The Size and Localization of Ribeye and GluR2 in the Auditory Inner Hair Cell Synapse of C57BL/6 Mice Are Affected by Short-Pulse Corticosterone in a Sex-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Animals
2.2. Experimental Flow
2.3. Cochlear Explant Culture
2.4. Exposure to Corticosterone
2.5. Quantification of the AMPA Receptor Subunit Concentrations in the Cochlear Tissues by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Treatment of Cochlear Explants with Glucocorticoid Receptor Antagonists
2.7. RNA Extraction and Semiquantitative Real-Time One-Step Reverse-Transcription–Polymerase Chain Reaction (sq rtRT-PCR)
2.8. Visualization of Hair Cells and SGNs
2.9. Immunofluorescent Detection of Cochlear Ribbon Synapses
2.10. Fluorescence Microscopy of Cochlear Explants
2.11. Confocal Microscopy
2.12. Quantification of Auditory Hair Cells and Spiral Ganglion Neurons
2.13. Quantitative Analysis of Synapses
2.14. Statistical Analyses
3. Results
3.1. Baseline Differences in Cochlear AMPA Receptor Subunit Concentrations Between Male and Female Mice
3.2. CORT Exposure Does Not Affect Cochlear AMPA Receptor Subunit Concentrations
3.3. Gria2 Gene Expression Remains Stable 3 and 6 h After the CORT Pulse
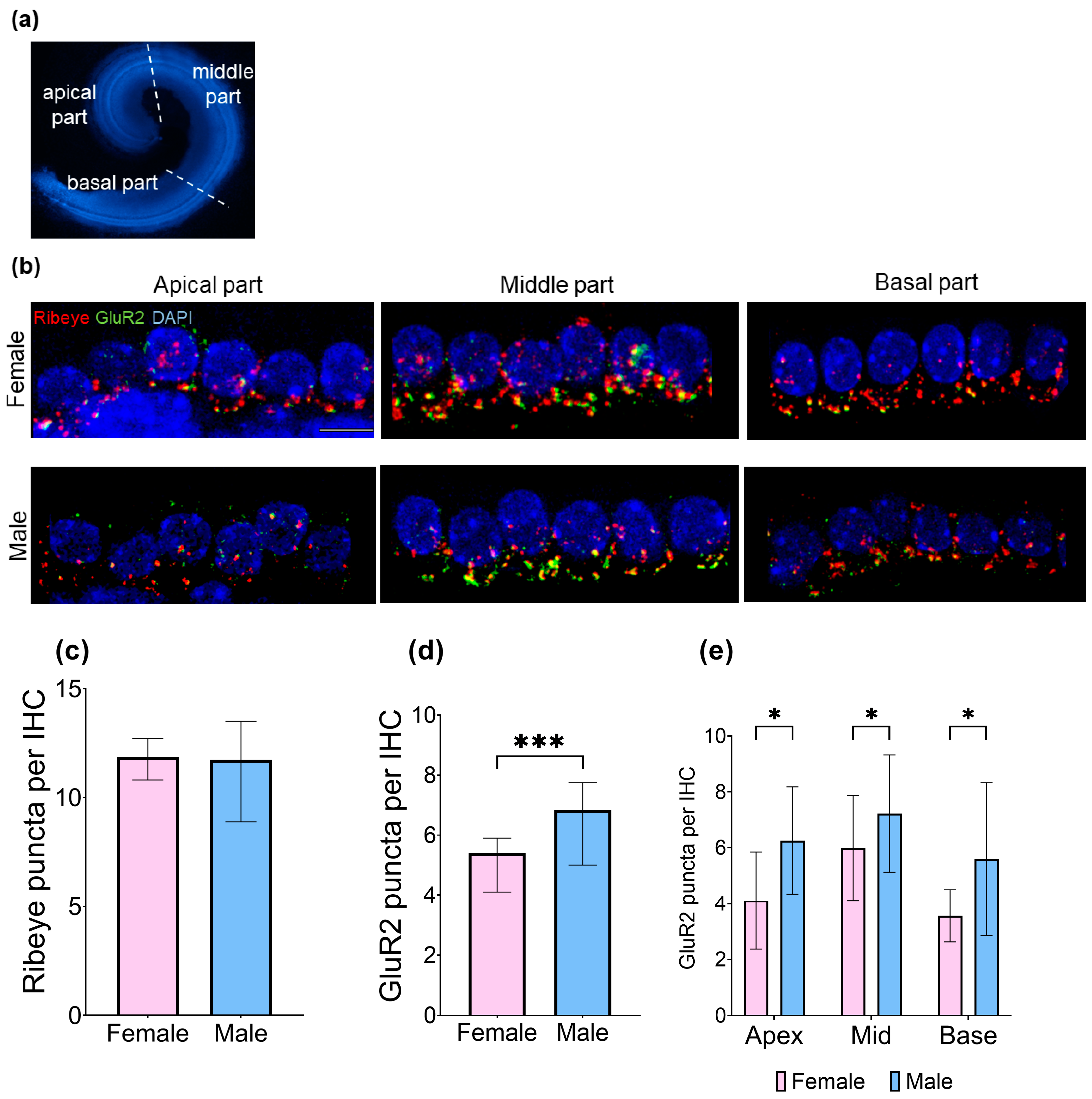
3.4. At Baseline, Male Cochleae Have More GluR2 Puncta than Females and an Equal Number of Ribeye
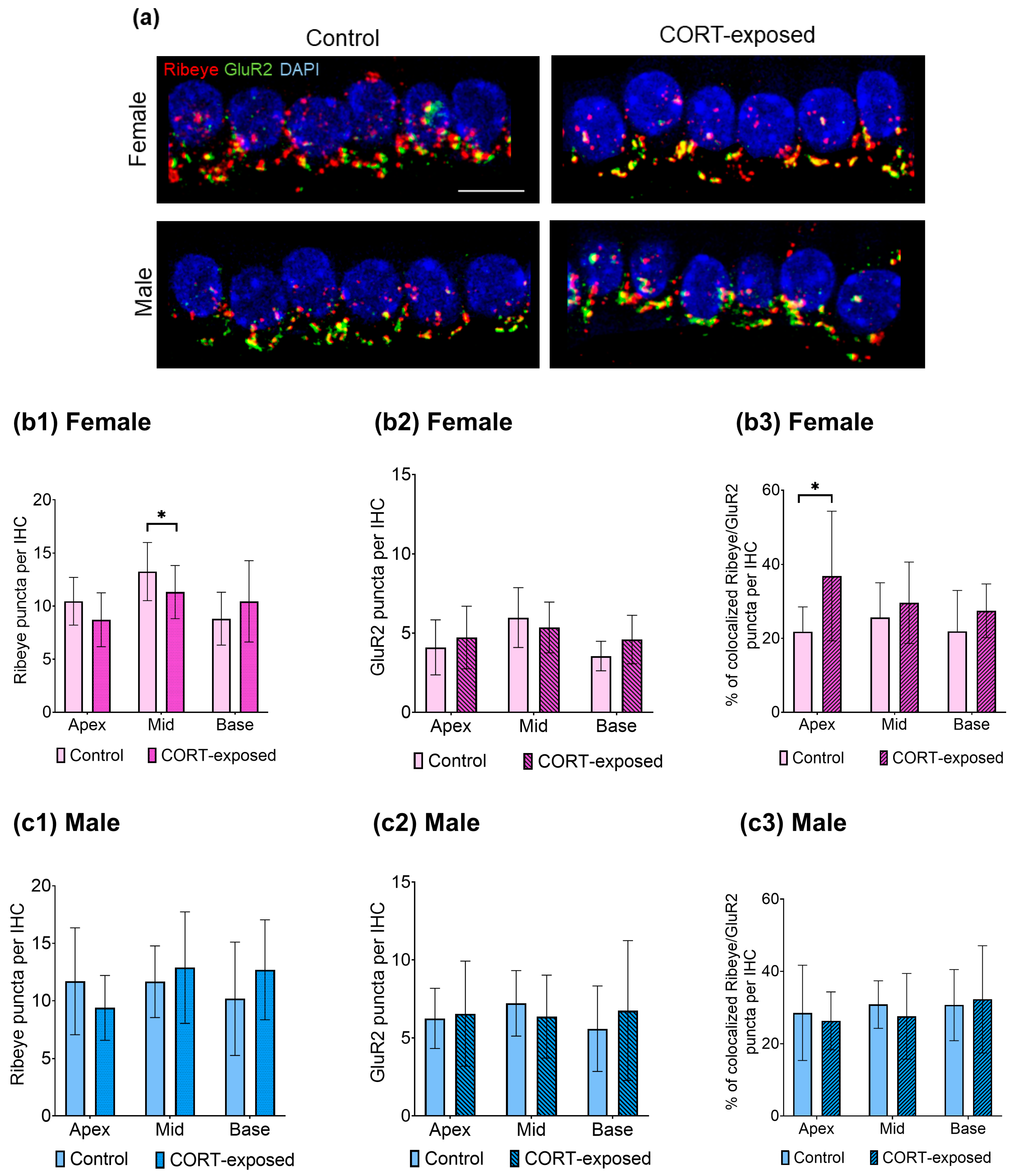
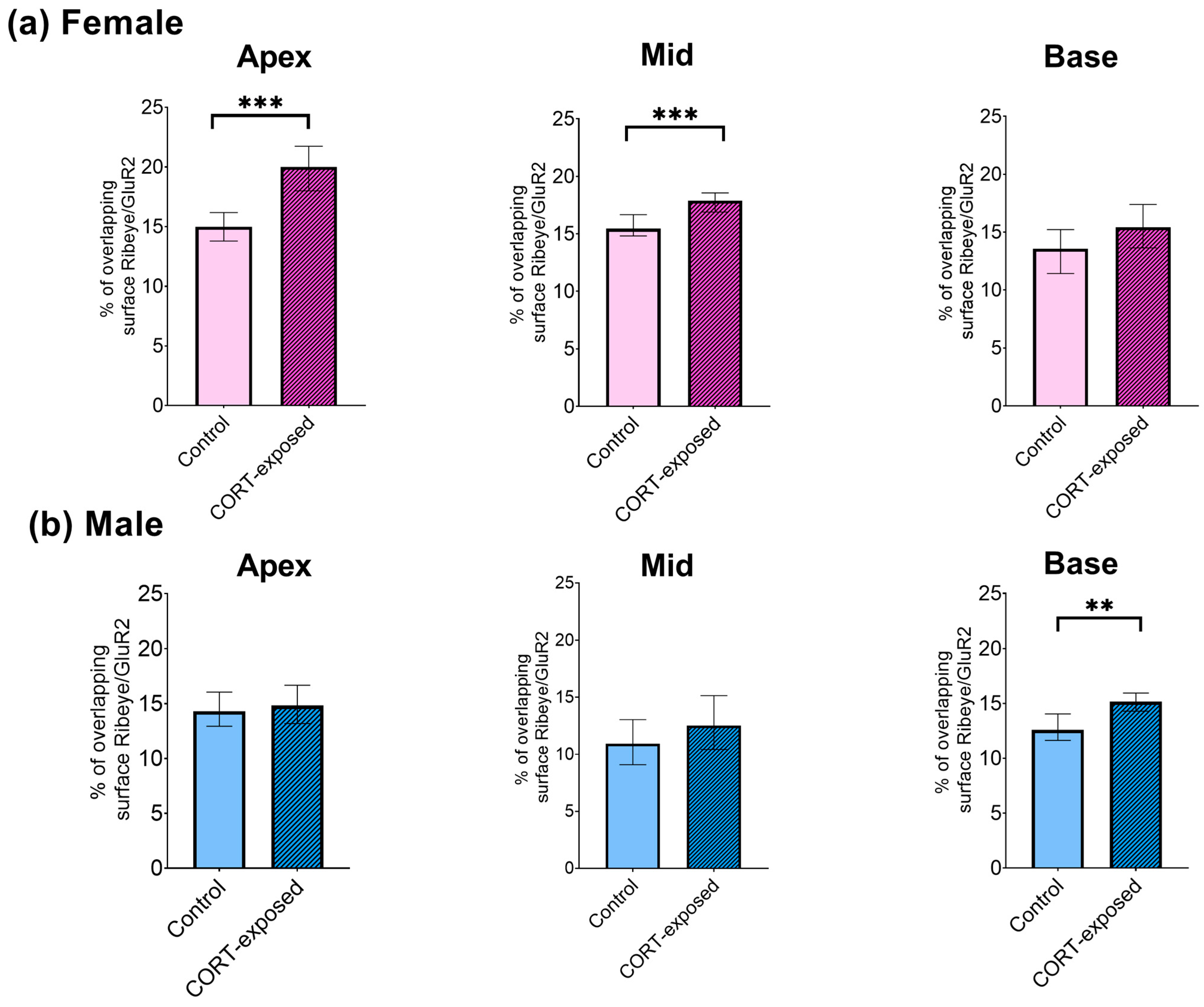
3.5. CORT Exposure Does Not Affect the Number of GluR2 Puncta in Males or Females but Significantly Reduces the Number of Ribeye Puncta in the Middle Cochlear Part and Enhances the Colocalization of Ribeye and GluR2 in the Apical Region of Female Cochleae
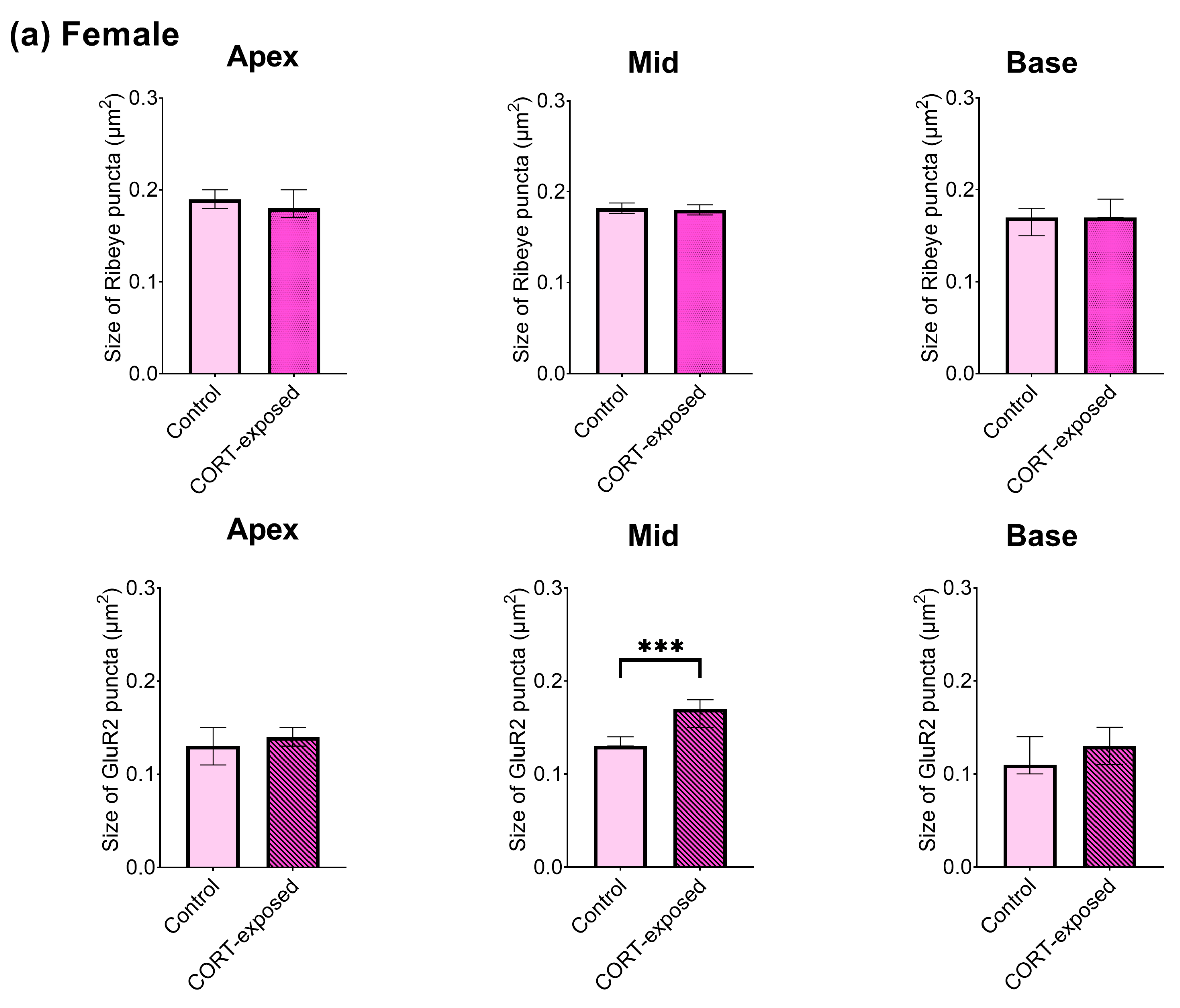
3.6. Baseline Size of GluR2 and Ribeye Puncta
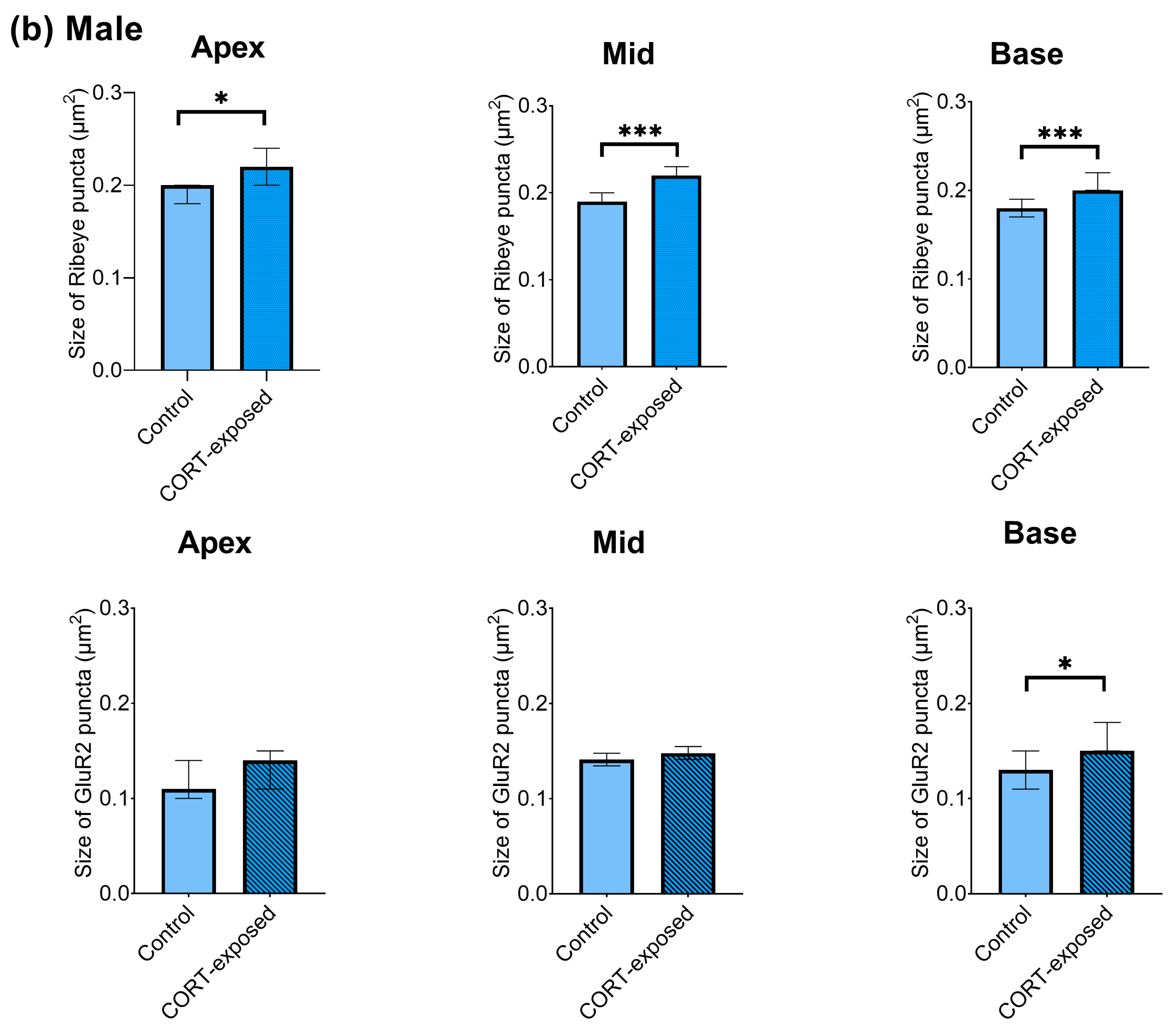
3.7. CORT Exposure Increases the Size of Ribeye Puncta in Males and Increases the Size of GluR2 Puncta in Female Cochlear Tissues
3.8. CORT-Induced Changes in Ribeye Size Are Mediated by the Glucocorticoid Receptor
4. Discussion
5. Limitations of the Study and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPAR | the ionotropic glutamate receptor AMPA |
| CORT | corticosterone |
| GluR1 | AMPA receptor GluR1 subunit |
| GluR2 | AMPA receptor GluR2 subunit |
| GluR3 | AMPA receptor GluR3 subunit |
| GluR4 | AMPA receptor GluR4 subunit |
| GR | glucocorticoid receptor |
| IHCs | inner hair cells |
| MR | mineralocorticoid receptor |
| SGNs | spiral ganglion neurons |
References
- Glowatzki, E.; Fuchs, P.A. Transmitter release at the hair cell ribbon synapse. Nat. Neurosci. 2002, 5, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.C.; Frank, T.; Khimich, D.; Hoch, G.; Riedel, D.; Chapochnikov, N.M.; Yarin, Y.M.; Harke, B.; Hell, S.W.; Egner, A.; et al. Tuning of synapse number, structure and function in the cochlea. Nat. Neurosci. 2009, 12, 444–453. [Google Scholar] [CrossRef]
- Rutherford, M.A. Resolving the structure of inner ear ribbon synapses with STED microscopy. Synapse 2015, 69, 242–255. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Waag, R.; Schläppi, C.; Germain, P.-L.; Bohacek, J. The Acute Stress Response in the Multiomic Era. Biol. Psychiatry 2021, 89, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Sarabdjitsingh, R.A.; Pasricha, N.; Smeets, J.A.; Kerkhofs, A.; Mikasova, L.; Karst, H.; Groc, L.; Joëls, M. Hippocampal Fast Glutamatergic Transmission Is Transiently Regulated by Corticosterone Pulsatility. PLoS ONE 2016, 11, e0145858. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Peng, W.-H.; Kan, H.-W.; Wu, C.-C.; Wang, D.-W.; Ho, Y.-C. Neurobiology of Depression: Chronic Stress Alters the Glutamatergic System in the Brain—Focusing on AMPA Receptor. Biomedicines 2022, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.M.; Werkman, T.R.; Craig, J.; Finnell, R.; Joëls, M.; Eberwine, J.H. Corticosteroid regulation of ion channel conductances and mRNA levels in individual hippocampal CA1 neurons. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2685–2696. [Google Scholar] [CrossRef]
- Martin, S.; Henley, J.M.; Holman, D.; Zhou, M.; Wiegert, O.; van Spronsen, M.; Joëls, M.; Hoogenraad, C.C.; Krugers, H.J. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS ONE 2009, 4, e4714. [Google Scholar] [CrossRef]
- Edlund, E.; Domarecka, E.; Olze, H.; Szczepek, A. A Scoping Review of Corticosterone-Induced Changes in Ionotropic Glutamate Receptor Levels and Localization in the Rodent Brain: Implications for the Auditory System. Brain Sci. 2025, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, W.; Xiong, H.; Hoogenraad, C.C.; Krugers, H.J. Stress and excitatory synapses: From health to disease. Neuroscience 2013, 248, 626–636. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Rizzo, S.; Laudani, S.; Ieraci, A.; Drago, F.; Leggio, G.M. Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner. Neurobiol. Stress 2023, 25, 100545. [Google Scholar] [CrossRef]
- Monfort, P.; Gomez-Gimenez, B.; Llansola, M.; Felipo, V. Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: Molecular mechanisms. ACS Chem. Neurosci. 2015, 6, 1420–1427. [Google Scholar] [CrossRef]
- Wickens, M.M.; Bangasser, D.A.; Briand, L.A. Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front. Mol. Neurosci. 2018, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Szczepek, A.J.; Dietz, G.P.H.; Reich, U.; Hegend, O.; Olze, H.; Mazurek, B. Differences in Stress-Induced Modulation of the Auditory System Between Wistar and Lewis Rats. Front. Neurosci. 2018, 12, 828. [Google Scholar] [CrossRef]
- Muchnik, C.; Hildesheimer, M.; Rubinstein, M. Effect of emotional stress on hearing. Arch. Otorhinolaryngol. 1980, 228, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Szczepek, A.J.; Haupt, H.; Mazurek, B. Geldanamycin induces production of heat shock protein 70 and partially attenuates ototoxicity caused by gentamicin in the organ of Corti explants. J. Biomed. Sci. 2009, 16, 79. [Google Scholar] [CrossRef]
- Karst, H.; Karten, Y.J.; Reichardt, H.M.; de Kloet, E.R.; Schütz, G.; Joëls, M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat. Neurosci. 2000, 3, 977–978. [Google Scholar] [CrossRef]
- Alfarez, D.N.; Wiegert, O.; Joëls, M.; Krugers, H.J. Corticosterone and stress reduce synaptic potentiation in mouse hippocampal slices with mild stimulation. Neuroscience 2002, 115, 1119–1126. [Google Scholar] [CrossRef]
- Groc, L.; Choquet, D.; Chaouloff, F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat. Neurosci. 2008, 11, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, M.; Smorodchenko, A.; Olze, H.; Szczepek, A.J. Identification and Characterization of TMEM119-Positive Cells in the Postnatal and Adult Murine Cochlea. Brain Sci. 2023, 13, 516. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monedero, R.; Liu, C.; Weisz, C.; Vyas, P.; Fuchs, P.A.; Glowatzki, E. GluA2-Containing AMPA Receptors Distinguish Ribbon-Associated from Ribbonless Afferent Contacts on Rat Cochlear Hair Cells. eNeuro 2016, 3, 1–17. [Google Scholar] [CrossRef]
- Reijntjes, D.O.J.; Breitzler, J.L.; Persic, D.; Pyott, S.J. Preparation of the intact rodent organ of Corti for RNAscope and immunolabeling, confocal microscopy, and quantitative analysis. STAR Protoc. 2021, 2, 100544. [Google Scholar] [CrossRef]
- Clancy, S.; Xie, N.; Muttikkal, T.E.; Wang, J.; Fateh, E.; Smith, M.; Wilson, P.; Smith, M.; Hogan, A.; Sutherland, A.; et al. Rac1 and Nectin3 are essential for PCP-directed axon guidance in the peripheral auditory system. bioRxiv 2024. [Google Scholar] [CrossRef]
- Gilles, J.F.; Dos Santos, M.; Boudier, T.; Bolte, S.; Heck, N. DiAna, an ImageJ tool for object-based 3D co-localization and distance analysis. Methods 2017, 115, 55–64. [Google Scholar] [CrossRef]
- Morsink, M.C.; Steenbergen, P.J.; Vos, J.B.; Karst, H.; Joëls, M.; De Kloet, E.R.; Datson, N.A. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J. Neuroendocr. 2006, 18, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Lozier, N.R.; Muscio, S.; Pal, I.; Cai, H.M.; Rubio, M.E. Sex differences in glutamate AMPA receptor subunits mRNA with fast gating kinetics in the mouse cochlea. Front. Syst. Neurosci. 2023, 17, 1100505. [Google Scholar] [CrossRef]
- Michanski, S.; Smaluch, K.; Steyer, A.M.; Chakrabarti, R.; Setz, C.; Oestreicher, D.; Fischer, C.; Möbius, W.; Moser, T.; Vogl, C.; et al. Mapping developmental maturation of inner hair cell ribbon synapses in the apical mouse cochlea. Proc. Natl. Acad. Sci. USA 2019, 116, 6415–6424. [Google Scholar] [CrossRef]
- Huang, L.C.; Barclay, M.; Lee, K.; Peter, S.; Housley, G.D.; Thorne, P.R.; Montgomery, J.M. Synaptic profiles during neurite extension, refinement and retraction in the developing cochlea. Neural Dev. 2012, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Palomero-Gallagher, N.; Bidmon, H.J.; Zilles, K. AMPA, kainate, and NMDA receptor densities in the hippocampus of untreated male rats and females in estrus and diestrus. J. Comp. Neurol. 2003, 459, 468–474. [Google Scholar] [CrossRef]
- Lozier, N.R.; Aizenstein, M.A.; Williams, E.D.; Rubio, M.E. Gonad-derived steroid hormones mediate a sex difference in the maturation of auditory encoding in the cochlea from adolescence to early adulthood in C57BL/6J mice. Hear. Res. 2025, 457, 109187. [Google Scholar] [CrossRef]
- Ismail Mohamad, N.; Santra, P.; Park, Y.; Matthews, I.R.; Taketa, E.; Chan, D.K. Synaptic ribbon dynamics after noise exposure in the hearing cochlea. Commun. Biol. 2024, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.Y.; Ceriani, F.; Olt, J.; Brown, S.D.M.; Holley, M.C.; Bowl, M.R.; Johnson, S.L.; Marcotti, W. Pathophysiological changes in inner hair cell ribbon synapses in the ageing mammalian cochlea. J. Physiol. 2020, 598, 4339–4355. [Google Scholar] [CrossRef]
- Becker, L.; Schnee, M.E.; Niwa, M.; Sun, W.; Maxeiner, S.; Talaei, S.; Kachar, B.; Rutherford, M.A.; Ricci, A.J. The presynaptic ribbon maintains vesicle populations at the hair cell afferent fiber synapse. eLife 2018, 7, e30241. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.M.; Xu, C.F.; Neubert, T.A.; Chao, M.V.; Garabedian, M.J.; Jeanneteau, F.D. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol. Cell. Biol. 2013, 33, 3700–3714. [Google Scholar] [CrossRef]
- Sarabdjitsingh, R.A.; Jezequel, J.; Pasricha, N.; Mikasova, L.; Kerkhofs, A.; Karst, H.; Groc, L.; Joëls, M. Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 14265–14270. [Google Scholar] [CrossRef]
- Zhou, M.; Hoogenraad, C.C.; Joëls, M.; Krugers, H.J. Combined β-adrenergic and corticosteroid receptor activation regulates AMPA receptor function in hippocampal neurons. J. Psychopharmacol. 2012, 26, 516–524. [Google Scholar] [CrossRef]
- Liberman, L.D.; Wang, H.; Liberman, M.C. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J. Neurosci. 2011, 31, 801–808. [Google Scholar] [CrossRef]
- Singer, W.; Kasini, K.; Manthey, M.; Eckert, P.; Armbruster, P.; Vogt, M.A.; Jaumann, M.; Dotta, M.; Yamahara, K.; Harasztosi, C.; et al. The glucocorticoid antagonist mifepristone attenuates sound-induced long-term deficits in auditory nerve response and central auditory processing in female rats. Faseb J. 2018, 32, 3005–3019. [Google Scholar] [CrossRef] [PubMed]
- Tahera, Y.; Meltser, I.; Johansson, P.; Hansson, A.C.; Canlon, B. Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology 2006, 147, 4430–4437. [Google Scholar] [CrossRef]
- Dagnino-Subiabre, A.; Terreros, G.; Carmona-Fontaine, C.; Zepeda, R.; Orellana, J.A.; Díaz-Véliz, G.; Mora, S.; Aboitiz, F. Chronic stress impairs acoustic conditioning more than visual conditioning in rats: Morphological and behavioural evidence. Neuroscience 2005, 135, 1067–1074. [Google Scholar] [CrossRef]
- Mazurek, B.; Haupt, H.; Joachim, R.; Klapp, B.F.; Stöver, T.; Szczepek, A.J. Stress induces transient auditory hypersensitivity in rats. Hear. Res. 2010, 259, 55–63. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Chia, C.; Wu, L.; Kujawa, S.G.; Liberman, M.C.; Goodrich, L.V. Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity. Cell 2018, 174, 1229–1246.e1217. [Google Scholar] [CrossRef] [PubMed]
- Eybalin, M.; Caicedo, A.; Renard, N.; Ruel, J.; Puel, J.L. Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur. J. Neurosci. 2004, 20, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.A.S.; Choleris, E.; Galea, L.A.M. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain 2019, 12, 22. [Google Scholar] [CrossRef]
- Landegger, L.D.; Dilwali, S.; Stankovic, K.M. Neonatal Murine Cochlear Explant Technique as an In Vitro Screening Tool in Hearing Research. J. Vis. Exp. 2017, 124, 55704. [Google Scholar] [CrossRef]
- Shu, Y.; Tao, Y.; Wang, Z.; Tang, Y.; Li, H.; Dai, P.; Gao, G.; Chen, Z.Y. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum. Gene Ther. 2016, 27, 687–699. [Google Scholar] [CrossRef]
- Szczepek, A.J.; Mazurek, B. Neurobiology of Stress-Induced Tinnitus. Curr. Top. Behav. Neurosci. 2021, 51, 327–347. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Park, J.S.; Basinou, V.; Weger, B.D.; Tserga, E.; Sarlus, H.; Magnusson, A.K.; Kadri, N.; Gachon, F.; Canlon, B. Circadian Regulation of Cochlear Sensitivity to Noise by Circulating Glucocorticoids. Curr. Biol. 2019, 29, 2477–2487.e2476. [Google Scholar] [CrossRef] [PubMed]



| Target | Supplier and Catalog Number | Sensitivity (the Minimum Detectable Level) |
|---|---|---|
| GluR1 | BIOZOL Diagnostica Vertrieb GmbH, Hamburg, Germany, cat. no. ASB-OKEH02153 | not provided |
| GluR2 | Hölzel Diagnostika Handels GmbH, Cologne, Germany, cat. no. SEE802Mu-96T | [0.057 ng/mL] |
| GluR3 | Hölzel Diagnostika Handels GmbH, Cologne, Germany, cat. no. SEE803Mu-96T | [0.062 ng/mL] |
| GluR4 | Hölzel Diagnostika Handels GmbH, Cologne, Germany, cat. no. SEE804Mu-96T | [0.054 ng/mL] |
| Target | Antibody Type | Supplier | Catalog No. | Working Dilution | |
|---|---|---|---|---|---|
| Primary antibodies | Ribeye A-domain | Guinea pig polyclonal | Synaptic Systems GmbH, Göttingen, Germany | 192104 | 1:5000 |
| GluR2 (clone 6C4) | Mouse monoclonal | Merck Millipore, Darmstadt, Germany | MAB397 | 1:200 | |
| Anti-neurofilament-200 | Mouse monoclonal | Sigma-Aldrich, Taufkirchen, Germany | N0142 | 1:400 | |
| Secondary antibody conjugates | Mouse IgG | Goat anti-mouse Alexa 488 IgG (H + L) | Thermo Fisher Scientific, Darmstadt, Germany | A1100 | 1:500 1:400 |
| Guinea pig IgG | Goat anti-guinea pig IgG (H + L) Alexa Fluor 647 | Thermo Fisher Scientific, Darmstadt, Germany | A21450 | 1:500 | |
| Other reagents | Prolong Gold DAPI | Mounting solution | Cell Signaling Technology Europe B.V, Frankfurt am Main, Germany | 8961S | undiluted |
| Phalloidin | iFluor594 | Santa Cruz Biotechnology Inc., Heidelberg, Germany | Sc363795 | 1:1000 |
| AMPA Subunits | Sex | No. of Samples Included in Statistical Analysis/No. of ELISA Analyzed Samples * | Mean ± SEM | Median | p-Value |
|---|---|---|---|---|---|
| GluR1 | Female | 8/10 | 22.62 ± 3.40 | 20.44 | ns 1 |
| Male | 9/10 | 33.23 ± 4.61 | 30.26 | ||
| GluR2 | Female | 10/10 | 2.82 ± 0.18 | 2.68 | <0.001 1 |
| Male | 9/10 | 6.16 ± 0.63 | 5.90 | ||
| GluR3 | Female | 4/10 | 2.48 ± 0.37 | 2.24 | ns 1 |
| Male | 6/10 | 2.99 ± 0.38 | 3.22 | ||
| GluR4 | Female | 0/10 | Not applicable | ||
| Male | 2/10 | 3.75 ± 0.39 | 3.75 |
| AMPA Subunits | Sex | Group | No. of Samples Included in Statistical Analysis/No. of ELISA Analyzed Samples * | Mean ± SEM | Median | p-Value |
|---|---|---|---|---|---|---|
| GluR1 | Female | Control | 8/10 | 22.62 ± 3.40 | 20.44 | ns 1 |
| CORT-exposed | 10/10 | 25.71 ± 3.73 | 22.03 | |||
| Male | Control | 9/10 | 33.23 ± 4.61 | 30.26 | ns 2 | |
| CORT-exposed | 9/10 | 31.37 ± 5.71 | 24.61 | |||
| GluR2 | Female | Control | 9/10 | 2.82 ± 0.18 | 2.68 | ns 2 |
| CORT-exposed | 10/10 | 2.86 ± 0.22 | 2.57 | |||
| Male | Control | 9/10 | 6.16 ± 0.63 | 5.90 | ns 1 | |
| CORT-exposed | 8/10 | 5.84 ± 0.43 | 5.69 | |||
| GluR3 | Female | Control | 4/10 | 2.48 ± 0.37 | 2.24 | ns 2 |
| CORT-exposed | 3/10 | 2.85 ± 0.21 | 2.80 | |||
| Male | Control | 6/10 | 2.99 ± 0.38 | 3.22 | ns 1 | |
| CORT-exposed | 6/10 | 2.79 ± 0.26 | 2.66 | |||
| GluR4 | Female | Control | 0/10 | Not applicable | ||
| CORT-exposed | 0/10 | |||||
| Male | Control | 2/10 | 3.75 ± 0.39 | 3.75 | Not applicable | |
| CORT-exposed | 4/10 | 3.62 ± 0.55 | 3.61 | |||
| CORT-exposed | 0/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domarecka, E.; Olze, H.; Szczepek, A.J. The Size and Localization of Ribeye and GluR2 in the Auditory Inner Hair Cell Synapse of C57BL/6 Mice Are Affected by Short-Pulse Corticosterone in a Sex-Dependent Manner. Brain Sci. 2025, 15, 441. https://doi.org/10.3390/brainsci15050441
Domarecka E, Olze H, Szczepek AJ. The Size and Localization of Ribeye and GluR2 in the Auditory Inner Hair Cell Synapse of C57BL/6 Mice Are Affected by Short-Pulse Corticosterone in a Sex-Dependent Manner. Brain Sciences. 2025; 15(5):441. https://doi.org/10.3390/brainsci15050441
Chicago/Turabian StyleDomarecka, Ewa, Heidi Olze, and Agnieszka J. Szczepek. 2025. "The Size and Localization of Ribeye and GluR2 in the Auditory Inner Hair Cell Synapse of C57BL/6 Mice Are Affected by Short-Pulse Corticosterone in a Sex-Dependent Manner" Brain Sciences 15, no. 5: 441. https://doi.org/10.3390/brainsci15050441
APA StyleDomarecka, E., Olze, H., & Szczepek, A. J. (2025). The Size and Localization of Ribeye and GluR2 in the Auditory Inner Hair Cell Synapse of C57BL/6 Mice Are Affected by Short-Pulse Corticosterone in a Sex-Dependent Manner. Brain Sciences, 15(5), 441. https://doi.org/10.3390/brainsci15050441







