Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Enrollment
2.2. Clinical Data Collection
2.3. Guidelines, Definitions, and Criteria Used in the Management of the Cohort of PLWH
2.4. Statistical Analysis
2.5. Ethical Issues
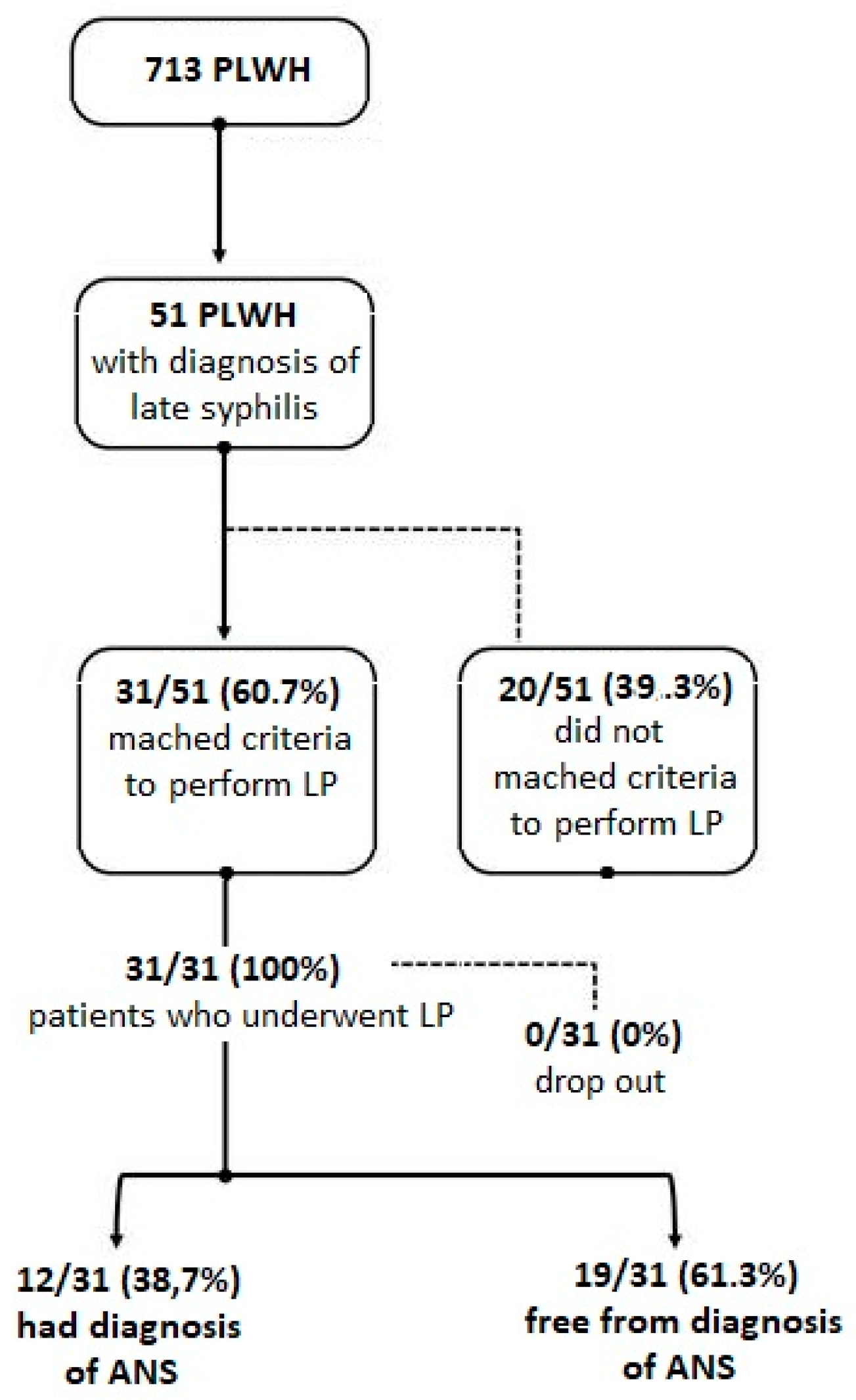
3. Results
3.1. Demographic and Clinical Characteristics of Patients Enrolled
3.2. Evaluation of ANS
3.3. Accuracy of Diagnostic Criteria Suggested to Perform LP for Suspicion of ANS
3.4. Correlation between Diagnosis of Asymptomatic NS and Nadir of CD4+
4. Discussion
5. Conclusions
Availability of Data and Material
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lynn, W.A.; Lightman, S. Syphilis and HIV: A dangerous combination. Lancet Infect. Dis. 2004, 4, 456–466. [Google Scholar] [CrossRef]
- Lang, R.; Read, R.; Krentz, H.B.; Peng, M.; Ramazani, S.; Vu, Q.; Gill, M.J. A retrospective study of the clinical features of new syphilis infections in an HIV-positive cohort in Alberta, Canada. BMJ Open 2018, 8, e021544. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.G.; Moore, R.D.; Rompalo, A.M.; Erbelding, E.J.; Zenilman, J.M.; Gebo, K.A. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS 2008, 22, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, E.; Vera, J.H.; Marks, M.; Barritt, A.W.; Ridha, B.H.; Lawrence, D. Neurosyphilis in patients with HIV. Pract. Neurol. 2018, 18, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Merins, V.; Hahn, K. Syphilis and neurosyphilis: HIV-coinfection and value of diagnostic parameters in cerebrospinal fluid. Eur. J. Med. Res. 2015, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.M.; Ghanem, K.G. Centers for disease control and prevention syphilis summit: Difficult clinical and patient management issues. Sex. Transm. Dis. 2018, 45, S10–S12. [Google Scholar] [CrossRef] [PubMed]
- Janier, Á.; Hegyi, V.; Dupin, N.; Unemo, M.; Tiplica, G.S.; Potočnik, M.; French, P.; Patel, R. European guideline on the management of syphilis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, A.; Ahmad, S.; Cousins, D.E.; Thng, C.M.; Vilar, F.J.; Higgins, S.P. Screening for asymptomatic neurosyphilis in HIV patients after treatment of early syphilis: An observational study. Sex. Transm. Infect. 2018, 94, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; Kressel, H.Y.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Zetola, N.M.; Klausner, J.D. Syphilis and HIV infection: An update. Clin. Infect. Dis. 2007, 44, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; d’Ettorre, G.; Gizzi, F.; Tierno, F.; Massetti, A.P.; Vullo, V. Ocular syphilis in an HIV-infected patient: Active disease or immune reconstitution syndrome? HAART Correl. Pathol. 2010, 7, 226–228. [Google Scholar]
- Ceccarelli, G.; d’Ettorre, G.; Carnevalini, M.; Mastroianni, C.M.; Vullo, V. Changes in natural course of syphilis in HIV infection. In Proceedings of the 3rd IAS Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil, 24–27 July 2005. Abstract no. TuPe1.2C08. [Google Scholar]
- Cai, S.N.; Long, J.; Chen, C.; Wan, G.; Lun, W.H. Incidence of asymptomatic neurosyphilis in serofast Chinese syphilis patients. Sci. Rep. 2017, 7, 15456. [Google Scholar] [CrossRef] [PubMed]
- Boulassel, M.R.; Chomont, N.; Pai, N.P.; Gilmore, N.; Sékaly, R.P.; Routy, J.P. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J. Clin. Virol. 2012, 53, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Massanella, M.; Puig, J.; Pérez-Alvarez, N.; Gallego-Escuredo, J.M.; Villarroya, J.; Villarroya, F.; Molto, J.; Santos, J.R.; Blanco, J.; et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: Clinical implications. Clin. Infect. Dis. 2010, 50, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Badiee, J.; Vaida, F.; Letendre, S.; Heaton, R.K.; Clifford, D.; Collier, A.C.; Gelman, B.; McArthur, J.; McCutchan, J.A.; et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011, 25, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Brothers, T.D.; Kirkland, S.; Theou, O.; Zona, S.; Malagoli, A.; Wallace, L.M.; Stentarelli, C.; Mussini, C.; Falutz, J.; Rockwood, K.; et al. Predictors of transitions in frailty severity and mortality among people aging with HIV. PLoS ONE 2017, 12, e0185352. [Google Scholar] [CrossRef] [PubMed]
- Landry, T.; Smyczek, P.; Cooper, R.; Gratrix, J.; Bertholet, L.; Read, R.; Romanowski, B.; Singh, A.E. Retrospective review of tertiary and neurosyphilis cases in Alberta, 1973–2017. BMJ Open 2019, 9, e025995. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, M.; Xu, D.; Kou, C.; Zhang, L.; Gao, J.; Qin, K.; Wu, W.; Zhang, X. Clinical and laboratory characteristics of symptomatic and asymptomatic neurosyphilis in HIV-negative patients: A retrospective study of 264 cases. Biomed. Res. Int. 2019, 2019, 2426313. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Total (n = 31) | Patients with ANS (n = 12) | Free for Diagnosis of ANS (n = 19) | OR | ||||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | (95% CI) | p-Value | |
| RPR ≥ 1:32 | 12/31 | (39) | 6/12 | (50) | 6/19 | (32) | 2.2 (0.5 to 9.6) | 0.307 |
| CD4+ ≤ 350 | 10/31 | (32) | 8/12 | (67) | 2/19 | (89) | 16.8 (2.5 to 70) | 0.003 |
| CD4+ ≤ 350 or RPR ≥ 1:32 | 14/31 | (45) | 8/12 | (67) | 6/19 | (32) | 10.8 (1.8 to 65) | 0.009 |
| CD4+ ≤ 350 and RPR ≥ 1:32 | 6/31 | (19) | 2/12 | (17) | 4/19 | (21) | 0.7 (0.11 to 4.9) | 0.763 |
| CD4+ ≤ 350 and/or RPR ≥ 1:32 | 20/31 | (64) | 10/12 | (83) | 10/19 | (53) | 4.5 (1 to 27) | 0.094 |
| Serological failure | 12/31 | (39) | 2/12 | (17) | 10/18 | (55) | 0.2 (0.1 to 1) | 0.057 |
| Patients with ANS | Free from Diagnosis of ANS | Sensitivity | Specificity | NPV | Accuracy | AUC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Condition | No. | No. | (%) | 95%CI | (%) | 95%CI | (%) | (%) | (%) |
| RPR ≥ 1:32 | Yes | 6 | 6 | 67% | 48% to 83% | 59% | 39% to 75% | 74% | 62% | 53% |
| No | 6 | 13 | ||||||||
| CD4+ ≤ 350 | Yes | 8 | 2 | 75% | 42% to 94% | 70% | 46% to 88% | 82% | 74% | 72% |
| No | 4 | 17 | ||||||||
| CD4+ ≤ 350 or RPR ≥ 1:32 | Yes | 8 | 6 | 83% | 36% to 98% | 61% | 41% to 79% | 94% | 82% | 76% |
| No | 4 | 13 | ||||||||
| CD4+ ≤ 350 and RPR ≥ 1:32 | Yes | 2 | 4 | 50% | 30% to 70% | 67% | 44% to 96% | 87% | 59% | 58% |
| No | 10 | 15 | ||||||||
| CD4+ ≤ 350 and/or RPR ≥ 1:32 | Yes | 10 | 10 | 62% | 51% to 98% | 83% | 42% to 79% | 94% | 82% | 73% |
| No | 2 | 9 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, G.; Borrazzo, C.; Lazzaro, A.; Innocenti, G.P.; Celani, L.; Cavallari, E.N.; Pinacchio, C.; Santinelli, L.; Mastroianni, C.M.; d’Ettorre, G. Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study. Brain Sci. 2019, 9, 278. https://doi.org/10.3390/brainsci9100278
Ceccarelli G, Borrazzo C, Lazzaro A, Innocenti GP, Celani L, Cavallari EN, Pinacchio C, Santinelli L, Mastroianni CM, d’Ettorre G. Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study. Brain Sciences. 2019; 9(10):278. https://doi.org/10.3390/brainsci9100278
Chicago/Turabian StyleCeccarelli, Giancarlo, Cristian Borrazzo, Alessandro Lazzaro, Giuseppe Pietro Innocenti, Luigi Celani, Eugenio Nelson Cavallari, Claudia Pinacchio, Letizia Santinelli, Claudio Maria Mastroianni, and Gabriella d’Ettorre. 2019. "Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study" Brain Sciences 9, no. 10: 278. https://doi.org/10.3390/brainsci9100278
APA StyleCeccarelli, G., Borrazzo, C., Lazzaro, A., Innocenti, G. P., Celani, L., Cavallari, E. N., Pinacchio, C., Santinelli, L., Mastroianni, C. M., & d’Ettorre, G. (2019). Diagnostic Issues of Asymptomatic Neurosyphilis in HIV-Positive Patients: A Retrospective Study. Brain Sciences, 9(10), 278. https://doi.org/10.3390/brainsci9100278








