Surface EEG Evidence for Cerebellar Control of Distal Upper Limbs in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
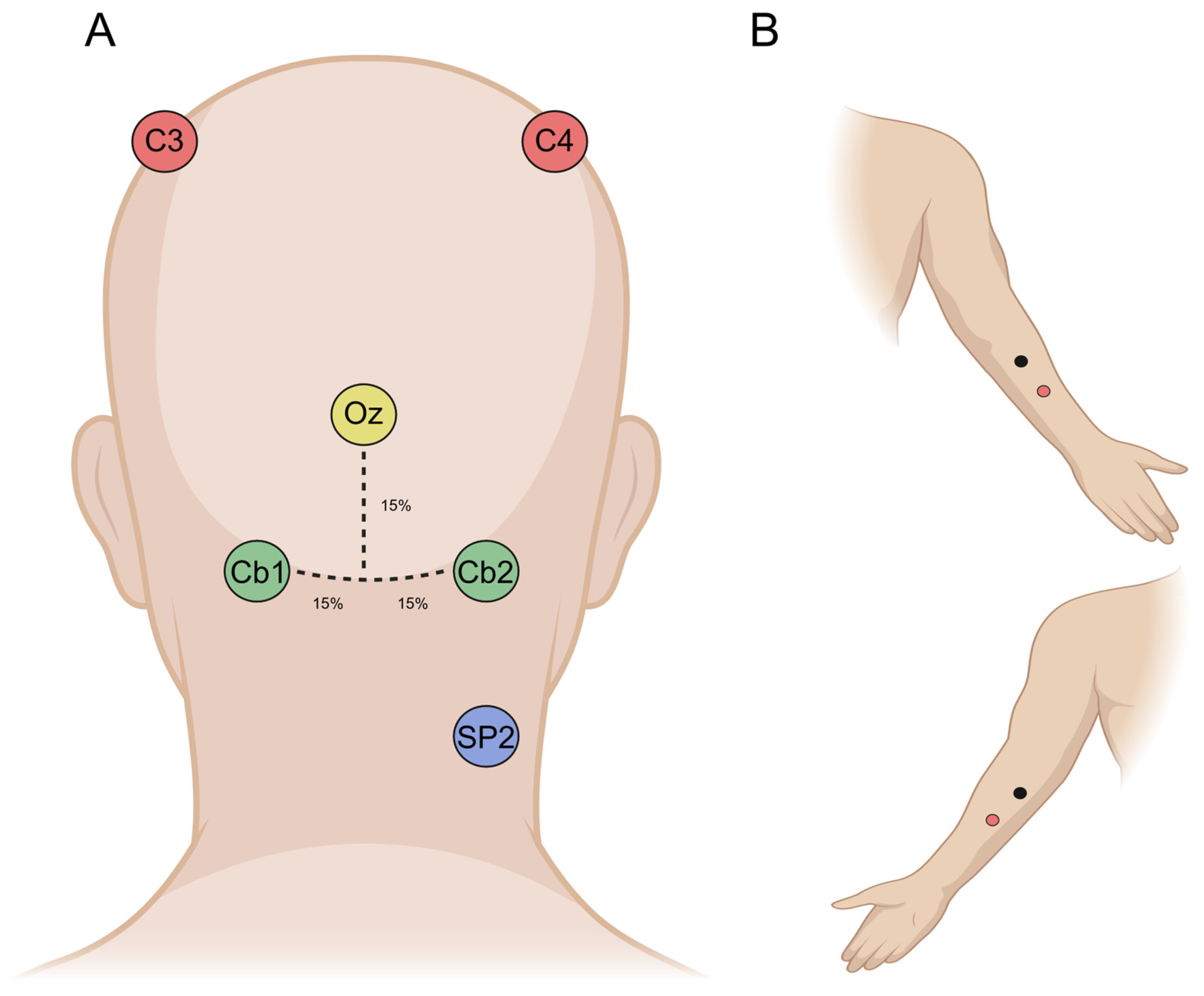
2.2. Experimental Design
2.3. Data Recording, Analysis, and Statistics
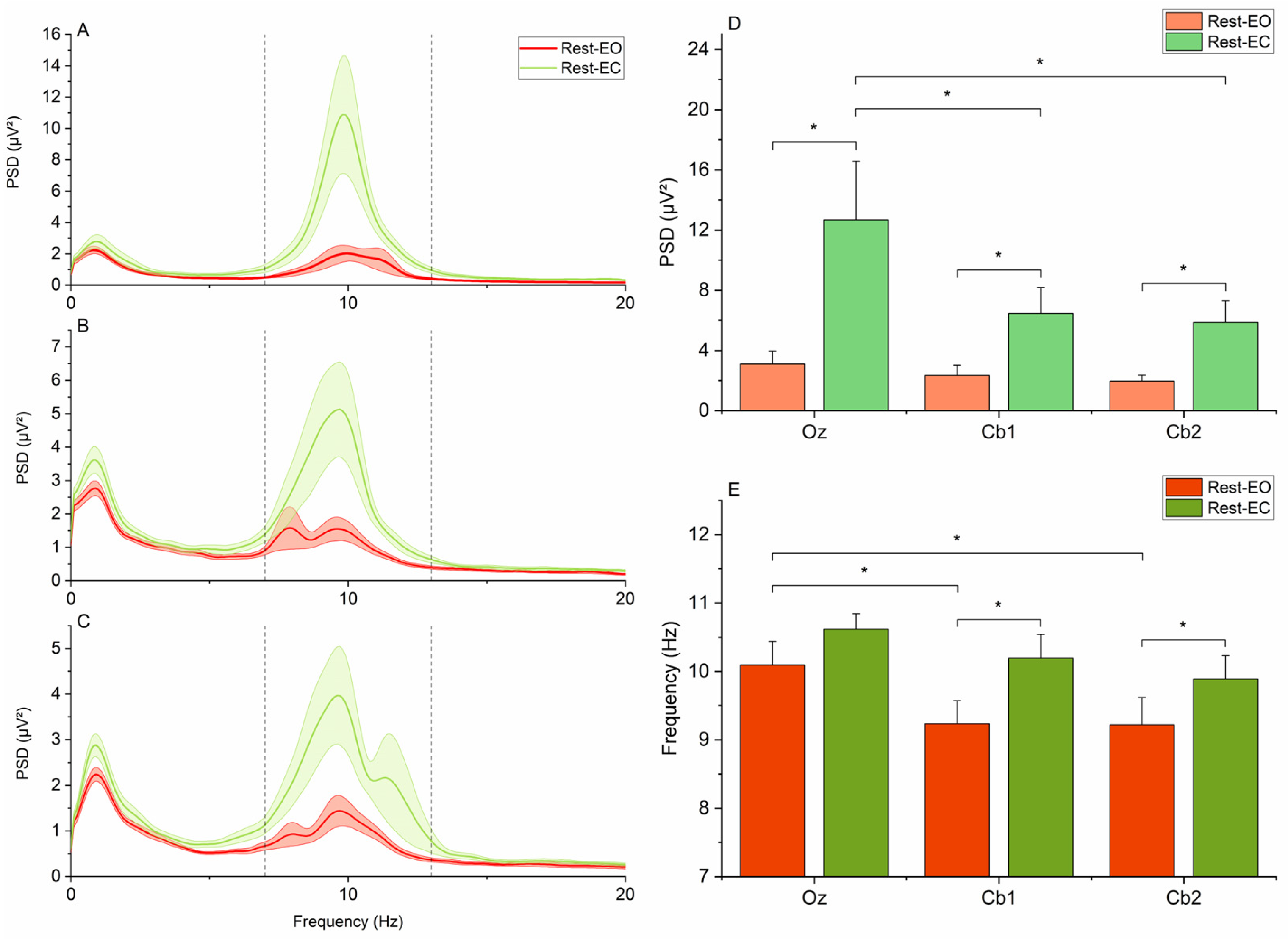
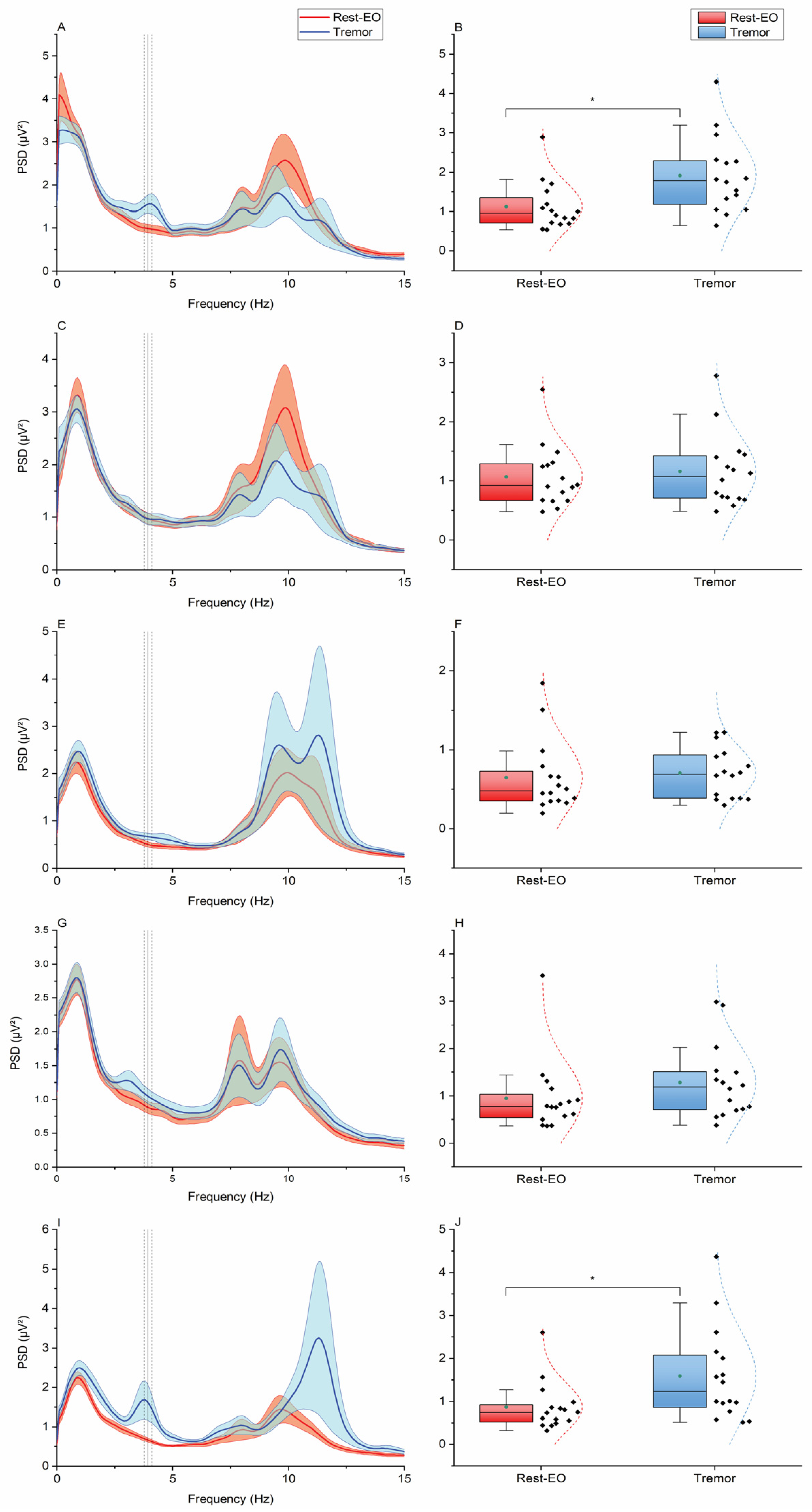
3. Results
4. Discussion
4.1. Contamination of ECeGs by Neck Muscle Activity and Occipital Alpha Rhythm
4.2. Relationship Between the ECeGs and Rhythmic Upper Limb Voluntary Movements
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECeG | Electrocerebellogram |
| EEG | Electroencephalogram |
| EMG | Electromyography |
| PSD | Power spectral density |
| RMS | Root mean square |
References
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. Physiology of the cerebellum. Handb. Clin. Neurol. 2018, 154, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Monaco, J.; Rocchi, L.; Ginatempo, F.; D’Angelo, E.; Rothwell, J.C. Cerebellar Theta-Burst Stimulation Impairs Memory Consolidation in Eyeblink Classical Conditioning. Neural Plast. 2018, 2018, 6856475. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Gerloff, C.; Altenmuller, E.; Dichgans, J. Disintegration and reorganization of cortical motor processing in two patients with cerebellar stroke. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 59–68. [Google Scholar] [CrossRef]
- Dalal, S.S.; Osipova, D.; Bertrand, O.; Jerbi, K. Oscillatory activity of the human cerebellum: The intracranial electrocerebellogram revisited. Neurosci. Biobehav. Rev. 2013, 37, 585–593. [Google Scholar] [CrossRef]
- de Solages, C.; Szapiro, G.; Brunel, N.; Hakim, V.; Isope, P.; Buisseret, P.; Rousseau, C.; Barbour, B.; Léna, C. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 2008, 58, 775–788. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Hoebeek, F.E.; Schonewille, M. Causes and consequences of oscillations in the cerebellar cortex. Neuron 2008, 58, 655–658. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Hoebeek, F.E.; Bosman, L.W.; Schonewille, M.; Witter, L.; Koekkoek, S.K. Spatiotemporal firing patterns in the cerebellum. Nat. Rev. Neurosci. 2011, 12, 327–344. [Google Scholar] [CrossRef]
- Sadnicka, A.; Rocchi, L.; Latorre, A.; Antelmi, E.; Teo, J.; Pareés, I.; Hoffland, B.S.; Brock, K.; Kornysheva, K.; Edwards, M.J.; et al. A Critical Investigation of Cerebellar Associative Learning in Isolated Dystonia. Mov. Disord. 2022, 37, 1187–1192. [Google Scholar] [CrossRef]
- Rocchi, L.; Latorre, A.; Ibanez Pereda, J.; Spampinato, D.; Brown, K.E.; Rothwell, J.; Bhatia, K. A case of congenital hypoplasia of the left cerebellar hemisphere and ipsilateral cortical myoclonus. Mov. Disord. 2019, 34, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.Y.; Spampinato, D.; Rocchi, L.; Hannah, R.; Teng, Y.; Di Santo, A.; Shoura, M.; Bhatia, K.; Rothwell, J.C. Two forms of short-interval intracortical inhibition in human motor cortex. Brain Stimul. 2021, 14, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.; Avci, E.; Rothwell, J.; Rocchi, L. Frequency-dependent modulation of cerebellar excitability during the application of non-invasive alternating current stimulation. Brain Stimul. 2021, 14, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.Y.; Spampinato, D.; Michell, K.; Mancuso, M.; Brown, K.; Ibáñez, J.; Di Santo, A.; Latorre, A.; Bhatia, K.; Rothwell, J.C.; et al. Reply to: “Reflecting the causes of variability of EEG responses elicited by cerebellar TMS”. Neuroimage 2023, 281, 120392. [Google Scholar] [CrossRef]
- Fong, P.Y.; Spampinato, D.; Michell, K.; Mancuso, M.; Brown, K.; Ibáñez, J.; Santo, A.D.; Latorre, A.; Bhatia, K.; Rothwell, J.C.; et al. EEG responses induced by cerebellar TMS at rest and during visuomotor adaptation. Neuroimage 2023, 275, 120188. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Colebatch, J.G. The human electrocerebellogram (ECeG) recorded non-invasively using scalp electrodes. Neurosci. Lett. 2018, 682, 124–131. [Google Scholar] [CrossRef]
- Niedermeyer, E. The electrocerebellogram. Clin. EEG Neurosci. 2004, 35, 112–115. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Colebatch, J.G. Vestibular cerebellar evoked potentials in humans and their modulation during optokinetic stimulation. J. Neurophysiol. 2018, 120, 3099–3109. [Google Scholar] [CrossRef]
- Todd, N.P.; Govender, S.; Colebatch, J.G. Modulation of the human electro-cerebellogram (ECeG) during vestibular and optokinetic stimulation. Neurosci. Lett. 2019, 712, 134497. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Lemieux, L.; Colebatch, J.G. Source analyses of axial and vestibular evoked potentials associated with brainstem-spinal reflexes show cerebellar and cortical contributions. Neurosci. Lett. 2021, 757, 135960. [Google Scholar] [CrossRef]
- Govender, S.; Todd, N.P.M.; Colebatch, J.G. Effects of posture on cerebellar evoked potentials (CEPs) following brief impulsive stimuli at the mastoid and trunk. Exp. Brain Res. 2022, 240, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.P.M.; Govender, S.; Colebatch, J.G. Non-invasive recording from the human cerebellum during a classical conditioning paradigm using the otolith-evoked blink reflex. Neurosci. Lett. 2021, 765, 136270. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.P.M.; Govender, S.; Keller, P.E.; Colebatch, J.G. Electrophysiological activity from over the cerebellum and cerebrum during eye blink conditioning in human subjects. Physiol. Rep. 2023, 11, e15642. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Keller, P.E.; Colebatch, J.G. Electrophysiological Activity from the Eye Muscles, Cerebellum and Cerebrum During Reflexive (Classical Pavlovian) Versus Voluntary (Ivanov-Smolensky) Eye-Blink Conditioning. Cerebellum 2024, 23, 1086–1100. [Google Scholar] [CrossRef]
- Bosch, T.J.; Espinoza, A.I.; Singh, A. Cerebellar oscillatory dysfunction during lower-limb movement in Parkinson’s disease with freezing of gait. Brain Res. 2023, 1808, 148334. [Google Scholar] [CrossRef]
- Bosch, T.J.; Kammermeier, S.; Groth, C.; Leedom, M.; Hanson, E.K.; Berg-Poppe, P.; Singh, A. Cortical and Cerebellar Oscillatory Responses to Postural Instability in Parkinson’s Disease. Front. Neurol. 2021, 12, 752271. [Google Scholar] [CrossRef]
- Pan, M.K.; Li, Y.S.; Wong, S.B.; Ni, C.L.; Wang, Y.M.; Liu, W.C.; Lu, L.Y.; Lee, J.C.; Cortes, E.P.; Vonsattel, J.G.; et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci. Transl. Med. 2020, 12, eaay1769. [Google Scholar] [CrossRef]
- Wong, S.B.; Wang, Y.M.; Lin, C.C.; Geng, S.K.; Vanegas-Arroyave, N.; Pullman, S.L.; Kuo, S.H.; Pan, M.K. Cerebellar Oscillations in Familial and Sporadic Essential Tremor. Cerebellum 2022, 21, 425–431. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Hochstrasser, D.; Keller, P.E.; Colebatch, J.G. Extended source analysis of movement related potentials (MRPs) for self-paced hand and foot movements demonstrates opposing cerebral and cerebellar laterality: A preliminary study. Neurosci. Lett. 2023, 815, 137476. [Google Scholar] [CrossRef]
- Todd, N.P.M.; Govender, S.; Hochstrasser, D.; Keller, P.E.; Colebatch, J.G. Distinct movement related changes in EEG and ECeG power during finger and foot movement. Neurosci. Lett. 2025, 853, 138207. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H.; Luders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Manli, W.; Junwen, G.; Tong, S.; Junjie, W.; Yikai, Y.; Yicheng, Z.; Yi, Z.; Xiaoyu, Y.; Xuepei, L.; Jingguo, Y.; et al. Enhancing automatic sleep stage classification with cerebellar EEG and machine learning techniques. Comput. Biol. Med. 2025, 185, 109515. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Figas, G.; Hadamus, A.; Błażkiewicz, M.; Kujawa, J. Symmetry of the Neck Muscles’ Activity in the Electromyography Signal during Basic Motion Patterns. Sensors 2023, 23, 4170. [Google Scholar] [CrossRef]
- van den Broek, S.P.; Reinders, F.; Donderwinkel, M.; Peters, M.J. Volume conduction effects in EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 522–534. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- D’Angelo, E.; Nieus, T.; Maffei, A.; Armano, S.; Rossi, P.; Taglietti, V.; Fontana, A.; Naldi, G. Theta-frequency bursting and resonance in cerebellar granule cells: Experimental evidence and modeling of a slow k+-dependent mechanism. J. Neurosci. 2001, 21, 759–770. [Google Scholar] [CrossRef]
- Tzvi, E.; Gajiyeva, L.; Bindel, L.; Hartwigsen, G.; Classen, J. Coherent theta oscillations in the cerebellum and supplementary motor area mediate visuomotor adaptation. Neuroimage 2022, 251, 118985. [Google Scholar] [CrossRef]
- Holdefer, R.N.; Miller, L.E.; Chen, L.L.; Houk, J.C. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J. Neurophysiol. 2000, 84, 585–590. [Google Scholar] [CrossRef]
- Manto, M. Mechanisms of human cerebellar dysmetria: Experimental evidence and current conceptual bases. J. Neuroeng. Rehabil. 2009, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Demanuele, C.; James, C.J.; Sonuga-Barke, E.J. Distinguishing low frequency oscillations within the 1/f spectral behaviour of electromagnetic brain signals. Behav. Brain Funct. 2007, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Peterson, E.J.; Voytek, B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 2017, 158, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.T.; Laird, A.R.; Meyerand, M.E. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage 2008, 42, 343–356. [Google Scholar] [CrossRef]
- Chan, R.C.; Huang, J.; Di, X. Dexterous movement complexity and cerebellar activation: A meta-analysis. Brain Res. Rev. 2009, 59, 316–323. [Google Scholar] [CrossRef]
- Conrad, B.; Brooks, V.B. Effects of dentate cooling on rapid alternating arm movements. J. Neurophysiol. 1974, 37, 792–804. [Google Scholar] [CrossRef]
- Flament, D.; Hore, J. Movement and electromyographic disorders associated with cerebellar dysmetria. J. Neurophysiol. 1986, 55, 1221–1233. [Google Scholar] [CrossRef]
- Thach, W.T.; Perry, J.G.; Kane, S.A.; Goodkin, H.P. Cerebellar nuclei: Rapid alternating movement, motor somatotopy, and a mechanism for the control of muscle synergy. Rev. Neurol. 1993, 149, 607–628. [Google Scholar]
- Berardelli, A.; Hallett, M.; Rothwell, J.C.; Agostino, R.; Manfredi, M.; Thompson, P.D.; Marsden, C.D. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain 1996, 119 Pt 2, 661–674. [Google Scholar] [CrossRef]
- Köster, B.; Deuschl, G.; Lauk, M.; Timmer, J.; Guschlbauer, B.; Lücking, C.H. Essential tremor and cerebellar dysfunction: Abnormal ballistic movements. J. Neurol. Neurosurg. Psychiatry 2002, 73, 400–405. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latorre, A.; Humaidan, K.; Sanna, M.; Lavena, M.L.; Pittalis, S.; Raimondi, C.; Casula, E.P.; Rocchi, L. Surface EEG Evidence for Cerebellar Control of Distal Upper Limbs in Humans. Brain Sci. 2025, 15, 440. https://doi.org/10.3390/brainsci15050440
Latorre A, Humaidan K, Sanna M, Lavena ML, Pittalis S, Raimondi C, Casula EP, Rocchi L. Surface EEG Evidence for Cerebellar Control of Distal Upper Limbs in Humans. Brain Sciences. 2025; 15(5):440. https://doi.org/10.3390/brainsci15050440
Chicago/Turabian StyleLatorre, Anna, Kais Humaidan, Mauro Sanna, Maria Lucrezia Lavena, Sara Pittalis, Clio Raimondi, Elias Paolo Casula, and Lorenzo Rocchi. 2025. "Surface EEG Evidence for Cerebellar Control of Distal Upper Limbs in Humans" Brain Sciences 15, no. 5: 440. https://doi.org/10.3390/brainsci15050440
APA StyleLatorre, A., Humaidan, K., Sanna, M., Lavena, M. L., Pittalis, S., Raimondi, C., Casula, E. P., & Rocchi, L. (2025). Surface EEG Evidence for Cerebellar Control of Distal Upper Limbs in Humans. Brain Sciences, 15(5), 440. https://doi.org/10.3390/brainsci15050440







