The Task at Hand: Fatigue-Associated Changes in Cortical Excitability During Writing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Experimental Set-Up and Recordings
2.3.1. Electromyography and Force Recordings
2.3.2. Transcranial Magnetic Stimulation
2.3.3. Motor Evoked Potentials (MEPs)
2.3.4. Cortical Silent Period
2.4. Statistical Analysis
3. Results
3.1. Baseline Measures
3.2. Effect of Task
Motor Evoked Potentials
3.3. Muscle Activity
3.4. Effect of Task on Fatigue
3.4.1. Motor Evoked Potentials
3.4.2. Muscle Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of Transcranial Magnetic Stimulation to the Understanding of Cortical Mechanisms Involved in Motor Control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, J.M. On Task: Considerations and Future Directions for Studies of Corticospinal Excitability in Exercise Neuroscience and Related Disciplines. Appl. Physiol. Nutr. Metab. 2018, 43, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, J. State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr. 2008, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Farina, S.; Tamburin, S.; Facchini, S.; Fiaschi, A.; Restivo, D.; Berardelli, A. Task-Dependent Modulation of Excitatory and Inhibitory Functions within the Human Primary Motor Cortex. Exp. Brain Res. 2003, 150, 222–229. [Google Scholar] [CrossRef]
- Datta, A.K.; Harrison, L.M.; Stephens, J.A. Task-Dependent Changes in the Size of Response to Magnetic Brain Stimulation in Human First Dorsal Interosseous Muscle. J. Physiol. 1989, 418, 13–23. [Google Scholar] [CrossRef]
- Bunday, K.L.; Tazoe, T.; Rothwell, J.C.; Perez, M.A. Subcortical Control of Precision Grip after Human Spinal Cord Injury. J. Neurosci. 2014, 34, 7341–7350. [Google Scholar] [CrossRef]
- Tazoe, T.; Perez, M.A. Cortical and Reticular Contributions to Human Precision and Power Grip. J. Physiol. 2017, 595, 2715–2730. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Kiernan, M.C.; Vucic, S. Motor Cortical Function and the Precision Grip. Physiol. Rep. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Muir, R.B.; Lemon, R.N. Corticospinal Neurons with a Special Role in Precision Grip. Brain Res. 1983, 261, 312–316. [Google Scholar] [CrossRef]
- Pearce, A.J.; Kidgell, D.J. Comparison of Corticomotor Excitability during Visuomotor Dynamic and Static Tasks. J. Sci. Med. Sport 2010, 13, 167–171. [Google Scholar] [CrossRef]
- Flament, B.Y.D.; Goldsmith, P.; Buckley, C.J.; Lemon, R.N. Task Dependence of Responses in First Dorsal Interosseous Muscle to Magnetic Brain Stimulation in Man. J. Physiol. 1993, 464, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Krakauer, J.W. The Uses and Interpretations of the Motor-Evoked Potential for Understanding Behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Gowen, E.; Miall, R.C. Differentiation between External and Internal Cuing: An FMRI Study Comparing Tracing with Drawing. Neuroimage 2007, 36, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.L.; Spivey, M.J.; Balasubramaniam, R. Corticospinal Excitability during the Processing of Handwritten and Typed Words and Non-Words. Neurosci. Lett. 2017, 651, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, M.; Thabit, M.N.; Koganemaru, S.; Nojima, I.; Fukuyama, H.; Mima, T. Writing’s Shadow: Corticospinal Activation during Letter Observation. J. Cogn. Neurosci. 2012, 24, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Gruet, M.; Temesi, J.; Rupp, T.; Levy, P.; Millet, G.Y.; Verges, S. Stimulation of the Motor Cortex and Corticospinal Tract to Assess Human Muscle Fatigue. Neuroscience 2013, 231, 384–399. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. Transcranial Magnetic Stimulation and Human Muscle Fatigue. Muscle Nerve 2001, 24. [Google Scholar] [CrossRef]
- Benwell, N.; Sacco, P.; Hammond, G.; Byrnes, M.L.; Mastaglia, F.L.; Thickbroom, G.W. Short-Interval Cortical Inhibition and Corticomotor Excitability with Fatiguing Hand Exercise: A Central Adaptation to Fatigue? Exp. Brain Res. 2006, 170, 191–198. [Google Scholar] [CrossRef]
- Liepert, J.; Kotterba, S.; Tegenthoff, M.; Malin, J.-P. Central Fatigue Assessed By Transcranial Magnetic Stimulation. Med. Sci. Sports Exerc. 1996, 19, 1166–1175. [Google Scholar] [CrossRef]
- Samii, A.; Wassermann, E.M.; Ikoma, K.; Mercuri, B.; George, M.S.; O’Fallon, A.; Dale, J.K.; Straus, S.E.; Hallett, M. Decreased Postexercise Facilitation of Motor Evoked Potentials in Patients with Chronic Fatigue Syndrome or Depression. Neurology 1996, 47, 1410–1414. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Cohen, L.G.; Hallett, M. Central Fatigue as Revealed by Postexercise Decrement of Motor Evoked Potentials. Muscle Nerve 1994, 17, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Sharples, S.A.; Gould, J.A.; Vandenberk, M.S.; Kalmar, J.M. Cortical Mechanisms of Central Fatigue and Sense of Effort. PLoS ONE 2016, 1–21. [Google Scholar] [CrossRef]
- Taylor, J.L.; Butler, J.E.; Allen, G.M.; Gandevia, S.C. Changes in Motor Cortical Excitability during Human Muscle Fatigue. J. Physiol. 1996, 490, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Butler, J.E.; Gandevia, S.C. Altered Responses of Human Elbow Flexors to Peripheral-Nerve and Cortical Stimulation during a Sustained Maximal Voluntary Contraction. Exp. Brain Res. 1999, 127, 108–115. [Google Scholar] [CrossRef]
- Dragovic, M.; Hammond, G. A Classification of Handedness Using the Annett Hand Preference Questionnaire. Br. J. Psychol. 2007, 98, 375–387. [Google Scholar] [CrossRef]
- Matamala, J.M.; Howells, J.; Dharmadasa, T.; Trinh, T.; Ma, Y.; Lera, L.; Vucic, S.; Burke, D.; Kiernan, M.C. Inter-Session Reliability of Short-Interval Intracortical Inhibition Measured by Threshold Tracking TMS. Neurosci. Lett. 2018, 674, 18–23. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Van Campen, A.D.; Neubert, F.; Wery, P.; Van Den Wildenberg, M.; Ridderinkhof, K.R.; Mars, R.B. Paired-Pulse Transcranial Magnetic Stimulation Reveals Probability-Dependent Changes in Functional Connectivity between Right Inferior Frontal Cortex and Primary Motor Cortex during Go/No-Go Performance. Front. Hum. Neurosci. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Darling, W.G.; Wolf, S.L.; Butler, A.J. Variability of Motor Potentials Evoked by Transcranial Magnetic Stimulation Depends on Muscle Activation. Exp. Brain Res. 2006, 174, 376–385. [Google Scholar] [CrossRef]
- Devanne, H.; Lavoie, B.A.; Capaday, C. Input-Output Properties and Gain Changes in the Human Corticospinal Pathway. Exp. Brain Res. 1997, 114, 329–338. [Google Scholar] [CrossRef]
- Kiers, L.; Cros, D.; Chiappa, K.H.; Fang, J. Variability of Motor Potentials Evoked by Transcranial Magnetic Stimulation. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials 1993, 89, 415–423. [Google Scholar] [CrossRef]
- Van Hedel, H.J.A.; Murer, C.; Dietz, V.; Curt, A. The Amplitude of Lower Leg Motor Evoked Potentials Is a Reliable Measure When Controlled for Torque and Motor Task. J. Neurol. 2007, 254, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kouchtir-Devanne, N.; Capaday, C.; Cassim, F.; Derambure, P.; Devanne, H. Task-Dependent Changes of Motor Cortical Network Excitability during Precision Grip Compared to Isolated Finger Contraction. J. Neurophysiol. 2012, 107, 1522–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsinger, C.L.; Harrington, D.L.; Rao, S.M. From Preparation to Online Control: Reappraisal of Neural Circuitry Mediating Internally Generated and Externally Guided Actions. Neuroimage 2006, 31, 1177–1187. [Google Scholar] [CrossRef]
- Debaere, F.; Wenderoth, N.; Sunaert, S.; Van Hecke, P.; Swinnen, S.P. Internal vs External Generation of Movements: Differential Neural Pathways Involved in Bimanual Coordination Performed in the Presence or Absence of Augmented Visual Feedback. Neuroimage 2003, 19, 764–776. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Gallea, C.; Najee-ullah, M.A.; Hallett, M. Functional Anatomy of Writing with the Dominant Hand. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Jones, D.; Christensen, C.A. Relationship between Automaticity in Handwriting and Students’ Ability to Generate Written Text. J. Educ. Psychol. 1999, 91, 44–49. [Google Scholar] [CrossRef]
- Devanne, H.; Cohen, L.G.; Kouchtir-Devanne, N.; Capaday, C. Integrated Motor Cortical Control of Task-Related Muscles during Pointing in Humans. J. Neurophysiol. 2002, 87, 3006–3017. [Google Scholar] [CrossRef]
- Boyadjian, A.; Tyc, F.; Allam, N.; Brasil-Neto, J.P. Writer’s Cramp: Cortical Excitability in Tasks Involving Proximo-Distal Coordination. Acta Physiol. Scand. 2011, 203, 321–330. [Google Scholar] [CrossRef]
- Gagné, M.; Schneider, C. Dynamic Changes in Corticospinal Control of Precision Grip during Wrist Movements. Brain Res. 2007, 1164, 32–43. [Google Scholar] [CrossRef]
- Perez, M.A.; Rothwell, J.C. Distinct Influence of Hand Posture on Cortical Activity during Human Grasping. J. Neurosci. 2015, 35, 4882–4889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmar, J.M.; Cafarelli, E. Central Fatigue and Transcranial Magnetic Stimulation: Effect of Caffeine and the Confound of Peripheral Transmission Failure. J. Neurosci. Methods 2004, 138, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Neto, J.P.; Pascual-Leone, A.; Valls-Solé, J.; Cammarota, A.; Cohen, L.G.; Hallett, M. Postexercise Depression of Motor Evoked Potentials: A Measure of Central Nervous System Fatigue. Exp. Brain Res. 1993, 93, 181–184. [Google Scholar] [CrossRef]
- Gandevia, S.C.; Petersen, N.; Butler, J.E.; Taylor, J.L. Impaired Response of Human Motoneurones to Corticospinal Stimulation after Voluntary Exercise. J. Physiol. 1999, 521, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, A.; Matsunaga, K.; Tanaka, N.; Rothwell, J.C. Muscle Fatigue Decreases Short-Interval Intracortical Inhibition after Exhaustive Intermittent Tasks. Clin. Neurophysiol. 2006, 117, 864–870. [Google Scholar] [CrossRef]
- Latella, C.; Hendy, A.; Vanderwesthuizen, D.; Teo, W. The Modulation of Corticospinal Excitability and Inhibition Following Acute Resistance Exercise in Males and Females Acute Resistance Exercise in Males and Females. Eur. J. Sport Sci. 2018, 1391. [Google Scholar] [CrossRef]
- Hunter, S.K.; McNeil, C.J.; Butler, J.E.; Gandevia, S.C.; Taylor, J.L. Short-Interval Cortical Inhibition and Intracortical Facilitation during Submaximal Voluntary Contractions Changes with Fatigue. Exp. Brain Res. 2016, 234, 2541–2551. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.S.; Hoffman, R.L.; Clark, B.C. Cortical and Spinal Mechanisms of Task Failure of Sustained Submaximal Fatiguing Contractions. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Sharples, S.A.; Kalmar, J.M. Modulation of Cortical Excitability and Interhemispheric Inhibition Prior to Rhythmic Unimanual Contractions. J. Neurosci. Methods 2012, 210, 178–186. [Google Scholar] [CrossRef]
- Popescu, I.M.; Vaidya, N.A. Isolated Inability to Write Cursively after Transient Ischemic Attack (TIA). Cogn. Behav. Neurol. 2007, 20, 131–135. [Google Scholar] [CrossRef]
- Matiello, M.; Zimmerman, E.; Caplan, D.; Cohen, A.B. Reversible Cursive Agraphia. Neurology 2015, 85, 95–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Abduction Day | Writing Day | |
|---|---|---|
| Maximal Force (N) | 19.49 ± 5.80 | 24.05 ± 11.38 |
| Maximal FDI RMS amplitude (mV) | 0.91 ± 0.38 | 0.94 ± 0.40 |
| Writing EMG (% max) | 18.7 ± 6.88 | 20.5 ± 9.62 |
| AMT (%MSO) | 39.1 ± 8.20 | 41.0 ± 7.30 |
| # of Fatiguing Trials | 43.50 ± 26.34 | 48.83 ± 45.34 |
| Abduction Task | Writing Task | Effects | ||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Pre-Fatigue | Post-Fatigue | % Change Effect Size | Pre-Ftg | Post-Ftg | % Change Effect Size | F Value § (Task) | F Value (Task × Fatigue) |
| Test MEP (n = 19) | 1.64 ± 1.11 | 1.54 ± 1.13 | −5.9% d = 0.09 | 1.85 ± 1.53 | 1.64 ± 1.65 | −11.2% d = 0.13 | F(1,18) = 0.60, p = 0.45, ηp2 = 0.03 | F(1,18) = 2.22, p = 0.15, ηp2 = 0.11 |
| SICI (n = 17) | 0.95 ± 0.22 | 0.94 ± 0.30 | −0.7% d = 0.03 | 0.82 ± 0.22 | 0.86 ± 0.21 | 4.1% d = 0.16 | F(1,16) = 4.40, p = 0.052, ηp2 = 0.22 | F(1,16) = 0.36, p = 0.56, ηp2 = 0.02 |
| ICF (n = 19) | 1.25 ± 0.33 | 1.19 ± 0.30 | −4.2% d = 0.16 | 1.13 ± 0.28 | 1.26 ± 0.33 | 11% d = 0.41 | F(1,18) = 1.69, p = 0.21, ηp2 = 0.09 | F(1,18) = 3.60, p = 0.07, ηp2 = 0.17 |
| Abduction Task | Writing Task | Effects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Method (n) | Pre-Fatigue | Post-Fatigue | % Change Effect Size | Pre-Fatigue | Post-Fatigue | % Change Effect Size | F Value § (Task × Style) | F Value (Task × Fatigue × Style) |
| Test MEP | Printers (8) | 1.49 ± 1.16 | 1.54 ± 0.82 | 3.0% d = 0.05 | 1.96 ± 1.54 | 1.77 ± 1.50 | −10.0% d = 0.13 | F(1,14) = 2.95, p = 0.11, ηp2 = 0.17 | F(1,14) = 0.77, p = 0.40, ηp2 = 0.05 |
| Cursive writers (8) | 1.93 ± 1.16 | 1.90 ± 1.43 | −1.4% d = 0.02 | 2.09 ± 1.78 | 1.86 ± 2.08 | −10.9% d = 0.12 | |||
| SICI | Printers (7) | 1.03 ± 0.28 | 1.05 ± 0.25 | 1.5% d = 0.06 | 0.71 ± 0.13 | 0.86 ± 0.16 | 20.4% d = 1.02 | F(1,13) = 8.00, p = 0.01, ηp2 = 0.38 | F(1,13) = 0.56, p = 0.47, ηp2 =0.04 |
| Cursive writers (8) | 0.87 ± 0.17 | 0.81 ± 0.33 | −6.6% d = 0.22 | 0.86 ± 0.26 | 0.82 ± 0.26 | −4.1% d = 0.13 | |||
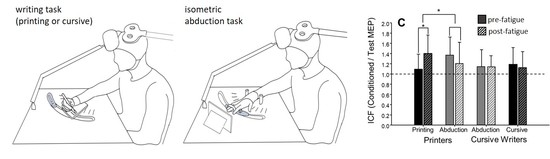
| ICF | Printers (8) | 1.37 ± 0.35 | 1.21 ± 0.41 | −12.0% d = 0.43 | 1.09 ± 0.29 | 1.40 ± 0.36 | 28.1% d = 0.94 | F(1,14) = 2.71, p = 0.12, ηp2 = 0.16 | F(1,14) = 9.90, p = 0.007, ηp2 =0.41 |
| Cursive Writers (8) | 1.15 ± 0.33 | 1.14 ± 0.21 | −0.4% d = 0.02 | 1.19 ± 0.33 | 1.12 ± 0.31 | −5.6% d = 0.21 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinelli, K.T.M.; Green, L.A.; Kalmar, J.M. The Task at Hand: Fatigue-Associated Changes in Cortical Excitability During Writing. Brain Sci. 2019, 9, 353. https://doi.org/10.3390/brainsci9120353
Cinelli KTM, Green LA, Kalmar JM. The Task at Hand: Fatigue-Associated Changes in Cortical Excitability During Writing. Brain Sciences. 2019; 9(12):353. https://doi.org/10.3390/brainsci9120353
Chicago/Turabian StyleCinelli, Kezia T. M., Lara A. Green, and Jayne M. Kalmar. 2019. "The Task at Hand: Fatigue-Associated Changes in Cortical Excitability During Writing" Brain Sciences 9, no. 12: 353. https://doi.org/10.3390/brainsci9120353
APA StyleCinelli, K. T. M., Green, L. A., & Kalmar, J. M. (2019). The Task at Hand: Fatigue-Associated Changes in Cortical Excitability During Writing. Brain Sciences, 9(12), 353. https://doi.org/10.3390/brainsci9120353