Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults
Abstract
:1. Active Navigation in Virtual Environments Benefits Older Adults’ Spatial Memory
2. Active vs. Passive Encoding of Spatial Information
3. Using Virtual Reality to Examine Spatial Memory
4. Method
4.1. Participants
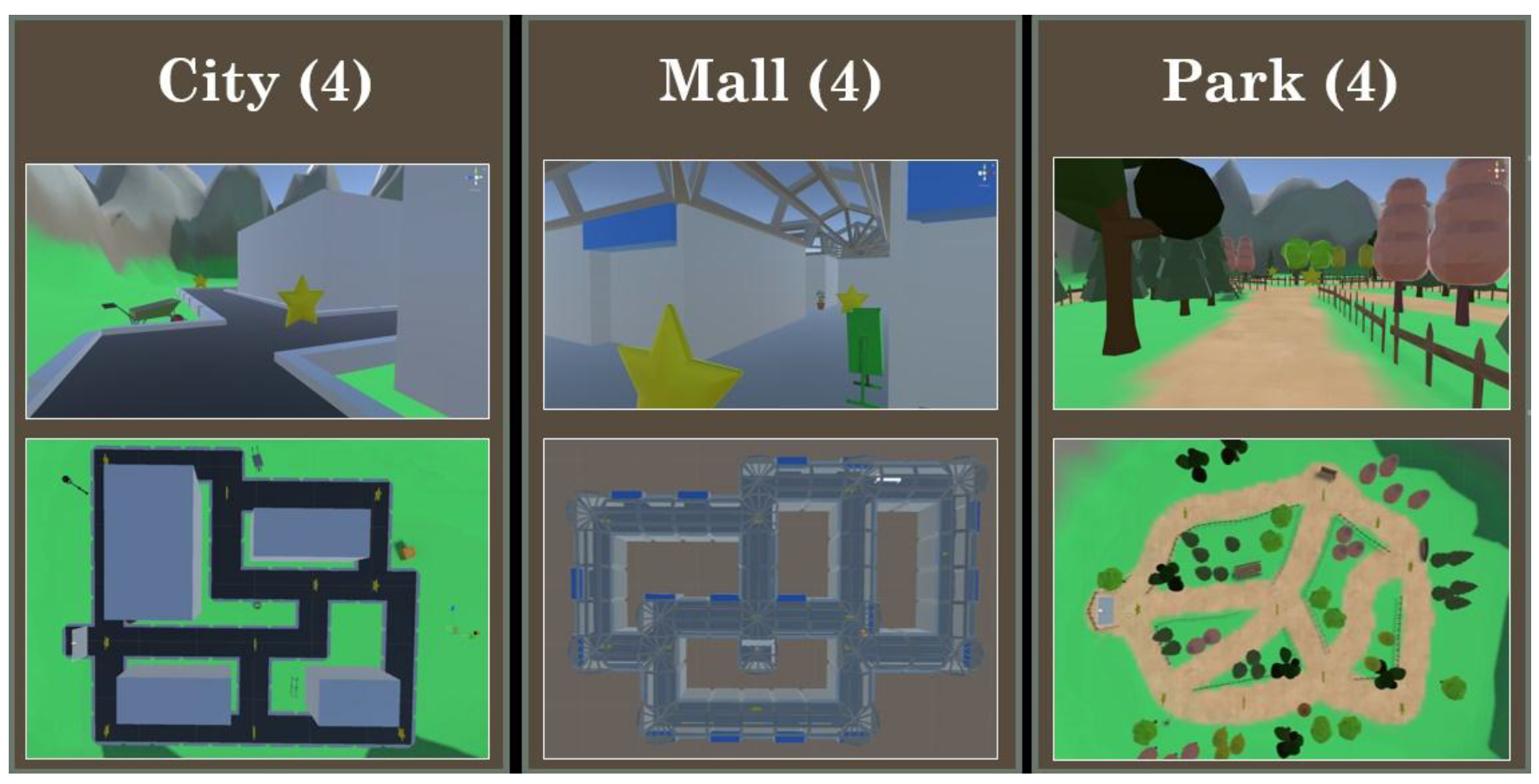
4.2. Materials
5. Procedure
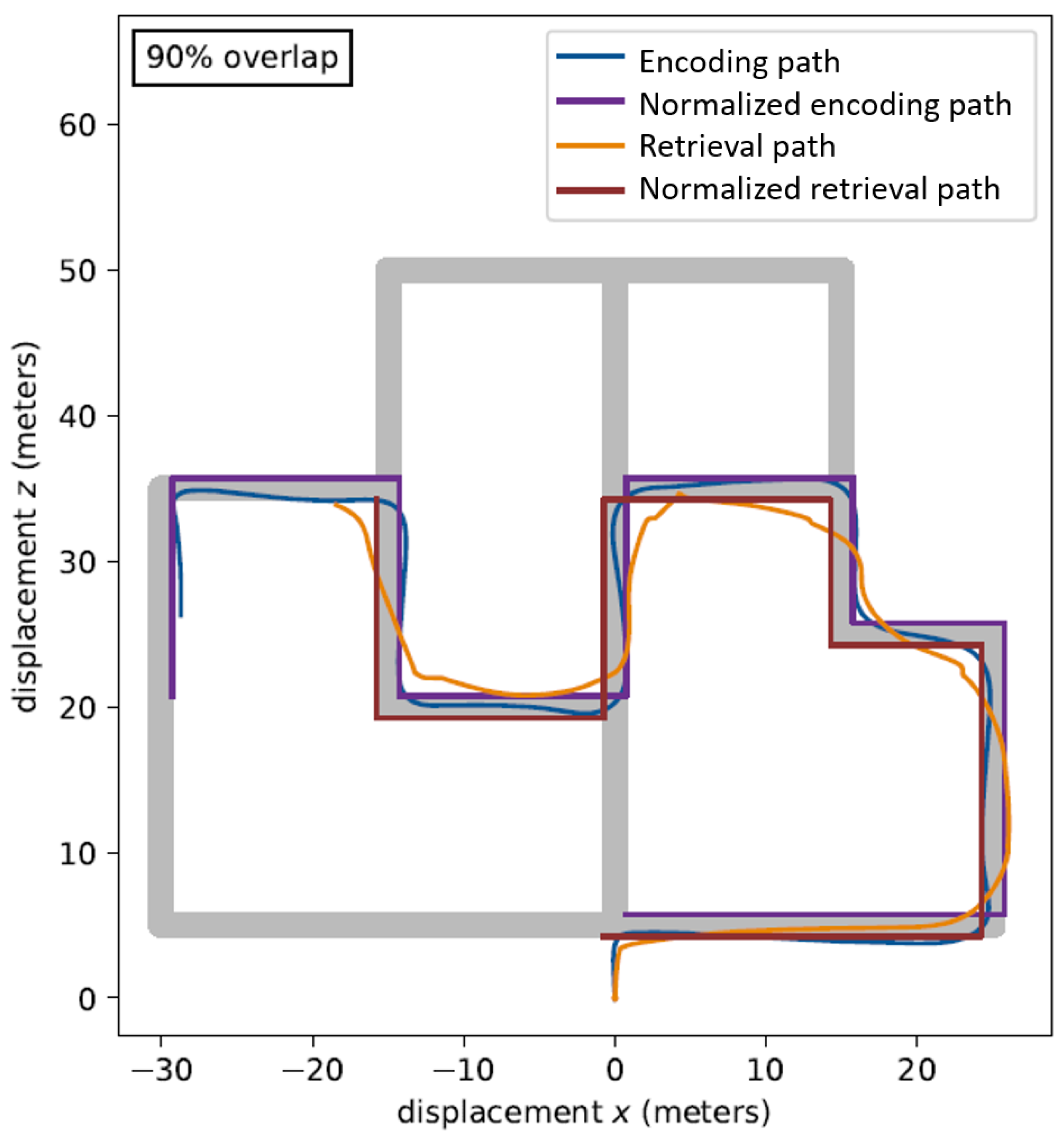
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirasic, K.C. Spatial cognition and behavior in young and elderly adults: Implications for learning new environments. Psychol. Aging 1991, 6, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wilkniss, S.M.; Jones, M.G.; Korol, D.L.; Gold, P.E.; Manning, C.A. Age-related differences in an ecologically based study of route learning. Psychol. Aging 1997, 12, 372–375. [Google Scholar] [CrossRef] [PubMed]
- De Beni, R.; Pazzaglia, F.; Gardini, S. The role of mental rotation and age in spatial perspective-taking tasks: When age does not impair perspective-taking performance. Appl. Cognit. Psychol. 2006, 20, 807–821. [Google Scholar] [CrossRef]
- Jonker, C.; Launer, L.J.; Hooijer, C.; Lindeboom, J. Memory Complaints and Memory Impairment in Older Individuals. J. Am. Geriatr. Soc. 1996, 44, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, S.; Algase, D.L.; Hong, G.-R.; Beattie, E.; Song, J.-A.; Yao, L. The Interrelatedness of Wandering and Wayfinding in a Community Sample of Persons with Dementia. Dement. Geriatr. Cogn. Disord. 2004, 17, 231–239. [Google Scholar]
- Henderson, V.W.; Mack, W.; Williams, B.W. Spatial Disorientation in Alzheimer’s Disease. Arch. Neurol. 1989, 46, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, V.; Duffy, C.J. Attentional dynamics and visual perception: mechanisms of spatial disorientation in Alzheimer’s disease. Brain 2003, 126, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Monacelli, A.M.; Cushman, L.A.; Kavcic, V.; Duffy, C.J. Spatial disorientation in Alzheimer’s disease: The remembrance of things passed. Neurology 2003, 61, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.-C.; Pai, M.; Jacobs, W.J. Topographical disorientation in community-residing patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2004, 19, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cogné, M.; Taillade, M.; N’Kaoua, B.; Tarruella, A.; Klinger, E.; Larrue, F.; Sauzéon, H.; Joseph, P.-A.; Sorita, E. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: A systematic literature review. Ann. Phys. Rehabil. Med. 2017, 60, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, E.R.; Warren, W.H. Active and passive contributions to spatial learning. Psychon. Bull. Rev. 2011, 19, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markant, D.B.; Ruggeri, A.; Gureckis, T.M.; Xu, F. Enhanced Memory as a Common Effect of Active Learning. Mind Brain Educ. 2016, 10, 142–152. [Google Scholar] [CrossRef]
- Chrastil, E.R.; Warren, W.H. Active and passive spatial learning in human navigation: Acquisition of survey knowledge. J. Exp. Psychol. Learn. Mem. Cognit. 2013, 39, 1520–1537. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Kemeny, A.; Gaunet, F.; Berthoz, A. Active, passive and snapshot exploration in a virtual environment: influence on scene memory, reorientation and path memory. Cogn. Brain Res. 2001, 11, 409–420. [Google Scholar] [Green Version]
- Jebara, N.; Orriols, E.; Zaoui, M.; Berthoz, A.; Piolino, P. Effects of Enactment in Episodic Memory: A Pilot Virtual Reality Study with Young and Elderly Adults. Front. Neuroinform. 2014, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Sandamas, G.; Foreman, N. Active Versus Passive Acquisition of Spatial Knowledge While Controlling a Vehicle in a Virtual Urban Space in Drivers and Non-Drivers. SAGE Open 2015, 5, 215824401559544. [Google Scholar] [CrossRef]
- Brooks, B.M. The Specificity of Memory Enhancement During Interaction with a Virtual Environment. Memory 1999, 7, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Sauzéon, H.; Pala, P.A.; Larrue, F.; Wallet, G.; Déjos, M.; Zheng, X.; Guitton, P.; N’Kaoua, B. The Use of Virtual Reality for Episodic Memory Assessment. Exp. Psychol. 2012, 59, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, H.D.; Engelkamp, J. Levels-of-processing effects in subject-performed tasks. Mem. Cogn. 1999, 27, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, T.; Backman, L.; Herlitz, A.; Nilsson, L.G.; Winblad, B.; Osterlind, P.O. Memory improvement at different stages of Alzheimer’s disease. Neuropsychologia 1989, 27, 737–742. [Google Scholar] [CrossRef]
- Silva, A.R.; Pinho, M.S.; Souchay, C.; Moulin, C.J.A. Evaluating the subject-performed task effect in healthy older adults: Relationship with neuropsychological tests. Socioaffect. Neurosci. Psychol. 2015, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Essen, J.D.; Von Essen, J.D.; Nilsson, L.-G. Memory effects of motor activation in subject-performed tasks and sign language. Psychon. Bull. Rev. 2003, 10, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Craik, F.I.M.; Routh, D.A.; Broadbent, D.E. On the Transfer of Information from Temporary to Permanent Memory [and Discussion]. Philos. Trans. R. Soc. B 1983, 302, 341–359. [Google Scholar] [CrossRef]
- Craik, F.I.M.; Jennings, J.M. Human memory. In The Handbook of Aging and Cognition; Craik, F.I.M., Salthouse, T.A., Eds.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1992; pp. 51–110. [Google Scholar]
- Plancher, G.; Tirard, A.; Gyselinck, V.; Nicolas, S.; Piolino, P. Using virtual reality to characterize episodic memory profiles in amnestic mild cognitive impairment and Alzheimer’s disease: Influence of active and passive encoding. Neuropsychologia 2012, 50, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Sauzéon, H.; N’Kaoua, B.; Pala, P.A.; Taillade, M.; Guitton, P. Age and active navigation effects on episodic memory: A virtual reality study. Br. J. Psychol. 2015, 107, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Taillade, M.; Sauzéon, H.; Dejos, M.; Pala, P.A.; Larrue, F.; Wallet, G.; Gross, C.; N’Kaoua, B. Executive and memory correlates of age-related differences in wayfinding performances using a virtual reality application. Aging Neuropsychol. Cognit. 2013, 20, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Z.; Lindenberger, U.; Freund, A.M.; Baltes, P.B. Walking While Memorizing: Age-Related Differences in Compensatory Behavior. Psychol. Sci. 2001, 12, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausdorff, J.M.; Yogev, G.; Springer, S.; Simon, E.S.; Giladi, N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Exp. Brain Res. 2005, 164, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, J.Z.; Linkenauger, S.A.; Proffitt, D. Comparing Decision-Making and Control for Learning a Virtual Environment: Backseat Drivers Learn Where They are Going. Proc. Hum. Factors Ergon. Soc. Ann. Meet. 2008, 52, 2117–2121. [Google Scholar] [CrossRef]
- Bakdash, J.; Linkenauger, S.; Stefanucci, J.; Witt, J.; Banton, T.; Proffitt, D. Perceived distance influences simulated walking time. J. Vis. 2010, 8, 1151. [Google Scholar] [CrossRef]
- Feyereisen, P. Enactment effects and integration processes in younger and older adults’ memory for actions. Memory 2009, 17, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Gutchess, A.H.; Kensinger, E.A.; Yoon, C.; Schacter, D.L. Ageing and the self-reference effect in memory. Memory 2007, 15, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, J.; Rozenberg, J.; Grolleau, P.; Piolino, P. The Self-Reference Effect on Episodic Memory Recollection in Young and Older Adults and Alzheimer’s Disease. Curr. Alzheimer Res. 2013, 10, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions. Contemp. Educ. Psychol. 2000, 25, 54–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashdan, T.B.; Silvia, P.J. Curiosity and Interest: The Benefits of Thriving on Novelty and Challenge. Curiosit. Interest Benefits Thriving Nov. Chall. 2012, 2, 367–374. [Google Scholar]
- Sakaki, M.; Yagi, A.; Murayama, K. Curiosity in old age: A possible key to achieving adaptive aging. Neurosci. Biobehav. Rev. 2018, 88, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Oudeyer, P.Y.; Gottlieb, J.; Lopes, M. Intrinsic motivation, curiosity, and learning: Theory and applications in educational technologies. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 229, pp. 257–284. [Google Scholar]
- Hegarty, M.; Montello, D.R.; Richardson, A.E.; Ishikawa, T.; Lovelace, K. Spatial abilities at different scales: Individual differences in aptitude-test performance and spatial-layout learning. Intelligence 2006, 34, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Moffat, S. Age differences in spatial memory in a virtual environment navigation task. Neurobiol. Aging 2001, 22, 787–796. [Google Scholar] [CrossRef]
- Moffat, S.D.; Resnick, S.M. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav. Neurosci. 2002, 116, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Cushman, L.A.; Stein, K.; Duffy, C.J. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology 2008, 71, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taillade, M.; N’Kaoua, B.; Sauzéon, H. Age-Related Differences and Cognitive Correlates of Self-Reported and Direct Navigation Performance: The Effect of Real and Virtual Test Conditions Manipulation. Front. Psychiatry 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H.; Bédirian, V. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Buttaro, M. Hooper Visual Organization Test; Springer Nature: Basingstoke, UK, 2017; Volume 28, pp. 1–3. [Google Scholar]
- Lopez, M.N.; Lazar, M.D.; Oh, S. Psychometric Properties of the Hooper Visual Organization Test. Assessment 2003, 10, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M. Development of a self-report measure of environmental spatial ability. Intelligence 2002, 30, 425–447. [Google Scholar] [CrossRef] [Green Version]
- Brown, J. Some tests of the decay theory of immediate memory. Q. J. Exp. Psychol. 1958, 10, 12–21. [Google Scholar] [CrossRef]
- Moffat, S.D.; Hampson, E.; Hatzipantelis, M. Navigation in a “Virtual” Maze: Sex Differences and Correlation with Psychometric Measures of Spatial Ability in Humans. Evol. Hum. Behav. 1998, 19, 73–87. [Google Scholar] [CrossRef]
- Miceli, A.; Salkind, N. Teoria Statistica Delle Classi e Calcolo Delle Probabilità. In Encyclopedia of Research Design; SAGE Publications: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ciaramelli, E. The role of ventromedial prefrontal cortex in navigation: A case of impaired wayfinding and rehabilitation. Neuropsychologia 2008, 46, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Kennedy, K.M.; Rodrigue, K.M.; Raz, N. Extrahippocampal Contributions to Age Differences in Human Spatial Navigation. Cereb. Cortex 2006, 17, 1274–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, S.L.; Tun, P.A.; Cox, L.C.; Colangelo, M.; Stewart, R.A.; Wingfield, A. Hearing loss and perceptual effort: downstream effects on older adults’ memory for speech. Q. J. Exp. Psychol. Sec. A 2005, 58, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Gazova, I.; Laczo, J.; Rubinova, E.; Mokrisova, I.; Hyncicova, E.; Andel, R.; Vyhnalek, M.; Sheardova, K.; Coulson, E.J.; Hort, J. Spatial navigation in young versus older adults. Front. Neuroinform. 2013, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Cipresso, P.; Morganti, F.; Riva, G. The role of egocentric and allocentric abilities in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2014, 16, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.W.; White, S.H. The Development of Spatial Representations of Large-Scale Environments. Adv. Child Dev. Behav. 1975, 10, 9–55. [Google Scholar] [PubMed]
- Guderian, S.; Dzieciol, A.M.; Gadian, D.G.; Jentschke, S.; Doeller, C.F.; Burgess, N.; Mishkin, M.; Vargha-Khadem, F. Hippocampal Volume Reduction in Humans Predicts Impaired Allocentric Spatial Memory in Virtual-Reality Navigation. J. Neurosci. 2015, 35, 14123–14131. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Martin, C.B. The Hippocampus Contributes to Allocentric Spatial Memory through Coherent Scene Representations. J. Neurosci. 2016, 36, 2555–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, K.S.; Barense, M.D.; Lee, A.C. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia 2010, 48, 831–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacter, D.L.; Addis, D.R.; Hassabis, D.; Martin, V.C.; Spreng, R.N.; Szpunar, K.K. The Future of Memory: Remembering, Imagining, and the Brain. Neuron 2012, 76, 677–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, E.A.; Mullally, S.L. The hippocampus: A manifesto for change. J. Exp. Psychol. Gen. 2013, 142, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Rendell, P.G.; Bailey, P.E.; Henry, J.D.; Phillips, L.H.; Gaskin, S.; Kliegel, M. Older adults have greater difficulty imagining future rather than atemporal experiences. Psychol. Aging 2012, 27, 1089–1098. [Google Scholar] [CrossRef] [PubMed]

| Active | Passive | |
|---|---|---|
| SBSD | r = −0.07, p = 0.62 | r = 0.11, p = 0.48 |
| Hooper | r = 0.51, p = 0.001 a,b | r = 0.42, p = 0.005 b |
| Number of stars passed during encoding | r = 0.39, p = 0.009 b | r = 0.09, p = 0.57 |
| Number of intersections passed during encoding | r = −0.006, p = 0.97 | r = 0.10, p = 0.52 |
| Length of the path travelled at encoding | r = −0.09, p = 0.57 | r = 0.03, p = 0.83 |
| Length of the path travelled at retrieval | r = −0.004, p = 0.98 | r = −0.13, p = 0.41 |
| Total duration of stopped movement during encoding | r = 0.06, p = 0.72 | r = 0.03, p = 0.86 |
| Number of stops during encoding | r = 0.14, p = 0.36 | r = −0.03, p = 0.85 |
| Distance of stops from the nearest intersection during encoding | r = −0.39, p = 0.01 b | r = −0.01, p = 0.96 |
| Total duration of stopped movement during retrieval | r = −0.23, p = 0.13 | r = 0.06, p = 0.70 |
| Number of stops during retrieval | r = 0.04, p = 0.82 | r = 0.01, p = 0.95 |
| Distance of stops from the nearest intersection during retrieval | r = −0.38, p = 0.01 b | r = −0.25, p = 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meade, M.E.; Meade, J.G.; Sauzeon, H.; Fernandes, M.A. Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults. Brain Sci. 2019, 9, 47. https://doi.org/10.3390/brainsci9030047
Meade ME, Meade JG, Sauzeon H, Fernandes MA. Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults. Brain Sciences. 2019; 9(3):47. https://doi.org/10.3390/brainsci9030047
Chicago/Turabian StyleMeade, Melissa E., John G. Meade, Hélène Sauzeon, and Myra A. Fernandes. 2019. "Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults" Brain Sciences 9, no. 3: 47. https://doi.org/10.3390/brainsci9030047
APA StyleMeade, M. E., Meade, J. G., Sauzeon, H., & Fernandes, M. A. (2019). Active Navigation in Virtual Environments Benefits Spatial Memory in Older Adults. Brain Sciences, 9(3), 47. https://doi.org/10.3390/brainsci9030047






