Clinical Role of Aspirin in Mood Disorders: A Systematic Review
Abstract
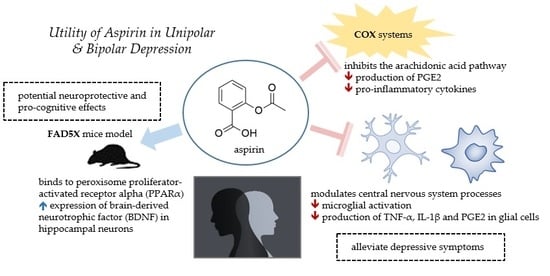
:1. Introduction
2. Methods
2.1. Search Strategy and Study Identification
2.2. Study Selection Criteria and Eligibility Criteria
2.3. Data Extraction and Risk of Bias
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Klein, D.N.; Santiago, N.J. Dysthymia and chronic depression: Introduction, classification, risk factors, and course. J. Clin. Psychol. 2003, 59, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Katakam, K.K.; Schou, A.; Hellmuth, S.G.; Stallknecht, S.E.; Leth-Møller, K.; Iversen, M.; Banke, M.B.; Petersen, I.J.; Klingenberg, S.L.; et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 2017, 17, 58. [Google Scholar]
- Khin, N.A.; Chen, Y.F.; Yang, Y.; Yang, P.; Laughren, T.P. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J. Clin. Psychiatry 2011, 72, 464–472. [Google Scholar] [CrossRef]
- Ng, Q.X.; Yeo, W.S.; Sivalingam, V. Lithium-associated Renal Dysfunction: To Stop or Not to Stop? Bipolar Disord. 2019. [Google Scholar] [CrossRef]
- Popovic, D.; Reinares, M.; Goikolea, J.M.; Bonnin, C.M.; Gonzalez-Pinto, A.; Vieta, E. Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur. Neuropsychopharmacol. 2012, 22, 339–346. [Google Scholar] [CrossRef]
- Bauer, M.; Glenn, T.; Alda, M.; Grof, P.; Sagduyu, K.; Bauer, R.; Lewitzka, U.; Whybrow, P.C. Comparison of pre-episode and pre-remission states using mood ratings from patients with bipolar disorder. Pharmacopsychiatry 2011, 44, S49–S53. [Google Scholar] [CrossRef]
- Ogawa, H.; Nakayama, M.; Morimoto, T.; Uemura, S.; Kanauchi, M.; Doi, N.; Jinnouchi, H.; Sugiyama, S.; Saito, Y. Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. JAMA 2008, 300, 2134–2141. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Garner, C.; Seibert, K.; Marnett, L.J. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 1998, 280, 1268–1270. [Google Scholar] [CrossRef]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Jung, H.G.; Myint, A.M.; Kim, H.; Park, S.H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Jacka, F.N.; Williams, L.J.; Henry, M.J.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Clinical implications of the cytokine hypothesis of depression: The association between use of statins and aspirin and the risk of major depression. Psychother. Psychosom. 2010, 79, 323. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wells, G.A. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 June 2019).
- Veronese, N.; Koyanagi, A.; Stubbs, B.; Solmi, M.; Fornaro, M.; Fernandes, B.S.; Muller, C.; Thompson, T.; Carvalho, A.F.; Maggi, S. Aspirin and incident depressive symptoms: A longitudinal cohort study over 8 years. Int. J. Geriatr. Psychiatr 2018, 33, e193–e198. [Google Scholar] [CrossRef]
- Saroukhani, S.; Emami-Parsa, M.; Modabbernia, A.; Ashrafi, M.; Farokhnia, M.; Hajiaghaee, R.; Akhondzadeh, S. Aspirin for treatment of lithium-associated sexual dysfunction in men: Randomized double-blind placebo-controlled study. Bipolar Disord. 2013, 15, 650–656. [Google Scholar] [CrossRef]
- Savitz, J.B.; Teague, T.K.; Misaki, M.; Macaluso, M.; Wurfel, B.E.; Meyer, M.; Drevets, D.; Yates, W.; Gleason, O.; Drevets, W.C.; et al. Treatment of bipolar depression with minocycline and/or aspirin: An adaptive, 2 × 2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl. Psychiatry 2018, 8, 27. [Google Scholar] [CrossRef]
- Stolk, P.; Souverein, P.C.; Wilting, I.; Leufkens, H.G.; Klein, D.F.; Rapoport, S.I.; Heerdink, E.R. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.J.; Pasco, J.A.; Mohebbi, M.; Jacka, F.N.; Stuart, A.L.; Venugopal, K.; O’Neil, A.; Berk, M. Statin and aspirin use and the risk of mood disorders among men. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef]
- Sidor, M.M.; MacQueen, G.M. An update on antidepressant use in bipolar depression. Curr. Psychiatry Rep. 2012, 14, 696–704. [Google Scholar] [CrossRef]
- Rao, J.S.; Harry, G.J.; Rapoport, S.I.; Kim, H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 2010, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Casolini, P.; Catalani, A.; Zuena, A.R.; Angelucci, L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J. Neurosci. Res. 2002, 68, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Compton, M.T.; Nemeroff, C.B. The treatment of bipolar depression. J. Clinic. Psychiatry 2000, 61, 57–67. [Google Scholar]
- Nahman, S.; Belmaker, R.H.; Azab, A.N. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Innate Immun. 2012, 18, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Feijó, D.P.; Batista-Silva, H.; Garcez, M.L.; Mina, F.; Belletini-Santos, T.; Krasilchik, L.R.; Luz, A.P.; Schiavo, G.L.; Quevedo, J. Lithium and memantine improve spatial memory impairment and neuroinflammation induced by β-amyloid 1-42 oligomers in rats. Neurobiol. Learn. Mem. 2017, 141, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.; Veerhuis, R.; Janssen, I.; van Elk, E.J.; Rozemuller, A.J.; Eikelenboom, P. The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E2 secretion by cultured human adult microglia: Implications for Alzheimer’s disease. Brain Res. 2002, 951, 218–226. [Google Scholar] [CrossRef]
- Söderlund, J.; Olsson, S.K.; Samuelsson, M.; Walther-Jallow, L.; Johansson, C.; Erhardt, S.; Landén, M.; Engberg, G. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J. Psychiatry Neurosci. JPN 2011, 36, 114. [Google Scholar] [CrossRef]
- Patel, D.; Roy, A.; Kundu, M.; Jana, M.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. Aspirin binds to PPARα to stimulate hippocampal plasticity and protect memory. Proc. Nat. Acad. Sci. USA 2018, 115, E7408–E7417. [Google Scholar] [CrossRef]
- MacQueen, G.M.; Memedovich, K.A. Cognitive dysfunction in major depression and bipolar disorder: A ssessment and treatment options. Psychiatry Clin. Neurosci. 2017, 71, 18–27. [Google Scholar] [CrossRef]
- Chang, C.W.; Horng, J.T.; Hsu, C.C.; Chen, J.M. Mean daily dosage of aspirin and the risk of incident Alzheimer’s dementia in patients with type 2 diabetes mellitus: A nationwide retrospective cohort study in Taiwan. J. Diabetes Res. 2016, 2016. [Google Scholar] [CrossRef]
- Lu, Y.; Ho, C.S.; McIntyre, R.S.; Wang, W.; Ho, R.C. Effects of vortioxetine and fluoxetine on the level of Brain Derived Neurotrophic Factors (BDNF) in the hippocampus of chronic unpredictable mild stress-induced depressive rats. Brain Res. Bull. 2018, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Hader, W.; Yong1, V.W. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. J. Leukoc. Biol. 2005, 78, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblat, J.D.; McIntyre, R.S. Efficacy and tolerability of minocycline for depression: A systematic review and meta-analysis of clinical trials. J. Affect. Disord. 2018, 227, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.A.; Mallien, A.S.; Pfeiffer, N.; Inta, I.; Gass, P.; Inta, D. Minocycline does not evoke anxiolytic and antidepressant-like effects in C57BL/6 mice. Behav. Brain Res. 2016, 301, 96–101. [Google Scholar] [CrossRef]
- Gao, R.; Li, X. Risk assessment and aspirin use in Asian and Western populations. Vasc. Health Risk Manag. 2010, 6, 943. [Google Scholar]
- Jonker, C.; Comijs, H.C.; Smit, J.H. Does aspirin or other NSAIDs reduce the risk of cognitive decline in elderly persons? Results from a population-based study. Neurobiol. Aging 2003, 24, 583–588. [Google Scholar] [CrossRef]
- Saito, Y.; Okada, S.; Ogawa, H.; Soejima, H.; Sakuma, M.; Nakayama, M.; Doi, N.; Jinnouchi, H.; Waki, M.; Masuda, I.; et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation 2017, 135, 659–670. [Google Scholar] [CrossRef]


| Author, Year | Study Design | Sample Size (N) | Study Population and Duration | Country of Origin | Conclusions |
|---|---|---|---|---|---|
| Saroukhani, 2013 [18] | Randomized, placebo-controlled, double-blind | 32 | Males with stable bipolar affective disorder (DSM-IV-TR) on maintenance lithium therapy; 6 weeks | Iran | Patients who received aspirin (240 mg/day) had significant improvements in total sexual function and erective function domain scores than placebo group. Baseline and endpoint serum lithium concentrations and mood symptoms remained stable throughout the duration of the study. |
| Savitz, 2018 [19] | Multi-site, randomized, placebo-controlled, double-blind | 99 | At least moderately depressed psychiatric outpatients with Bipolar I, II or NOS (DSM-IV-TR criteria); 6 weeks | United States | Active minocycline (100 mg twice daily) and aspirin (81 mg twice daily) significantly improved depressive symptoms. There was a main effect of aspirin on treatment response. |
| Stolk, 2010 [20] | Retrospective linkage record | 5145 | Patients ≥18 years old, who had been dispensed at least five prescriptions for lithium; 10-year period of observation | Netherlands | Presumably, low-dose ASA (30 or 80 mg/day) significantly reduced the relative risk of clinical deteriorations in patients on lithium (adjusted incidence density of medication events (dose increase or drug change) was 0.84, 95% CI: 0.75 to 0.94). |
| Pasco, 2010 [14] | Study 1: Nested case–control | 386 | Community-dwelling females; followed for 10 years; population derived from the Geelong Osteoporosis Study | Australia | ASA use associated with protective effect against major depression (age-adjusted OR 0.18, 95% CI: 0.02 to 1.39, p = 0.1). |
| Study 2: Retrospective cohort | 345 | Reduced risk of major depression in individuals with history of ASA and statin exposure (HR 0.20, 95% CI: 0.04 to 0.85, p = 0.03). | |||
| Veronese, 2018 [17] | Longitudinal cohort | 4070 | Community-dwelling adults; followed for 8 years; population derived from ongoing multicenter, longitudinal Osteoarthritis Initiative (OAI) study | United States | Adjusting for confounders, ASA use did not protect against incident depressive symptoms over the study period of 8 years (HR 1.12; 95% CI: 0.78 to 1.62, p = 0.54). |
| Williams, 2016 [21] | Study 1: Nested case–control | 937 | Community-dwelling males, 24–98 years old; followed for 5 years; population derived from ongoing Geelong Osteoporosis Study | Australia | After adjustment for age and antidepressant use, exposure to ASA was associated with a reduced likelihood of major depression (OR 0.4, 95% CI: 0.2 to 0.9, p = 0.03). |
| Study 2: Retrospective cohort | 836 | Reduced risk of major depression in individuals with history of ASA and statin use (HR 0.55, 95% CI: 0.23 to 1.32, p = 0.18). |
| Study | Representativeness of the Exposed Cohort a | Selection of the Non-Exposed Cohort a | Ascertainment of Exposure a | Demonstration that Outcome of Interest was Not Present at Start of Study a | Comparability of Cohorts b | Assessment of Outcome a | Follow-up Duration a | Follow-up Adequacy a |
|---|---|---|---|---|---|---|---|---|
| Pasco, 2010 (Study 2) [14] | * | * | * | * | ** | * | * | * |
| Veronese, 2018 [17] | * | * | * | * | ** | * | * | * |
| Williams, 2016 (Study 2) [21] | * | * | * | * | ** | * | * | * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.X.; Ramamoorthy, K.; Loke, W.; Lee, M.W.L.; Yeo, W.S.; Lim, D.Y.; Sivalingam, V. Clinical Role of Aspirin in Mood Disorders: A Systematic Review. Brain Sci. 2019, 9, 296. https://doi.org/10.3390/brainsci9110296
Ng QX, Ramamoorthy K, Loke W, Lee MWL, Yeo WS, Lim DY, Sivalingam V. Clinical Role of Aspirin in Mood Disorders: A Systematic Review. Brain Sciences. 2019; 9(11):296. https://doi.org/10.3390/brainsci9110296
Chicago/Turabian StyleNg, Qin Xiang, Krishnapriya Ramamoorthy, Wayren Loke, Matthew Wei Liang Lee, Wee Song Yeo, Donovan Yutong Lim, and Vivekanandan Sivalingam. 2019. "Clinical Role of Aspirin in Mood Disorders: A Systematic Review" Brain Sciences 9, no. 11: 296. https://doi.org/10.3390/brainsci9110296
APA StyleNg, Q. X., Ramamoorthy, K., Loke, W., Lee, M. W. L., Yeo, W. S., Lim, D. Y., & Sivalingam, V. (2019). Clinical Role of Aspirin in Mood Disorders: A Systematic Review. Brain Sciences, 9(11), 296. https://doi.org/10.3390/brainsci9110296