How Therapeutic Tapping Can Alter Neural Correlates of Emotional Prosody Processing in Anxiety
Abstract
1. Introduction
1.1. Altered Emotional Processing as a Key Aspect of Anxiety Disorders
1.2. The Late Positive Potential (LPP) as an Electrophysiological Correlate of Emotion Regulation
1.3. Neural Effects of Therapeutic Interventions on Emotional Processing
1.4. Effects of Tapping in Anxiety Patients
1.5. Aims of the Study and Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Acoustic Stimuli
2.2.2. Standardized Psychological Tests and Additional Questionnaires
2.3. Therapeutic Interventions
2.4. Procedure
2.5. EEG Recordings
2.6. Data Analyses
EEG Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
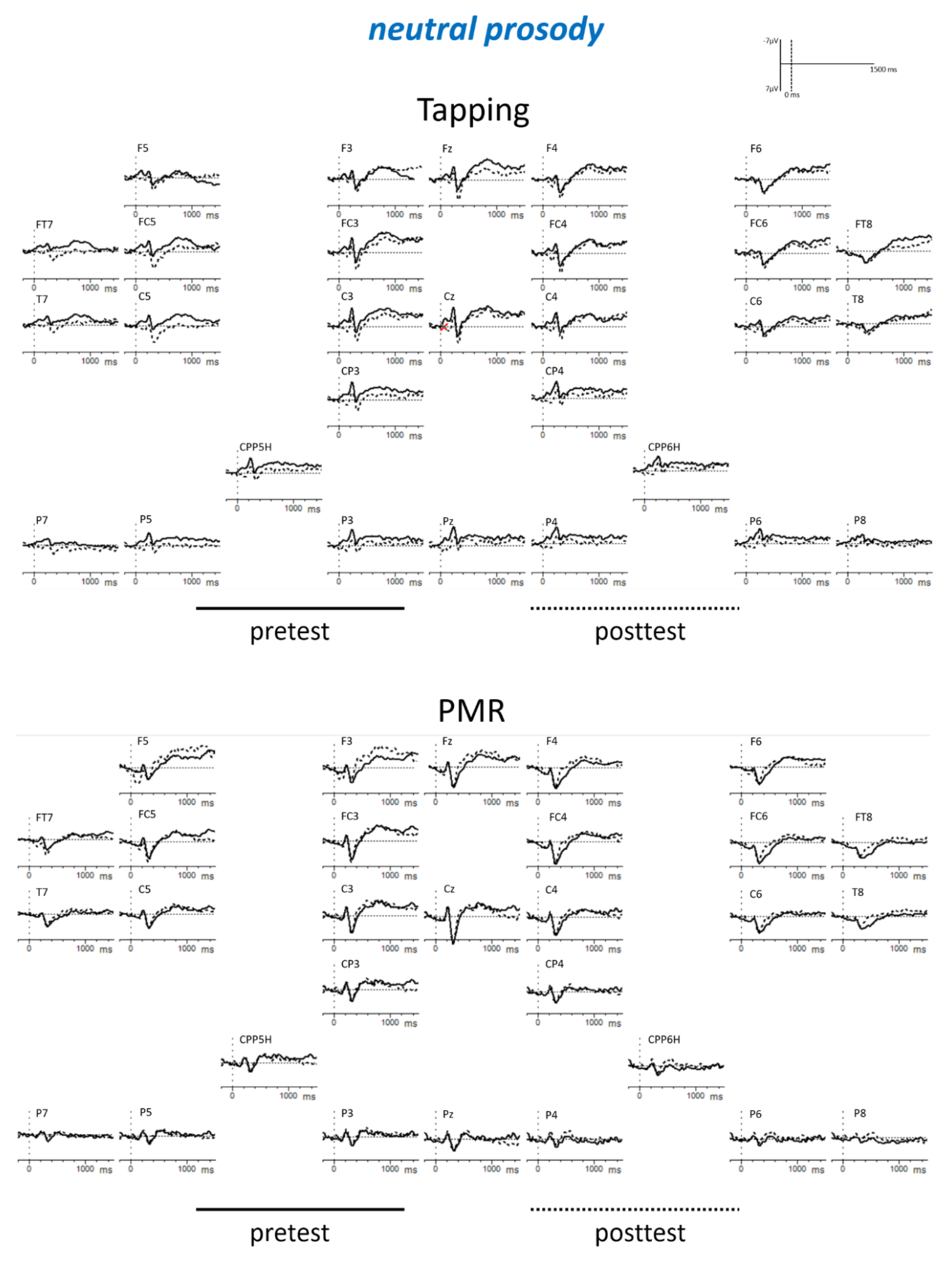


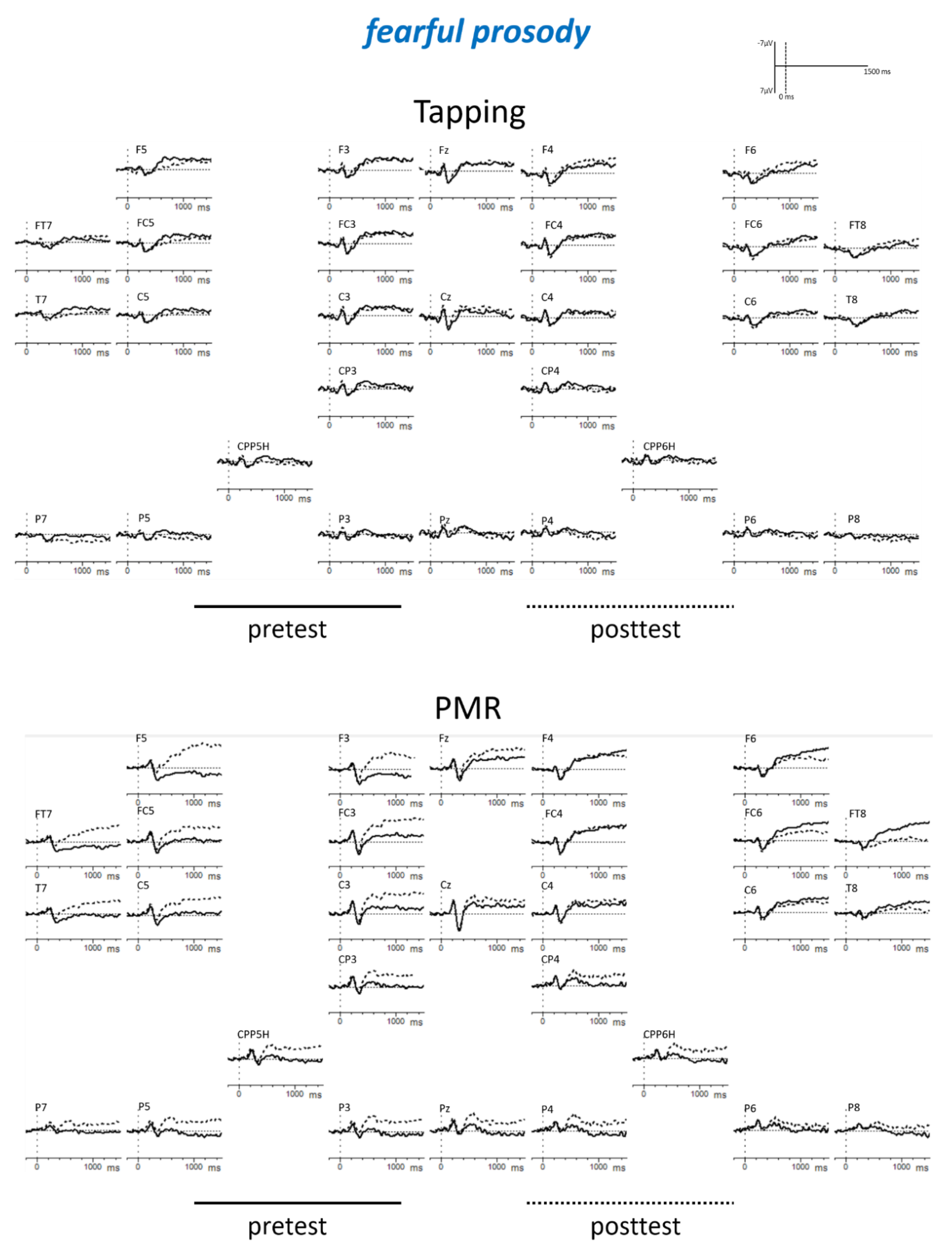
Appendix B
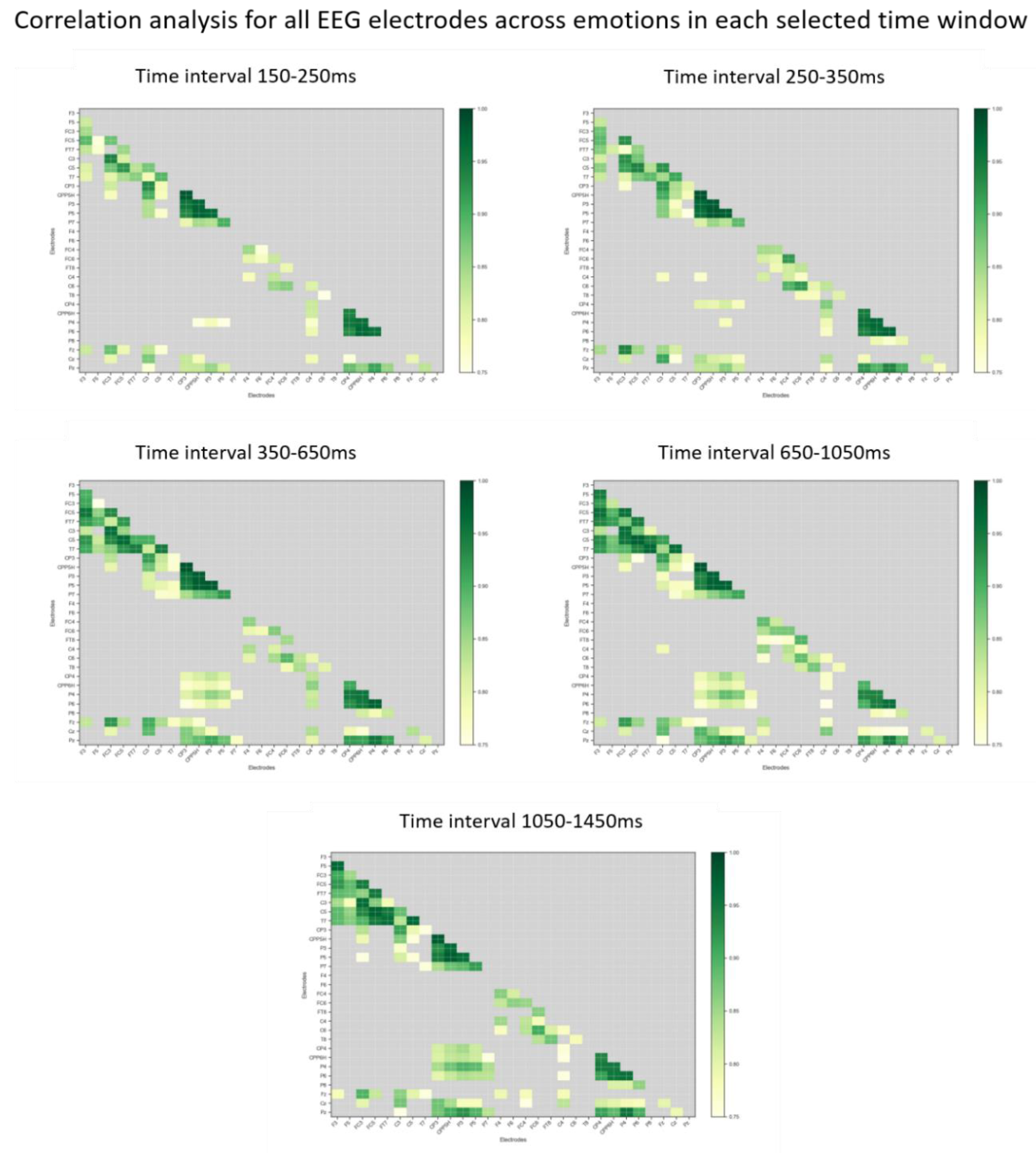
References
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; De Graaf, R.; Demyttenaere, K.; Gasquet, I.; De Girolamo, G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007, 6, 168–176. [Google Scholar] [PubMed]
- Wittchen, H.-U.; Essau, C.A.; Krieg, J.-C. Anxiety Disorders: Similarities and Differences of Comorbidity in Treated and Untreated Groups. Br. J. Psychiatry 1991, 159, 23–33. [Google Scholar] [CrossRef]
- Robinson, J.; Sareen, J.; Cox, B.J.; Bolton, J. Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample. J. Anxiety Disord. 2009, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Morrison, A.; Jazaieri, H.; Brozovich, F.; Heimberg, R.; Gross, J.J. Group CBT versus MBSR for Social Anxiety Disorder: A Randomized Controlled Trial. J. Consult Clin. Psychol. 2016, 84, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Hawk, C.; Brooks, A.J.; Toukolehto, O.; Wren, M.; Dinter; Stein, P. Psychological Trauma Symptom Improvement in Veterans Using Emotional Freedom Techniques: A Randomized Controlled Trial. J. Nerv. Ment. Dis. 2013, 201, 153. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.; Brooks, A.J.; Rowe, J.E. The Immediate Effect of a Brief Energy Psychology Intervention (Emotional Freedom Techniques) on Specific Phobias: A Pilot Study. Explore 2011, 7, 155–161. [Google Scholar] [CrossRef]
- Craig, G.; Fowlie, A. Emotional Freedom Techniques: Self-Published Manual; The Sea Ranch: Sonoma County, CA, USA, 1995. [Google Scholar]
- Callahan, R.J. Five Minute Phobia Cure: Dr. Callahan’s Treatment for Fears, Phobias and Self-Sabotage; Enterprise Publishing: Wilmington, DE, USA, 1985. [Google Scholar]
- Clond, M. Emotional Freedom Techniques for Anxiety: A Systematic Review with Meta-analysis. J. Nerv. Ment. Dis. 2016, 204, 388. [Google Scholar] [CrossRef] [PubMed]
- Mennin, D.S.; McLaughlin, K.A.; Flanagan, T.J. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. J. Anxiety Disord. 2009, 23, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Hallion, L.S.; Ruscio, A.M. Should uncontrollable worry be removed from the definition of GAD? A test of incremental validity. J. Abnorm. Psychol. 2013, 122, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Hallion, L.S.; Tolin, D.F.; Assaf, M.; Goethe, J.; Diefenbach, G.J. Cognitive Control in Generalized Anxiety Disorder: Relation of Inhibition Impairments to Worry and Anxiety Severity. Cogn. Ther. Res. 2017, 41, 610–618. [Google Scholar] [CrossRef]
- Quadflieg, S.; Wendt, B.; Mohr, A.; Miltner, W.H.R.; Straube, T. Recognition and evaluation of emotional prosody in individuals with generalized social phobia: A pilot study. Behav. Res. Ther. 2007, 45, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, B.; Verschuere, B.; Tibboel, H.; De Houwer, J.; Crombez, G.; Koster, E.H.W. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychol. Bull. 2014, 140, 682–721. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.; Clarke, P.J.F. The Attentional Bias Modification Approach to Anxiety Intervention. Clin. Psychol. Sci. 2015, 3, 58–78. [Google Scholar] [CrossRef]
- Beauregard, M. Mind does really matter: Evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog. Neurobiol. 2007, 81, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.G.H.; Rugg, M.D. Electrophysiology of Mind: Event-related brain potentials and Cognition; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Donchin, E.; Ritter, W.; McCallum, W.C. Cognitive psychophysiology: The endogenous components of the ERP. In Event-Related Brain Potentials in Man; Callaway, E., Tueting, P., Koslow, S.H., Eds.; Academic Press: New York, NY, USA, 1978; pp. 349–411. [Google Scholar]
- Chronaki, G.; Broyd, S.J.; Garner, M.; Benikos, N.; Thompson, M.J.J.; Sonuga-Barke, E.J.S.; Hadwin, J.A. The Moderating Effect of Self-Reported State and Trait Anxiety on the Late Positive Potential to Emotional Faces in 6–11-Year-Old Children. Front. Psychol. 2018, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Pell, M.D.; Rothermich, K.; Liu, P.; Paulmann, S.; Sethi, S.; Rigoulot, S. Preferential decoding of emotion from human non-linguistic vocalizations versus speech prosody. Biol. Psychol. 2015, 111, 14–25. [Google Scholar] [CrossRef]
- Paulmann, S.; Bleichner, M.; Kotz, S.A.E. Valence, arousal, and task effects in emotional prosody processing. Front. Psychol. 2013, 4, 345. [Google Scholar] [CrossRef]
- Wickens, S.; Perry, C. What Do You Mean by That?! An Electrophysiological Study of Emotional and Attitudinal Prosody. PLoS ONE 2015, 10, e0132947. [Google Scholar] [CrossRef]
- Grossmann, T.; Vaish, A.; Franz, J.; Schroeder, R.; Stoneking, M.; Friederici, A.D. Emotional Voice Processing: Investigating the Role of Genetic Variation in the Serotonin Transporter across Development. PLoS ONE 2013, 8, e68377. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.M.; Rushby, J.A.; McDonald, S.; Dimoska-Di Marco, A.; Swift, J. Emotion in voice matters: Neural correlates of emotional prosody perception. Int. J. Psychophysiol. 2013, 89, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Frühholz, S.; Ceravolo, L.; Grandjean, D. Specific Brain Networks during Explicit and Implicit Decoding of Emotional Prosody. Cereb. Cortex 2012, 22, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Borod, J.C. Interhemispheric and intrahemispheric control of emotion: A focus on unilateral brain damage. J. Consult. Clin. Psychol. 1992, 60, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.; Bradley, M.M.; Jones, J.; Okun, M.S.; Perlstein, W.M.; Bowers, D. The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia 2013, 51, 960–966. [Google Scholar] [CrossRef][Green Version]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.M.; Birbaumer, N.; Lang, P.J. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef]
- Bradley, M.M. Natural selective attention: Orienting and emotion. Psychophysiology 2009, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.R.; Moser, J.S.; Huppert, J.D.; Simons, R.F. What’s in a Face? J. Psychophysiol. 2013, 27, 27–38. [Google Scholar] [CrossRef]
- Zhou, H.; Dai, B.; Rossi, S.; Li, J. Electrophysiological Evidence for Elimination of the Positive Bias in Elderly Adults with Depressive Symptoms. Front. Psychiatry 2018, 9, 62. [Google Scholar] [CrossRef]
- Weinberg, A.; Hajcak, G. Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion 2010, 10, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Bradley, M.M.; Hauk, O.; Rockstroh, B.; Elbert, T.; Lang, P.J. Large-scale neural correlates of affective picture processing. Psychophysiology 2002, 39, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; MacNamara, A.; Olvet, D.M. Event-Related Potentials, Emotion, and Emotion Regulation: An Integrative Review. Dev. Neuropsychol. 2010, 35, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Flaisch, T.; Stockburger, J.; Junghöfer, M. Emotion and attention: Event-related brain potential studies. In Progress in Brain Research; Anders, S., Ende, G., Junghofer, M., Kissler, J., Wildgruber, D., Eds.; Understanding Emotions; Elsevier: Amsterdam, The Netherlands, 2006; Volume 156, pp. 31–51. [Google Scholar]
- Hajcak, G.; Olvet, D.M. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion 2008, 8, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings; University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Schupp, H.T.; Öhman, A.; Junghöfer, M.; Weike, A.I.; Stockburger, J.; Hamm, A.O. The Facilitated Processing of Threatening Faces: An ERP Analysis. Emotion 2004, 4, 189–200. [Google Scholar] [CrossRef]
- Flaisch, T.; Häcker, F.; Renner, B.; Schupp, H.T. Emotion and the processing of symbolic gestures: An event-related brain potential study. Soc. Cogn. Affect. Neurosci. 2011, 6, 109–118. [Google Scholar] [CrossRef]
- Schupp, H.T.; Junghöfer, M.; Weike, A.I.; Hamm, A.O. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology 2004, 41, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dennis, T.A.; Hajcak, G. The late positive potential: A neurophysiological marker for emotion regulation in children. J. Child Psychol. Psychiatry 2009, 50, 1373–1383. [Google Scholar] [CrossRef]
- Horan, W.P.; Wynn, J.K.; Kring, A.M.; Simons, R.F.; Green, M.F. Electrophysiological correlates of emotional responding in schizophrenia. J. Abnorm. Psychol. 2010, 119, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Marissen, M.A.E.; Meuleman, L.; Franken, I.H.A. Altered emotional information processing in borderline personality disorder: An electrophysiological study. Psychiatry Res. Neuroimaging 2010, 181, 226–232. [Google Scholar] [CrossRef]
- Thiruchselvam, R.; Blechert, J.; Sheppes, G.; Rydstrom, A.; Gross, J.J. The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biol. Psychol. 2011, 87, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; McGinnis-Deweese, M.; Keil, A.; Ding, M. Neural Substrate of the Late Positive Potential in Emotional Processing. J. Neurosci. 2012, 32, 14563–14572. [Google Scholar] [CrossRef]
- Moratti, S.; Saugar, C.; Strange, B.A. Prefrontal-Occipitoparietal Coupling Underlies Late Latency Human Neuronal Responses to Emotion. J. Neurosci. 2011, 31, 17278–17286. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, H.; Fitzgerald, D.A.; Phan, K.L. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kinney, K.L.; Burkhouse, K.L.; Klumpp, H. Self-report and neurophysiological indicators of emotion processing and regulation in social anxiety disorder. Biol. Psychol. 2019, 142, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.; Polglase, K.; Andrews, H.B.; Carrington, P.; Baker, A.H. Evaluation of a meridian-based intervention, Emotional Freedom Techniques (EFT), for reducing specific phobias of small animals. J. Clin. Psychol. 2003, 59, 943–966. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, A.H.; Karan, O.C. A Randomized Controlled Comparison of Emotional Freedom Technique and Cognitive-Behavioral Therapy to Reduce Adolescent Anxiety: A Pilot Study. J. Altern. Complement. Med. 2016, 23, 102–108. [Google Scholar] [CrossRef]
- Boath, E.; Good, R.; Tsaroucha, A.; Stewart, T.; Pitch, S.; Boughey, A.J. Tapping your way to success: Using Emotional Freedom Techniques (EFT) to reduce anxiety and improve communication skills in social work students. Soc. Work Educ. 2017, 36, 715–730. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992; ISBN 978-7-117-01957-6. [Google Scholar]
- Margraf, J. Mini-DIPS: Diagnostisches Kurz-Interview bei Psychischen Störungen; Springer-Verlag: Berlin, Germany, 2013; ISBN 978-3-662-08774-9. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory-II; Harcourt Assessment Inc.: San Antonio, TX, USA, 1996; Volume 78. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. BDI-II Beck-Depressions-inventar; Harcourt Test Services: Frankfurt/Main, Germany, 2006. [Google Scholar]
- Garb, H.N. Clinical judgment, clinical training, and professional experience. Psychol. Bull. 1989, 105, 387–396. [Google Scholar] [CrossRef]
- Leutgeb, V.; Schäfer, A.; Schienle, A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biol. Psychol. 2009, 82, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Hämmerli, K.; Gubser, N.; Andersson, G.; Caspar, F. Internet-Based Treatment of Depression: A Randomized Controlled Trial Comparing Guided with Unguided Self-Help. Cogn. Behav. Ther. 2011, 40, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Fehm, L.; Margraf, J. Thought suppression: specificity in agoraphobia versus broad impairment in social phobia? Behav. Res. Ther. 2002, 40, 57–66. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Gillberg, M. Subjective and Objective Sleepiness in the Active Individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Craig, G. The EFT manual; Energy Psychology Press: Santa Rosa, CA, USA, 2011. [Google Scholar]
- Sezgin, N.; Özcan, B. The effect of progressive muscular relaxation and Emotional Freedom Techniques on test anxiety in high school students: A randomized blind controlled study. Energy Psychol. Theory Res. Treat. 2009, 1, 23–29. [Google Scholar] [CrossRef]
- Jacobson, E. Progressive muscle relaxation. J. Abnorm. Psychol. 1938, 75, 18. [Google Scholar]
- Conrad, A.; Roth, W.T. Muscle relaxation therapy for anxiety disorders: It works but how? J. Anxiety Disord. 2007, 21, 243–264. [Google Scholar] [CrossRef]
- Huntley, C.D.; Young, B.; Temple, J.; Longworth, M.; Smith, C.T.; Jha, V.; Fisher, P.L. The efficacy of interventions for test-anxious university students: A meta-analysis of randomized controlled trials. J. Anxiety Disord. 2019, 63, 36–50. [Google Scholar] [CrossRef] [PubMed]
- De Lorent, L.; Agorastos, A.; Yassouridis, A.; Kellner, M.; Muhtz, C. Auricular Acupuncture Versus Progressive Muscle Relaxation in Patients with Anxiety Disorders or Major Depressive Disorder: A Prospective Parallel Group Clinical Trial. J. Acupunct. Meridian Stud. 2016, 9, 191–199. [Google Scholar] [CrossRef]
- Obrig, H.; Villringer, A. Beyond the visible--imaging the human brain with light. J. Cereb. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Berking, M.; Wupperman, P.; Reichardt, A.; Pejic, T.; Dippel, A.; Znoj, H. Emotion-regulation skills as a treatment target in psychotherapy. Behav. Res. Ther. 2008, 46, 1230–1237. [Google Scholar] [CrossRef]
- Foti, D.; Hajcak, G.; Dien, J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology 2009, 46, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, A.; MacNamara, A.; Fitzgerald, K.D.; Monk, C.S.; Phan, K.L. Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. J. Abnorm. Child Psychol. 2015, 43, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Gadea, M.; Espert, R.; Salvador, A.; Martí-Bonmatí, L. The sad, the angry, and the asymmetrical brain: Dichotic Listening studies of negative affect and depression. Brain Cogn. 2011, 76, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhuang, M.; Jie, J.; Wu, X.; Zheng, X. State Anxiety Down-Regulates Empathic Responses: Electrophysiological Evidence. Front. Hum. Neurosci. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.J.; Atchley, R.A.; Young, K.M. Valence and arousal influence the late positive potential during central and lateralized presentation of images. Laterality: Asymmetries of Body, Brain and Cognition 2017, 22, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; Labek, K.; Taubner, S.; Kessler, H.; Pokorny, D.; Kächele, H.; Cierpka, M.; Roth, G.; Pogarell, O.; Karch, S. Modulation of Gamma Band Activity and Late Positive Potential in Patients with Chronic Depression after Psychodynamic Psychotherapy. Psychother Psychosom 2018, 87, 252–254. [Google Scholar] [CrossRef] [PubMed]
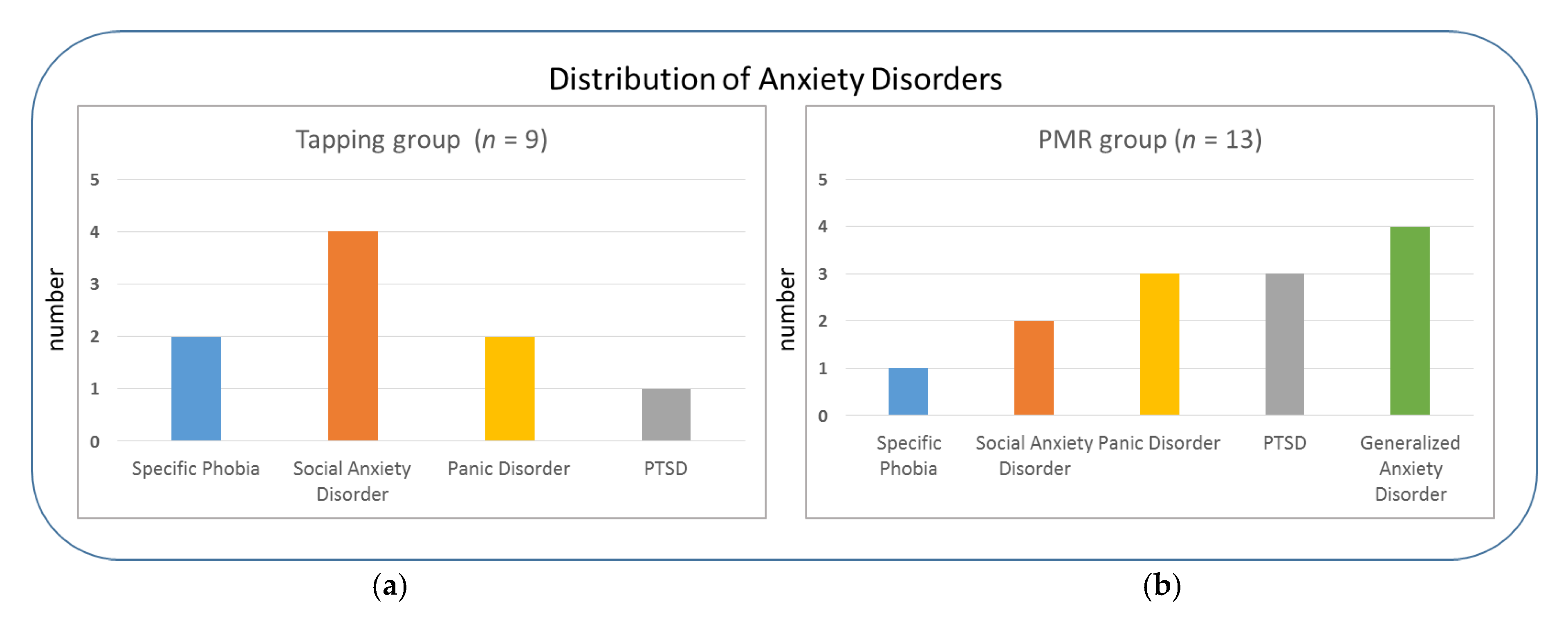






| Tapping (n = 9) | PMR (n = 13) | df | t | p | |
|---|---|---|---|---|---|
| Age (Mean) | 28.56 (Range 20–52) | 28.85 (Range 19–43) | 20 | −0.078 | 0.939 |
| BDI-II score | 13.0 | 13.23 | 18 | −0.064 | 0.950 |
| KSS score | 4.25 | 4.69 | 19 | −0.631 | 0.536 |
| NAS | 2.13 | 2.31 | 19 | −0.325 | 0.748 |
| PANAS | 29.67 | 26.85 | 20 | 0.944 | 0.356 |
| Group | Additional Anxiety and/or Depressive Symptoms | Current Psychiatric Medication | Current or Past Psychotherapy |
|---|---|---|---|
| Tapping | 78% 1 | 67% | 67% |
| PMR (control) | 85% 2 | 62% | 85% |
| 1050–1450 ms Left Centro-Parietal ROI (C3CP3C5) | df | t | p |
|---|---|---|---|
| emotion*time | 3.60 | 1.387 | 0.257 |
| emotion*time*group | 3.60 | 3.456 | 0.025 |
| Tapping: angry: post < pre PMR: fearful: post < pre PMR: posttest: fearful < angry Posttest: fearful: PMR < Tapping | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, N.; Steber, S.; Seebacher, J.; von Prittwitz, Q.; Bliem, H.R.; Rossi, S. How Therapeutic Tapping Can Alter Neural Correlates of Emotional Prosody Processing in Anxiety. Brain Sci. 2019, 9, 206. https://doi.org/10.3390/brainsci9080206
König N, Steber S, Seebacher J, von Prittwitz Q, Bliem HR, Rossi S. How Therapeutic Tapping Can Alter Neural Correlates of Emotional Prosody Processing in Anxiety. Brain Sciences. 2019; 9(8):206. https://doi.org/10.3390/brainsci9080206
Chicago/Turabian StyleKönig, Nicola, Sarah Steber, Josef Seebacher, Quinten von Prittwitz, Harald R. Bliem, and Sonja Rossi. 2019. "How Therapeutic Tapping Can Alter Neural Correlates of Emotional Prosody Processing in Anxiety" Brain Sciences 9, no. 8: 206. https://doi.org/10.3390/brainsci9080206
APA StyleKönig, N., Steber, S., Seebacher, J., von Prittwitz, Q., Bliem, H. R., & Rossi, S. (2019). How Therapeutic Tapping Can Alter Neural Correlates of Emotional Prosody Processing in Anxiety. Brain Sciences, 9(8), 206. https://doi.org/10.3390/brainsci9080206





