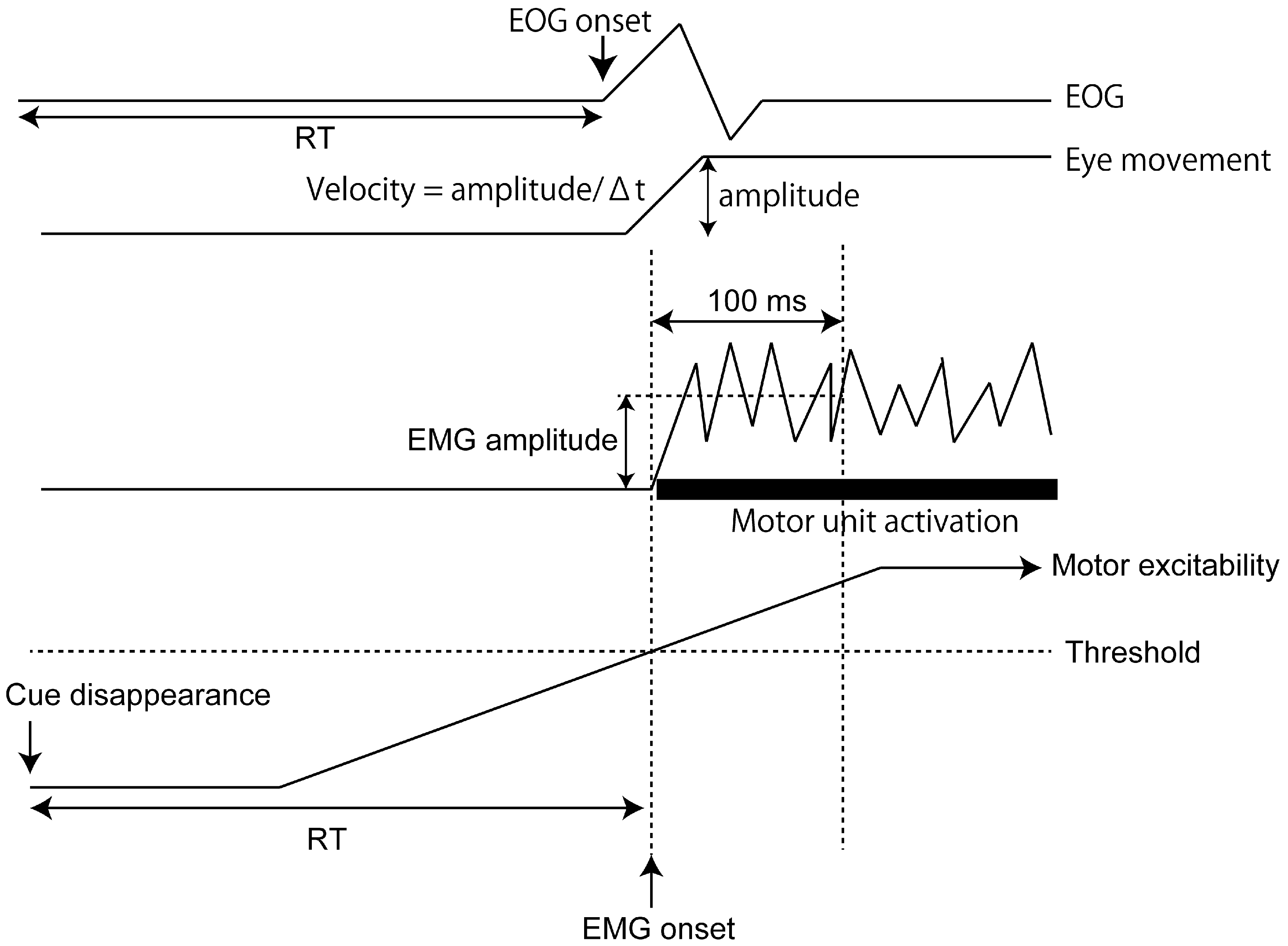
2.4. Data Analysis
3. Results
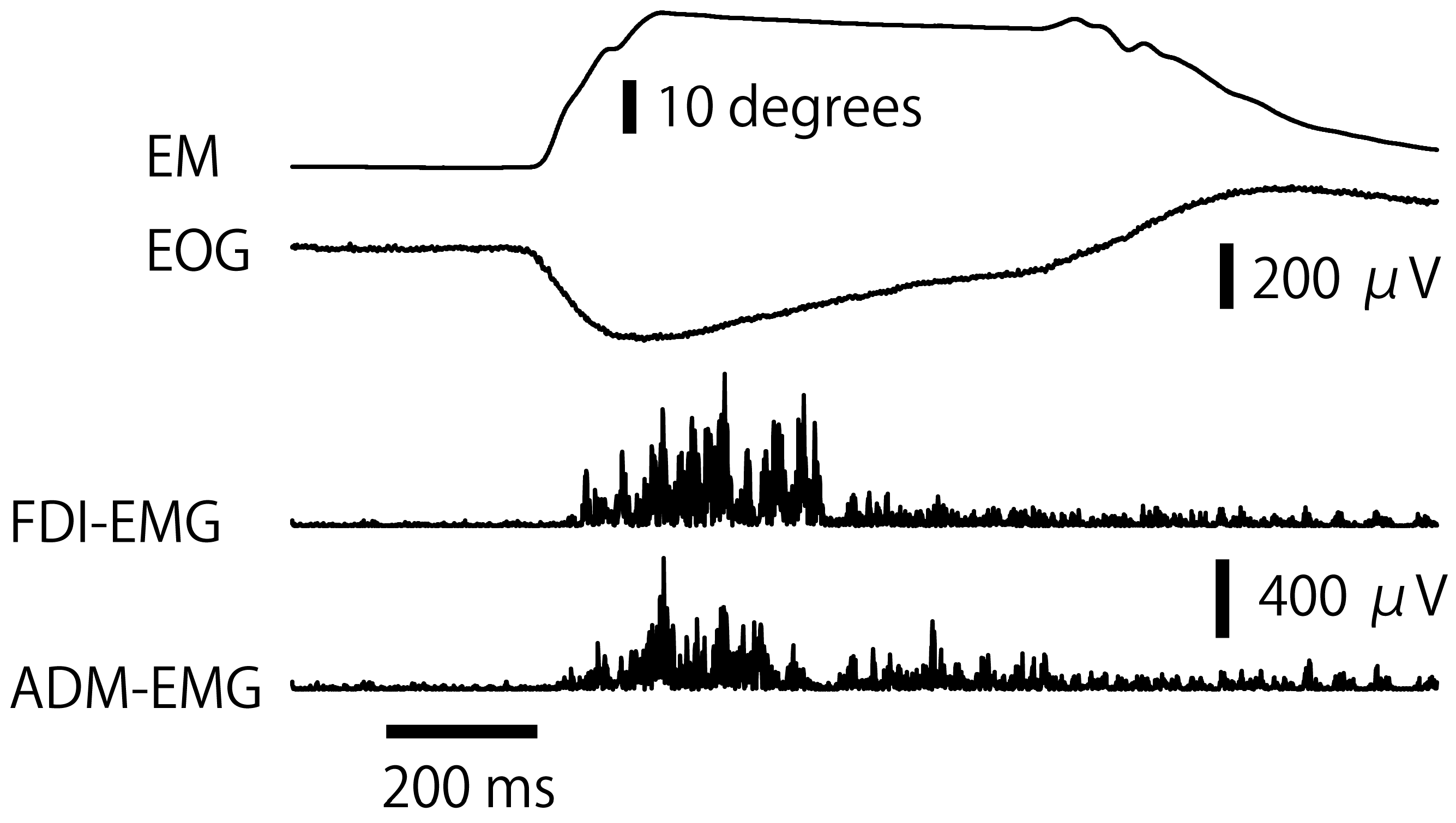
3.1. Eye Movement
3.2. RT of EMG Response
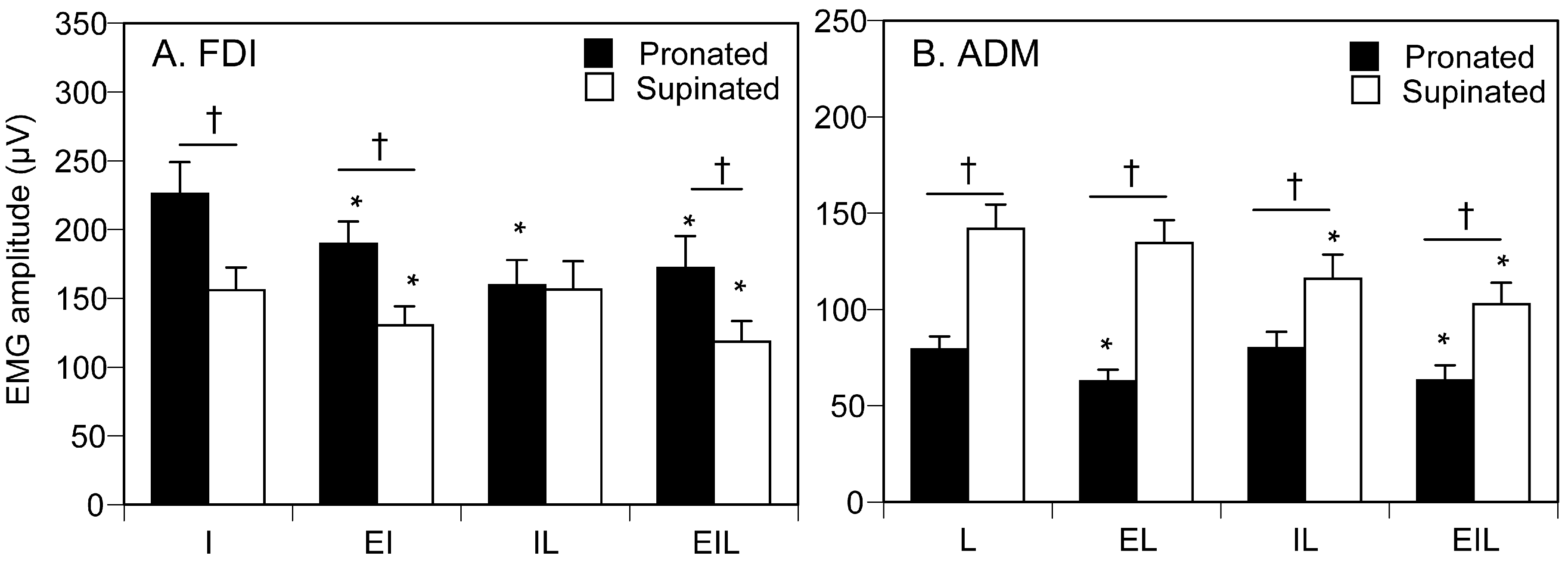
3.3. EMG Amplitude
4. Discussion
4.1. Proprioceptive Influence
4.2. Central Motor Drive to Finger
4.3. Motor Excitability
4.4. Central Motor Drive to Eyes
4.5. Influence of Finger on Eyes
4.6. Between Fingers Interaction
4.7. Limitations
4.8. Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engel, K.C.; Anderson, J.H.; Soechting, J.F. Similarity in the response of smooth pursuit and manual tracking to a change in the direction of target motion. J. Neurophysiol. 2000, 84, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.C.; Soechting, J.F. Interactions between ocular motor and manual responses during two-dimensional tracking. Prog. Brain Res. 2003, 142, 141–153. [Google Scholar] [PubMed]
- Snyder, L.H.; Batista, A.P.; Andersen, R.A. Saccade-related activity in the parietal reach region. J. Neurophysiol. 2000, 83, 1099–1102. [Google Scholar] [CrossRef]
- Sailer, U.; Eggert, T.; Ditterich, J.; Straube, A. Spatial and temporal aspects of eye-hand coordination across different tasks. Exp. Brain Res. 2000, 134, 163–173. [Google Scholar] [CrossRef]
- Horino, H.; Mori, N.; Matsugi, A.; Kamata, N.; Hiraoka, K. The effect of eye movement on the control of arm movement to a target. Somatosens. Mot. Res. 2013, 30, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Abrams, R.A.; Meyer, D.E.; Kornblum, S. Eye-hand coordination: Oculomotor control in rapid aimed limb movements. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16, 248. [Google Scholar] [CrossRef]
- Miall, R.C.; Reckess, G.Z. The cerebellum and the timing of coordinated eye and hand tracking. Brain Cogn. 2002, 48, 212–226. [Google Scholar] [CrossRef]
- Gueugneau, N.; Crognier, L.; Papaxanthis, C. The influence of eye movements on the temporal features of executed and imagined arm movements. Brain Res. 2008, 1187, 95–102. [Google Scholar] [CrossRef]
- Maioli, C.; Falciati, L.; Gianesini, T. Pursuit eye movements involve a covert motor plan for manual tracking. J. Neurosci. 2007, 27, 7168–7173. [Google Scholar] [CrossRef]
- Hiraoka, K.; Ae, M.; Ogura, N.; Komuratani, S.; Sano, C.; Shiomi, K.; Morita, Y.; Yokoyama, H. Smooth pursuit eye movement preferentially facilitates motor-evoked potential elicited by anterior–posterior current in the brain. Neuroreport 2014, 25, 279–283. [Google Scholar] [CrossRef]
- Chujo, Y.; Jono, Y.; Tani, K.; Nomura, Y.; Hiraoka, K. Corticospinal excitability in the hand muscles is decreased during eye movement with visual occlusion. Percept. Mot. Skills 2016, 122, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Neggers, S.F.; Bekkering, H. Ocular gaze is anchored to the target of an ongoing pointing movement. J. Neurophysiol. 2000, 83, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Jono, Y.; Iwata, Y.; Kinoshita, A.; Hiraoka, K. Common Motor Drive Triggers Response of Prime Movers When Two Fingers Simultaneously Respond to a Cue. Brain Sci. 2021, 11, 700. [Google Scholar] [CrossRef]
- Jono, Y.; Chujo, Y.; Nomura, Y.; Tani, K.; Nikaido, Y.; Hatanaka, R.; Hiraoka, K. The effect of tonic contraction of the finger muscle on the motor cortical representation of the contracting adjacent muscle. Somatosens. Mot. Res. 2015, 32, 114–121. [Google Scholar] [CrossRef]
- Jono, Y.; Iwata, Y.; Mizusawa, H.; Hiraoka, K. Change in excitability of corticospinal pathway and GABA-mediated inhibitory circuits of primary motor cortex induced by contraction of adjacent hand muscle. Brain Topogr. 2016, 29, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Latash, M.L.; Zatsiorsky, V.M. Finger interaction during multi-finger tasks involving finger addition and removal. Exp. Brain Res. 2003, 150, 230–236. [Google Scholar] [CrossRef]
- Beck, S.; Richardson, S.P.; Shamim, E.A.; Dang, N.; Schubert, M.; Hallett, M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J. Neurosci. 2008, 28, 10363–10369. [Google Scholar] [CrossRef]
- Tinazzi, M.; Farina, S.; Tamburin, S.; Facchini, S.; Fiaschi, A.; Restivo, D.; Berardelli, A. Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp. Brain Res. 2003, 150, 222–229. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Hallett, M. Disturbed surround inhibition in focal hand dystonia. Ann. Neurol. 2004, 56, 595–599. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Hallett, M. Surround inhibition in human motor system. Exp. Brain Res. 2004, 158, 397–404. [Google Scholar] [CrossRef]
- Beck, S.; Hallett, M. Surround inhibition in the motor system. Exp. Brain Res. 2011, 210, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zatsiorsky, V.M.; Li, Z.M.; Latash, M.L. Coordinated force production in multi-finger tasks: Finger interaction and neural network modeling. Biol. Cybern. 1998, 79, 139–150. [Google Scholar] [CrossRef]
- Zatsiorsky, V.M.; Li, Z.M.; Latash, M.L. Enslaving effects in multi-finger force production. Exp. Brain Res. 2000, 131, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Brown, R.G.; Marsden, D. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson’s disease. Brain 1992, 115, 539–564. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Matsugi, A.; Kamata, N.; Iwata, A. Premovement facilitation of corticospinal excitability before simple and sequential movement. Percept. Mot. Skills 2010, 111, 129–140. [Google Scholar] [CrossRef]
- Hiraoka, K.; Notani, M.; Iwata, A.; Minamida, F.; Abe, K. Premovement facilitation of corticospinal excitability in patients with Parkinson’s disease. Int. J. Neurosci. 2010, 120, 104–109. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Valls-Solé, J.; Brasil-Neto, J.P.; Cohen, L.G.; Hallett, M. Akinesia in Parkinson’s disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology 1994, 44, 884–891. [Google Scholar] [CrossRef]
- Rossini, P.M.; Zarola, F.; Stalberg, E.; Caramia, M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988, 458, 20–30. [Google Scholar] [CrossRef]
- Starr, A.; Caramia, M.; Zarola, F.; Rossini, P.M. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 26–32. [Google Scholar] [CrossRef]
- Hussain, S.J.; Vollmer, M.K.; Iturrate, I.; Quentin, R. Voluntary motor command release coincides with restricted sensorimotor beta rhythm phases. J. Neurosci. 2022, 42, 5771–5781. [Google Scholar] [CrossRef]
- Farina, D.; Holobar, A.; Merletti, R.; Enoka, R.M. Decoding the neural drive to muscles from the surface electromyogram. Clin. Neurophysiol. 2010, 121, 1616–1623. [Google Scholar] [CrossRef]
- Flanders, M.; Cordo, P. Kinesthetic and visual control of a bimanual task: Specifying the amplitude of movement. J. Neurosci. 1989, 9, 447–453. [Google Scholar] [CrossRef]
- Garcia, M.A.C.; de Carvalho, T.S.; Matsuda, R.H.; Baffa, O.; Imbiriba, L.A.; Souza, V.H. Forearm posture affects the corticospinal excitability of intrinsic and extrinsic hand muscles in dominant and nondominant sides. J. Appl. Biomech. 2024, 40, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hamill, P.V. NCHS Growth Curves for Children: Birth-18 Years, United States (No. 165); US Department of Health, Education, and Welfare, Public Health Service, National Center for Health Statistics: Hyattsville, MD, USA, 1997. [Google Scholar]
- Thomas, J.R.; French, K.E. Gender differences across age in motor performance: A meta-analysis. Psychol. Bull. 1985, 98, 260. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Häger-Ross, C.; Schieber, M.H. Quantifying the independence of human finger movements: Comparisons of digits, hands, and movement frequencies. J. Neurosci. 2000, 20, 8542–8550. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.T.; Hammond, G.R. Independence of force production by digits of the human hand. Neurosci. Lett. 2000, 290, 53–56. [Google Scholar] [CrossRef]
- Slobounov, S.; Johnston, J.; Chiang, H.; Ray, W.J. Motor-related cortical potentials accompanying enslaving effect in single versus combination of fingers force production tasks. Clin. Neurophysiol. 2002, 113, 1444–1453. [Google Scholar] [CrossRef]
- Aoki, T.; Furuya, S.; Kinoshita, H. Finger-tapping ability in male and female pianists and nonmusician controls. Motor Control 2005, 9, 23–39. [Google Scholar] [CrossRef]
- Hodges, P.W.; Bui, B.H. A comparison of computer-based methods for the determination of onset of muscle contraction us-ing electromyography. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 511–519. [Google Scholar]
- Delahunty, E.T.; Bisset, L.M.; Kavanagh, J.J. Intracortical motor networks are affected in both the contralateral and ipsilat-eral hemisphere during single limb cold water immersion. Exp. Physiol. 2019, 104, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.; Barlaam, F.; Vaugoyeau, M.; Assaiante, C. Neurodevelopment of posture-movement coordination from late childhood to adulthood as assessed from bimanual load-lifting task: An event-related potential study. Neuroscience 2021, 457, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; O’Sullivan, E.; Park, M.S. The reaction times and symmetry indices in the bilateral trunk and limb muscles in con-trol subjects and subjects with low back pain that persisted two months or longer. Eur. Spine J. 2021, 30, 2975–2982. [Google Scholar] [CrossRef]
- Van Boxtel, G.J.; Geraats, L.H.; Van den Berg-Lenssen, M.M.; Brunia, C.H. Detection of EMG onset in ERP research. Psychphysiology 1993, 30, 405–412. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Botter, A.; Vieira, T.M.; Peolsson, A.; Petzke, F.; Davey, P.; Falla, D. Spatial variation and inconsistency between estimates of onset of muscle activation from EMG and ultrasound. Sci. Rep. 2017, 7, 42011. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Bisset, L.M.; Tsao, H. Deficits in reaction time due to increased motor time of peroneus longus in people with chronic ankle instability. J. Biomech. 2012, 45, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Crotty, E.D.; Furlong, L.M.; Hayes, K.; Harrison, A.J. Onset detection in surface electromyographic signals across isometric explosive and ramped contractions: A comparison of computer-based methods. Physiol. Meas. 2021, 42, 035010. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kaneko, F.; Ohashi, Y.; Kohno, Y. Neural mechanism of selective finger movement independent of synergistic movement. Exp. Brain Res. 2019, 237, 3485–3492. [Google Scholar] [CrossRef]
- Burden, A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J. Electromyogr. Kinesiol. 2010, 20, 1023–1035. [Google Scholar] [CrossRef]
- Sousa, A.S.; Tavares, J.M.R. Surface electromyographic amplitude normalization methods: A review. In Electromyography: New Developments, Procedures and Applications; Nova Biomedical: Waltham, MA, USA, 2012. [Google Scholar]
- Kim, H.; Tobisawa, S.; Park, H.; Kim, J.; Lee, J.; Shin, D. Aging-induced degradation in tracking performance in three-dimensional movement. SICE J. Control Meas. Syst. Integr. 2024, 17, 239–246. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, S.; Gao, H.; Hamada, N.; Hiraoka, K. A Study of the Central Motor Drives Interactions Between the Eyes, and an Index Finger, and a Little Finger. Brain Sci. 2025, 15, 422. https://doi.org/10.3390/brainsci15040422
Fukuda S, Gao H, Hamada N, Hiraoka K. A Study of the Central Motor Drives Interactions Between the Eyes, and an Index Finger, and a Little Finger. Brain Sciences. 2025; 15(4):422. https://doi.org/10.3390/brainsci15040422
Chicago/Turabian StyleFukuda, Shiho, Han Gao, Naoki Hamada, and Koichi Hiraoka. 2025. "A Study of the Central Motor Drives Interactions Between the Eyes, and an Index Finger, and a Little Finger" Brain Sciences 15, no. 4: 422. https://doi.org/10.3390/brainsci15040422
APA StyleFukuda, S., Gao, H., Hamada, N., & Hiraoka, K. (2025). A Study of the Central Motor Drives Interactions Between the Eyes, and an Index Finger, and a Little Finger. Brain Sciences, 15(4), 422. https://doi.org/10.3390/brainsci15040422




