Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring
Abstract
:1. Introduction
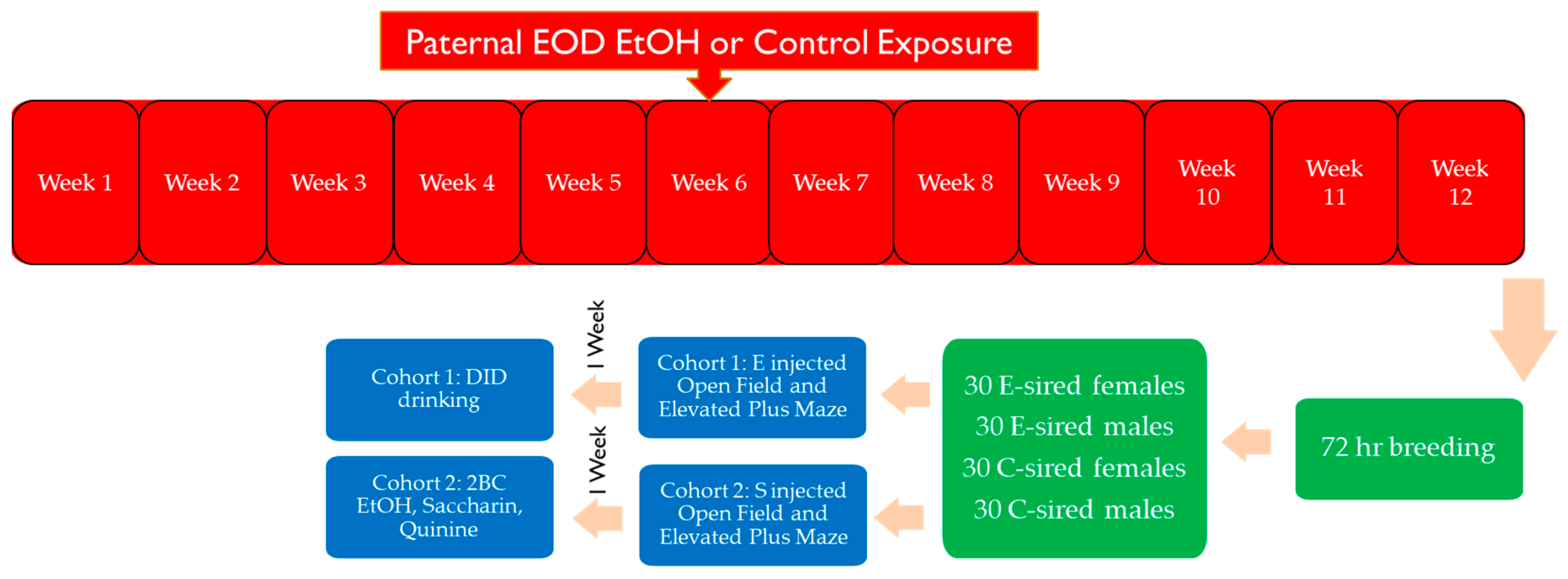
2. Materials and Methods
2.1. Animals
2.2. Every-Other-Day 2 Bottle Choice (EOD 2BC) EtOH Exposure
2.3. Selection of Sires for Breeding
2.4. Behavioral Assays
2.5. Drinking in the Dark Assay
2.6. Continuous 2BC Drinking Assay
2.7. Statistical Analysis
3. Results
3.1. Paternal Preconception EOD 2BC Drinking
3.2. Breeding Results
3.3. Offspring Behavioral Assays
3.3.1. Open Field
3.3.2. Elevated Plus Maze
3.4. Drinking in the Dark Assay
3.5. 2BC Drinking Assay
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Methodological Summary and Definitions; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2016.
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Claus, E.D.; Ewing, S.W.F.; Filbey, F.M.; Sabbineni, A.; Hutchison, K.E. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 2011, 36, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Finegersh, A.; Rompala, G.R.; Martin, D.I.; Homanics, G.E. Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol 2015, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, B.; Neale, M.C.; Kendler, K.S. The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychol. Med. 2015, 45, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Cichon, S.; Treutlein, J.; Ridinger, M.; Mattheisen, M.; Hoffmann, P.; Herms, S.; Wodarz, N.; Soyka, M.; Zill, P.; et al. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict. Biol. 2012, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Park, B.L.; Kim, J.W.; Cheong, H.S.; Kim, L.H.; Lee, B.C.; Seo, C.H.; Kang, T.-C.; Nam, Y.-W.; Kim, G.-B.; Shin, H.D.; et al. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: From GWAS to replication. Hum. Genet. 2013, 132, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.B.; Kranzler, H.R. Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol. Clin. Exp. Res. 2015, 39, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Kalsi, G.; Holmans, P.A.; Sanders, A.R.; Aggen, S.H.; Dick, D.M.; Aliev, F.; Shi, J.; Levinson, D.F.; Gejman, P.V. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol. Clin. Exp. Res. 2011, 35, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Bierut, L.J.; Agrawal, A.; Bucholz, K.K.; Doheny, K.F.; Laurie, C.; Pugh, E.; Fisher, S.; Fox, L.; Howells, W.; Bertelsen, S.; et al. A genome-wide association study of alcohol dependence. Proc. Natl. Acad. Sci. USA 2010, 107, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, J.M.; Geske, J.R.; Schneekloth, T.D.; Frye, M.A.; Cunningham, J.M.; Choi, D.-S.; Tapp, C.L.; Lewis, B.R.; Drews, M.S.; Pietrzak, T.L.; et al. Replication of Genome Wide Association Studies of Alcohol Dependence: Support for Association with Variation in ADH1C. PLoS ONE 2013, 8, e58798. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Mounier, A.; Wolfe, C.M.; Lin, Q.; Lefterov, I.; Homanics, G.E. Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Front. Genet. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sadri-Vakili, G.; Pierce, R.C. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.G.; Ressler, K.J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.C.; Franklin, T.B.; Vizi, S.; Mansuy, I.M. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front. Behav. Neurosci. 2011, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bygren, L.O.; Tinghög, P.; Carstensen, J.; Edvinsson, S.; Kaati, G.; Pembrey, M.E.; Sjöström, M. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, S.; Li, S.; Feng, R.; Na, L.; Chu, X.; Wu, X.; Niu, Y.; Sun, Z.; Han, T.; et al. Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: A population-based cohort study of families in Suihua, China. Am. J. Clin. Nutr. 2017, 105, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Heredia, N.; Senut, M.-C.; Land, S.; Hollocher, K.; Lu, X.; Dereski, M.O.; Ruden, D.M. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 2015, 5, 14466. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.L.; Yetter, N.; DeSomer, H. Intergenerational transmission of paternal trauma among US Civil War ex-POWs. Proc. Natl. Acad. Sci. USA 2018, 115, 11215–11220. [Google Scholar] [CrossRef] [PubMed]
- Tracey, R.; Manikkam, M.; Guerrero-Bosagna, C.; Skinner, M.K. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod. Toxicol. 2013, 36, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [PubMed]
- van Steenwyk, G.; Roszkowski, M.; Manuella, F.; Franklin, T.B.; Mansuy, I.M. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: Evidence in the 4th generation. Environ. Epigenet. 2018, 4, dvy023. [Google Scholar] [CrossRef] [PubMed]
- Cropley, J.E.; Eaton, S.A.; Aiken, A.; Young, P.E.; Giannoulatou, E.; Ho, J.W.K.; Buckland, M.E.; Keam, S.P.; Hutvagner, G.; Humphreys, D.T.; et al. Male-lineage transmission of an acquired metabolic phenotype induced by grand-paternal obesity. Mol. Metab. 2016, 5, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Homanics, G.E. Inter-generational effects of alcohol: A review of epigenetics through paternal preconception alcohol exposure. Alcohol. Clin. Exp. Res. 2019. under review. [Google Scholar]
- Kim, P.; Choi, C.S.; Park, J.H.; Joo, S.H.; Kim, S.Y.; Ko, H.M.; Kim, K.C.; Jeon, S.J.; Park, S.H.; Han, S.-H.; et al. Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. J. Neurosci. Res. 2014, 92, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Meek, L.R.; Myren, K.; Sturm, J.; Burau, D. Acute paternal alcohol use affects offspring development and adult behavior. Physiol. Behav. 2007, 91, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Diao, L.; Liu, J.; Jiang, N.; Zhang, J.; Wang, H.; Zhou, W.; Huang, G.; Ma, D. Paternal ethanol exposure and behavioral abnormities in offspring: Associated alterations in imprinted gene methylation. Neuropharmacology 2014, 81, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Lee, J.A. Paternal Alcohol Exposure Affects Offspring Behavior but not Body or Organ Weights in Mice. Alcohol. Clin. Exp. Res. 1988, 12, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Finegersh, A.; Homanics, G.E. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE 2014, 9, e99078. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Finegersh, A.; Slater, M. Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol 2017, 60, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Simons, A.; Kihle, B.; Homanics, G.E. Paternal Preconception Chronic Variable Stress Confers Attenuated Ethanol Drinking Behavior Selectively to Male Offspring in a Pre-Stress Environment Dependent Manner. Front. Behav. Neurosci. 2018, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Melendez, R.I. Intermittent (Every-Other-Day) Drinking Induces Rapid Escalation of Ethanol Intake and Preference in Adolescent and Adult C57BL/6J Mice. Alcohol. Clin. Exp. Res. 2011, 35, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Hwa, L.S.; Chu, A.; Levinson, S.A.; Kayyali, T.M.; DeBold, J.F.; Miczek, K.A. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol. Clin. Exp. Res. 2011, 35, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.C.; Lopez, M.F. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol. Clin. Exp. Res. 2004, 28, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.E.; Navarro, M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol 2014, 48, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Rompala, G.R.; Finegersh, A.; Homanics, G.E. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol 2016, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. Res. 2013, 33, 9003–9012. [Google Scholar] [CrossRef] [PubMed]
- Rivier, C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic–pituitary–adrenal axis. Front. Neuroendocrinol. 2014, 35, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Finegersh, A.; Ferguson, C.; Maxwell, S.; Mazariegos, D.; Farrell, D.; Homanics, G.E. Repeated vapor ethanol exposure induces transient histone modifications in the brain that are modified by genotype and brain region. Front. Mol. Neurosci. 2015, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.N.; Yoneyama, N.; Fretwell, A.M.; Snelling, C.; Tanchuck, M.A.; Finn, D.A. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm. Behav. 2010, 58, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ruby, C.L.; Palmer, K.N.; Zhang, J.; Risinger, M.O.; Butkowski, M.A.; Swartzwelder, H.S. Differential Sensitivity to Ethanol-Induced Circadian Rhythm Disruption in Adolescent and Adult Mice. Alcohol. Clin. Exp. Res. 2017, 41, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Shahanoor, Z.; Sultana, R.; Baker, M.R.; Romeo, R.D. Neuroendocrine stress reactivity of male C57BL/6N mice following chronic oral corticosterone exposure during adulthood or adolescence. Psychoneuroendocrinology 2017, 86, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.S.; Fabio, M.C.; Miranda-Morales, R.S.; Virgolini, M.B.; De Giovanni, L.N.; Hansen, C.; Wille-Bille, A.; Nizhnikov, M.E.; Spear, L.P.; Pautassi, R.M. Age-related effects of chronic restraint stress on ethanol drinking, ethanol-induced sedation, and on basal and stress-induced anxiety response. Alcohol 2016, 51, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.D.; Kaplowitz, E.T.; Ho, A.; Franco, D. The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice. Psychoneuroendocrinology 2013, 38, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 2008, 29, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]













© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beeler, E.; Nobile, Z.L.; Homanics, G.E. Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring. Brain Sci. 2019, 9, 56. https://doi.org/10.3390/brainsci9030056
Beeler E, Nobile ZL, Homanics GE. Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring. Brain Sciences. 2019; 9(3):56. https://doi.org/10.3390/brainsci9030056
Chicago/Turabian StyleBeeler, Erik, Zachary L. Nobile, and Gregg E. Homanics. 2019. "Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring" Brain Sciences 9, no. 3: 56. https://doi.org/10.3390/brainsci9030056
APA StyleBeeler, E., Nobile, Z. L., & Homanics, G. E. (2019). Paternal Preconception Every-Other-Day Ethanol Drinking Alters Behavior and Ethanol Consumption in Offspring. Brain Sciences, 9(3), 56. https://doi.org/10.3390/brainsci9030056





