Papain Affects the Percentage and Morphology of Microglia in Hippocampal Neuron–Glial Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cell Cultures
2.2. Fluorescent Microscopy
2.2.1. Calcium Imaging
2.2.2. Vital Microglia Identification
2.3. Immunocytochemical Staining
2.4. Scratch Assay
2.5. Data Analysis
2.6. Substances
3. Results
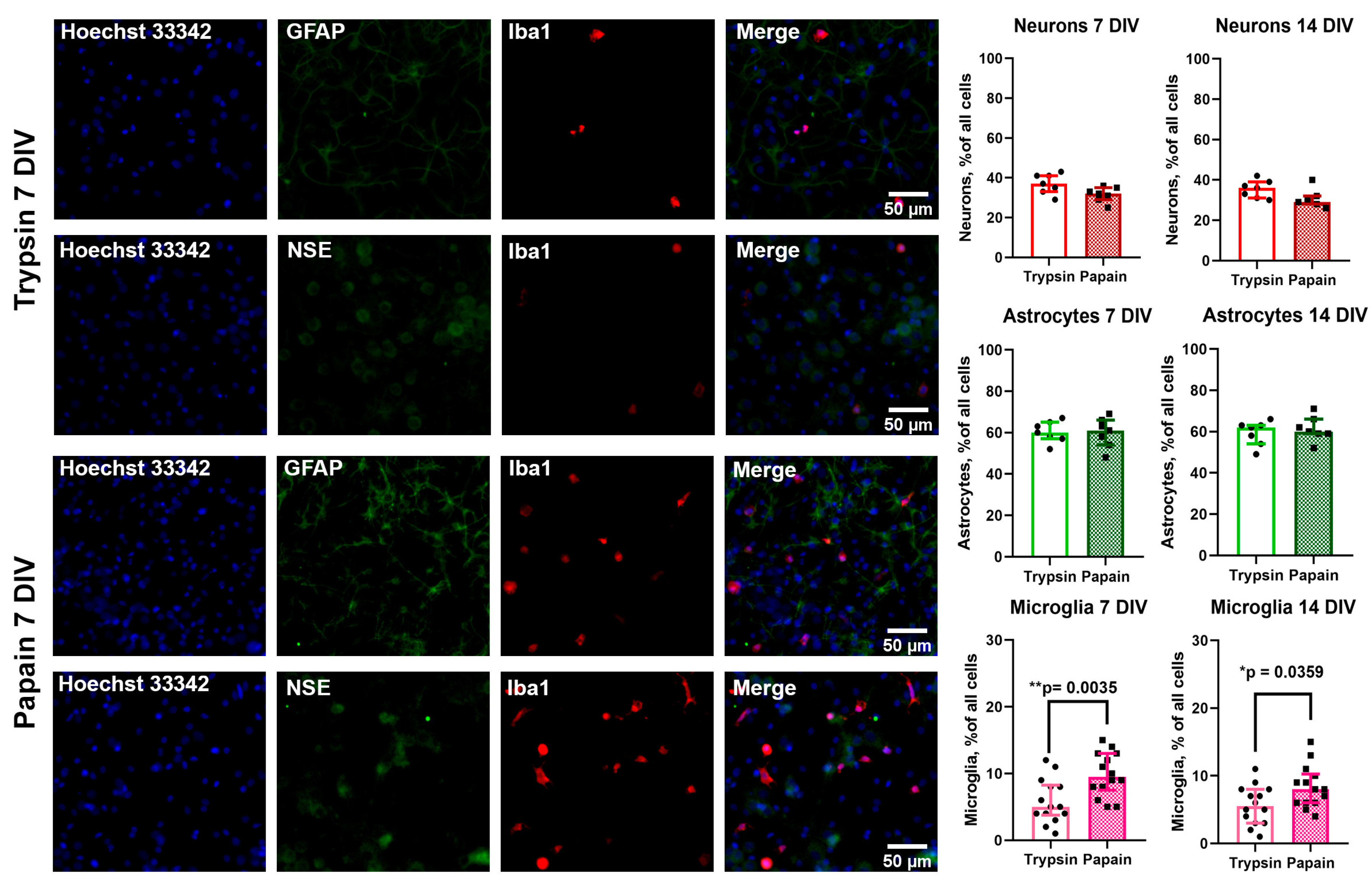
3.1. Comparison of Population Composition of Cultures Prepared Using Trypsin and Papain
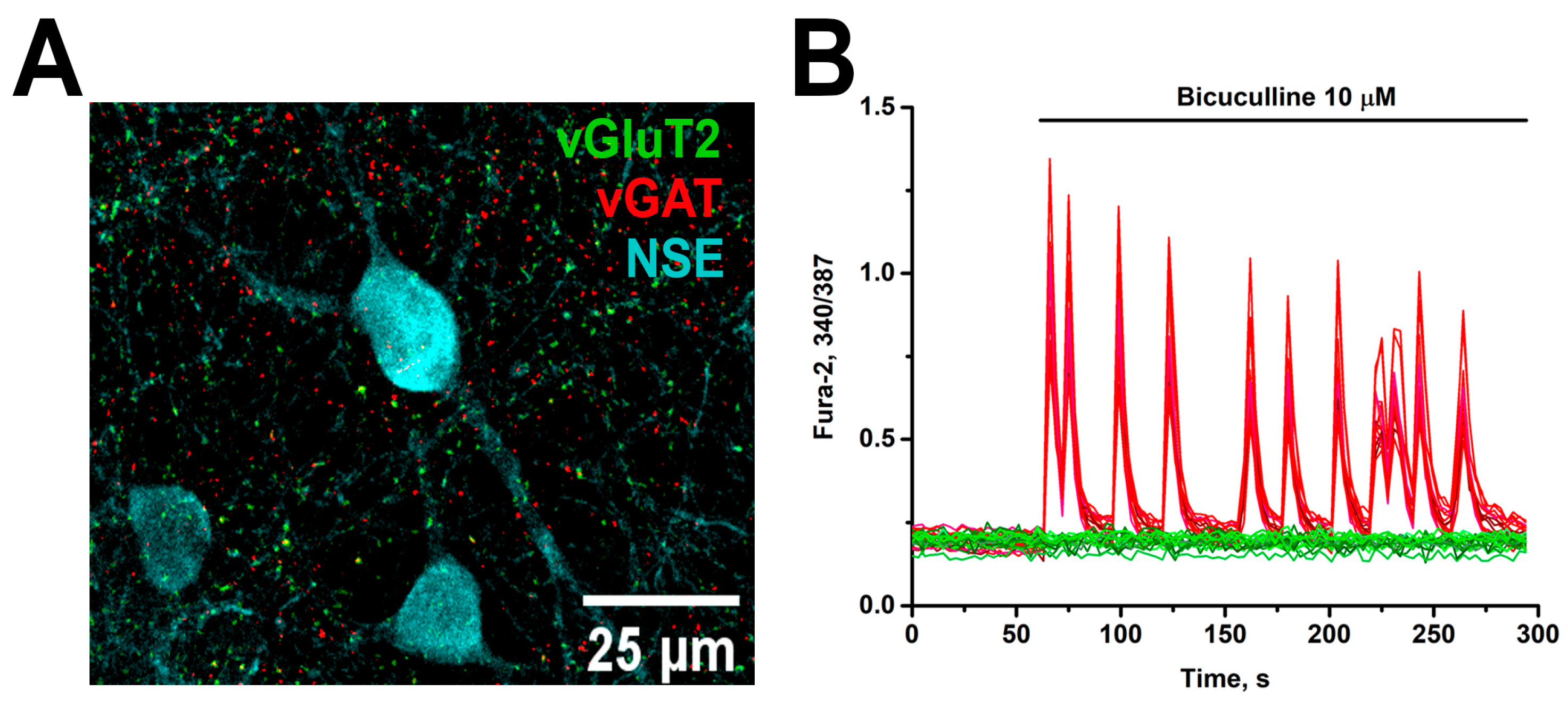
3.2. Papain Does Not Affect the Synaptogenesis and Maturation of Neuronal Networks
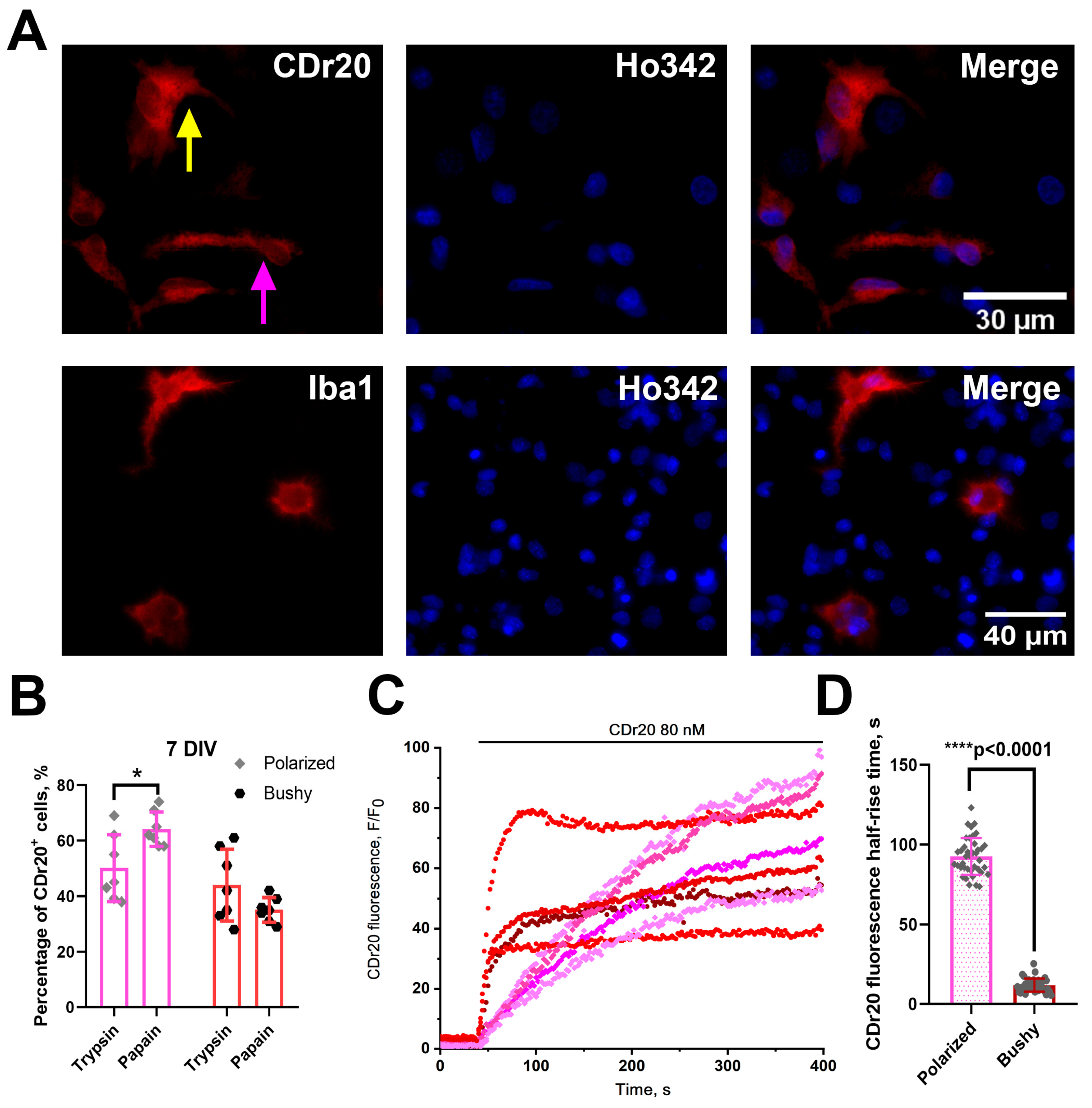
3.3. Microglia Morphology in PAP Cultures Differs from Microglia Morphology in TRY Cultures
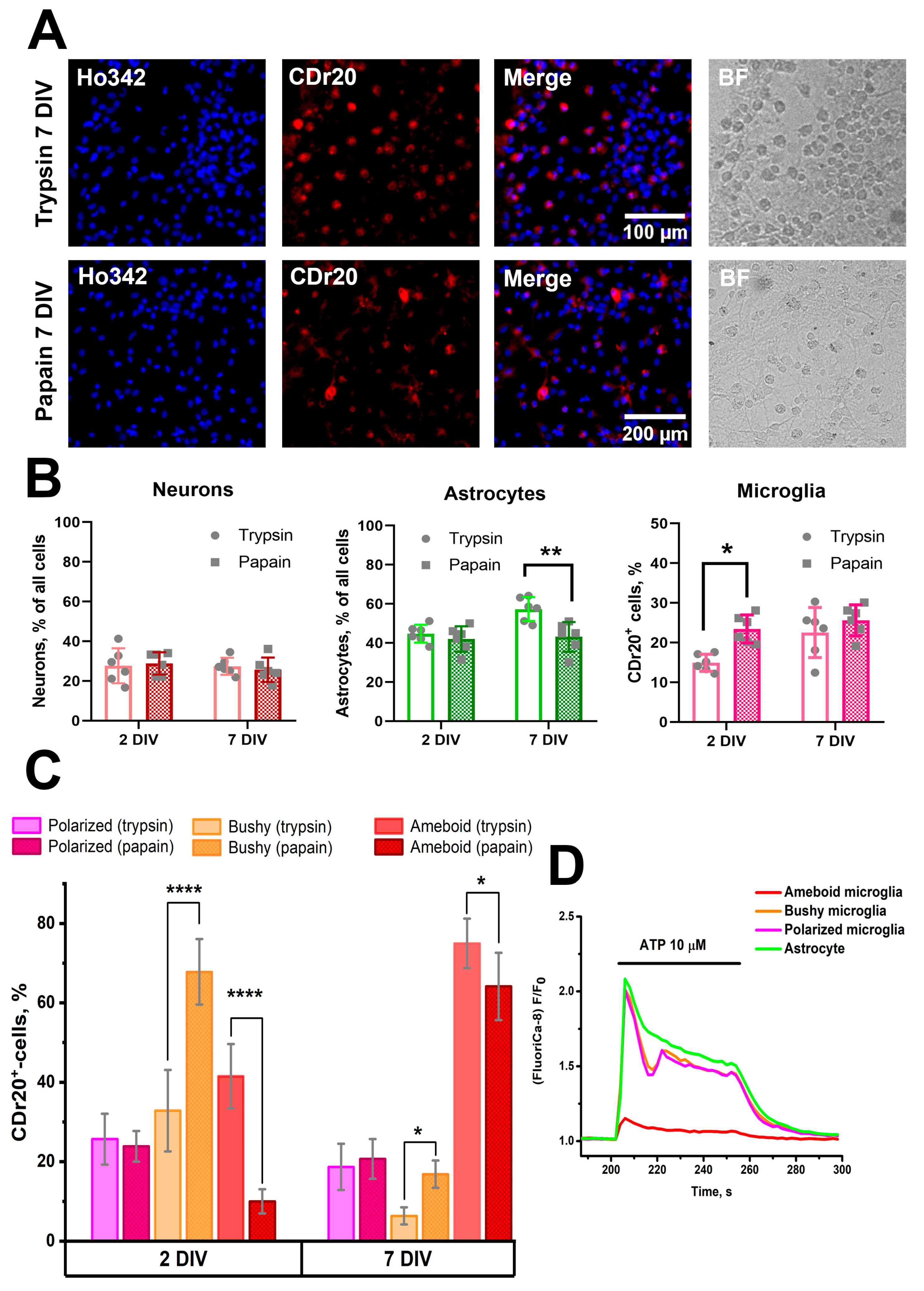
3.4. Effect of the Combination of TGFβ+MCSF+Cholesterol Factors on the Population Composition of PAP and TRY Cultures Obtained Using Papain and Trypsin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadwal, S.; Heneka, M.T. Microglia heterogeneity in health and disease. FEBS Open Bio 2024, 14, 217–229. [Google Scholar] [CrossRef] [PubMed]
- De Deus, J.L.; Faborode, O.S.; Nandi, S. Synaptic Pruning by Microglia: Lessons from Genetic Studies in Mice. Dev. Neurosci. 2024, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Pesti, I.; Légrádi, Á.; Farkas, E. Primary microglia cell cultures in translational research: Strengths and limitations. J. Biotechnol. 2024, 386, 10–18. [Google Scholar] [CrossRef]
- Aktories, P.; Petry, P.; Kierdorf, K. Microglia in a Dish-Which Techniques Are on the Menu for Functional Studies? Front. Cell. Neurosci. 2022, 16, 908315. [Google Scholar] [CrossRef]
- Roqué, P.J.; Costa, L.G. Co-Culture of Neurons and Microglia. Curr. Protoc. Toxicol. 2017, 74, 11.24.1–11.24.17. [Google Scholar] [CrossRef]
- Dessi, F.; Pollard, H.; Moreau, J.; Ben-Ari, Y.; Charriaut-Marlangue, C. Cytosine arabinoside induces apoptosis in cerebellar neurons in culture. J. Neurochem. 1995, 64, 1980–1987. [Google Scholar] [CrossRef]
- Besirli, C.G.; Deckwerth, T.L.; Crowder, R.J.; Freeman, R.S.; Johnson, E.M. Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 2003, 10, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Adachi, M.; Hatano, M.; Inahata, N.; Nagao, T.; Fukushima, N. Cytosine arabinoside induces phosphorylation of histone H2AX in hippocampal neurons via a noncanonical pathway. Neurochem. Int. 2021, 142, 104933. [Google Scholar] [CrossRef]
- Du, F.; Zhu, L.; Qian, Z.; Wu, X.M.; Yung, W.; Xu, W.Y.; Ya, K. Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim. Biophys. Acta 2008, 1782, 658–663. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef]
- Goshi, N.; Kim, H.; Seker, E. Primary Cortical Cell Tri-Culture-Based Screening of Neuroinflammatory Response in Toll-like Receptor Activation. Biomedicines 2022, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Le, B.; Goshi, N.; Zhu, K.; Grodzki, A.C.; Lein, P.J.; Zhao, M.; Seker, E. Primary cortical cell tri-culture to study effects of amyloid-β on microglia function and neuroinflammatory response. J. Alzheimer’s Dis. 2024, 102, 730–741. [Google Scholar] [CrossRef]
- Woolf, Z.; Stevenson, T.J.; Lee, K.; Jung, Y.; Park, T.I.H.; Curtis, M.A.; Montgomery, J.M.; Dragunow, M. Isolation of adult mouse microglia using their in vitro adherent properties. STAR Protoc. 2021, 2, 100518. [Google Scholar] [CrossRef]
- Brewer, G.J. Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods 1997, 71, 143–155. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Du, R.-L.; Xiao, Q.-X.; Geng, M.-J. Differences between cultured cortical neurons by trypsin and papain digestion. Ibrain 2022, 8, 93–99. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Zinchenko, V.P.; Sergeev, A.I.; Teplov, I.Y.; Mal’tseva, V.N.; Kosenkov, A.M. Activation of alpha-2 adrenergic receptors stimulates GABA release by astrocytes. Glia 2020, 68, 1114–1130. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Kosenkov, A.M.; Zinchenko, V.P.; Kairat, B.K.; Malibayeva, A.E.; Tuleukhanov, S.T. Identification of Neurons Containing Calcium-Permeable AMPA and Kainate Receptors Using Ca2+ Imaging. Bio Protoc. 2025, 15, e5199. [Google Scholar] [CrossRef] [PubMed]
- Laryushkin, D.P.; Maiorov, S.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. Role of L-Type Voltage-Gated Calcium Channels in Epileptiform Activity of Neurons. Int. J. Mol. Sci. 2021, 22, 10342. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Kosenkov, A.M.; Gaidin, S.G.; Sergeev, A.I.; Dolgacheva, L.P.; Tuleukhanov, S.T. Properties of GABAergic Neurons Containing Calcium-Permeable Kainate and AMPA-Receptors. Life 2021, 11, 1309. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Maiorov, S.A.; Laryushkin, D.P.; Zinchenko, V.P.; Kosenkov, A.M. A novel approach for vital visualization and studying of neurons containing Ca2+-permeable AMPA receptors. J. Neurochem. 2023, 164, 583–597. [Google Scholar] [CrossRef]
- Laryushkin, D.P.; Maiorov, S.A.; Zinchenko, V.P.; Mal’tseva, V.N.; Gaidin, S.G.; Kosenkov, A.M. Of the Mechanisms of Paroxysmal Depolarization Shifts: Generation and Maintenance of Bicuculline-Induced Paroxysmal Activity in Rat Hippocampal Cell Cultures. Int. J. Mol. Sci. 2023, 24, 10991. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Teplov, I.Y.; Kosenkov, A.M.; Gaidin, S.G.; Kairat, B.K.; Tuleukhanov, S.T. Participation of calcium-permeable AMPA receptors in the regulation of epileptiform activity of hippocampal neurons. Front. Synaptic Neurosci. 2024, 16, 1349984. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Grol, M.W.; Grutter, P.H.; Dixon, S.J.; Komarova, S.V. Modeling Interactions among Individual P2 Receptors to Explain Complex Response Patterns over a Wide Range of ATP Concentrations. Front. Physiol. 2016, 7, 294. [Google Scholar] [CrossRef]
- Kim, B.; Fukuda, M.; Lee, J.-Y.; Su, D.; Sanu, S.; Silvin, A.; Khoo, A.T.T.; Kwon, T.; Liu, X.; Chi, W.; et al. Visualizing Microglia with a Fluorescence Turn-On Ugt1a7c Substrate. Angew. Chem. Int. Ed. Engl. 2019, 58, 7972–7976. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Del Puerto, A.; Wandosell, F.; Garrido, J.J. Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front. Cell. Neurosci. 2013, 7, 197. [Google Scholar] [CrossRef]
- Kleinsimlinghaus, K.; Marx, R.; Serdar, M.; Bendix, I.; Dietzel, I.D. Strategies for repair of white matter: Influence of osmolarity and microglia on proliferation and apoptosis of oligodendrocyte precursor cells in different basal culture media. Front. Cell. Neurosci. 2013, 7, 277. [Google Scholar] [CrossRef]
- Vigneault, É.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of vesicular glutamate transporters in the human brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef]
- He, Y.; Taylor, N.; Yao, X.; Bhattacharya, A. Mouse primary microglia respond differently to LPS and poly(I:C) in vitro. Sci. Rep. 2021, 11, 10447. [Google Scholar] [CrossRef]
- Cuní-López, C.; Stewart, R.; Quek, H.; White, A.R. Recent Advances in Microglia Modelling to Address Translational Outcomes in Neurodegenerative Diseases. Cells 2022, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, J.; Xian, X.; Chen, Q. Isolation, culture, and characterization of duck primary neurons. Poult. Sci. 2023, 102, 102485. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Streit, W.J.; Bechmann, I. Beyond Activation: Characterizing Microglial Functional Phenotypes. Cells 2021, 10, 2236. [Google Scholar] [CrossRef]
- Green, T.R.F.; Rowe, R.K. Quantifying microglial morphology: An insight into function. Clin. Exp. Immunol. 2024, 216, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lier, J.; Winter, K.; Bleher, J.; Grammig, J.; Mueller, W.C.; Streit, W.; Bechmann, I. Loss of IBA1-Expression in brains from individuals with obesity and hepatic dysfunction. Brain Res. 2019, 1710, 220–229. [Google Scholar] [CrossRef]
- Waller, R.; Baxter, L.; Fillingham, D.J.; Coelho, S.; Pozo, J.M.; Mozumder, M.; Frangi, A.F.; Ince, P.G.; Simpson, J.E.; Highley, J.R. Iba-1-/CD68+ microglia are a prominent feature of age-associated deep subcortical white matter lesions. PLoS ONE 2019, 14, e0210888. [Google Scholar] [CrossRef]
- Fritze, J.; Muralidharan, C.; Stamp, E.; Ahlenius, H. Microglia undergo disease-associated transcriptional activation and CX3C motif chemokine receptor 1 expression regulates neurogenesis in the aged brain. Dev. Neurobiol. 2024, 84, 128–141. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Kim, W.; Kim, M.; Kim, B. Unraveling the enigma: Housekeeping gene Ugt1a7c as a universal biomarker for microglia. Front. Psychiatry 2024, 15, 1364201. [Google Scholar] [CrossRef]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.T.; van Roon-Mom, W.M.C.; Höllt, T.; van der Weerd, L. Co-expression patterns of microglia markers Iba1, TMEM119 and P2RY12 in Alzheimer’s disease. Neurobiol. Dis. 2022, 167, 105684. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kann, O.; Ohlemeyer, C.; Hanisch, U.-K.; Kettenmann, H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): Suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 2003, 23, 4410–4419. [Google Scholar] [CrossRef] [PubMed]
- Palomba, N.P.; Martinello, K.; Cocozza, G.; Casciato, S.; Mascia, A.; Di Gennaro, G.; Morace, R.; Esposito, V.; Wulff, H.; Limatola, C.; et al. ATP-evoked intracellular Ca2+ transients shape the ionic permeability of human microglia from epileptic temporal cortex. J. Neuroinflamm. 2021, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Beaino, W.; Janssen, B.; Kooij, G.; van der Pol, S.M.A.; van Het Hof, B.; van Horssen, J.; Windhorst, A.D.; de Vries, H.E. Purinergic receptors P2Y12R and P2X7R: Potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflamm. 2017, 14, 259. [Google Scholar] [CrossRef]
- Fei, X.; Yuan, W.; Zhao, Y.; Wang, H.; Bai, S.; Huang, Q. Papain Ameliorates the MPAs Formation-Mediated Activation of Monocytes by Inhibiting Cox-2 Expression via Regulating the MAPKs and PI3K/Akt Signal Pathway. BioMed Res. Int. 2018, 2018, 3632084. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Kang, H.-A.; Cominguez, D.C.; Kim, S.-H.; An, H.-J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9885. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, C.; Zhao, Y.; Huang, Q.; Yuan, W.; Wu, Y.; Fei, X. Papain ameliorates monocyte-platelet aggregate formation-mediated inflammatory responses in monocytes by upregulating miRNA-146a transcription. PLoS ONE 2022, 17, e0278059. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kang, Y.-M.; Lee, M.; An, H.-J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. [Google Scholar] [CrossRef] [PubMed]
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e8. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Dando, O.; Emelianova, K.; He, X.; McKay, S.; Hardingham, G.E.; Qiu, J. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep. 2021, 34, 108882. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.R.; Biundo, F.; Gökhan, Ş.; Chitu, V. Differential regulation of microglial states by colony stimulating factors. Front. Cell. Neurosci. 2023, 17, 1275935. [Google Scholar] [CrossRef] [PubMed]
- Frei, K.; Nohava, K.; Malipiero, U.V.; Schwerdel, C.; Fontana, A. Production of macrophage colony-stimulating factor by astrocytes and brain macrophages. J. Neuroimmunol. 1992, 40, 189–195. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumozov, I.A.; Mal’tseva, V.N.; Maiorov, S.A.; Kosenkov, A.M.; Gaidin, S.G. Papain Affects the Percentage and Morphology of Microglia in Hippocampal Neuron–Glial Cultures. Brain Sci. 2025, 15, 442. https://doi.org/10.3390/brainsci15050442
Tumozov IA, Mal’tseva VN, Maiorov SA, Kosenkov AM, Gaidin SG. Papain Affects the Percentage and Morphology of Microglia in Hippocampal Neuron–Glial Cultures. Brain Sciences. 2025; 15(5):442. https://doi.org/10.3390/brainsci15050442
Chicago/Turabian StyleTumozov, Ivan A., Valentina N. Mal’tseva, Sergei A. Maiorov, Artem M. Kosenkov, and Sergei G. Gaidin. 2025. "Papain Affects the Percentage and Morphology of Microglia in Hippocampal Neuron–Glial Cultures" Brain Sciences 15, no. 5: 442. https://doi.org/10.3390/brainsci15050442
APA StyleTumozov, I. A., Mal’tseva, V. N., Maiorov, S. A., Kosenkov, A. M., & Gaidin, S. G. (2025). Papain Affects the Percentage and Morphology of Microglia in Hippocampal Neuron–Glial Cultures. Brain Sciences, 15(5), 442. https://doi.org/10.3390/brainsci15050442







