Abstract
Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative diseases worldwide. In an effort to search for new strategies for treating AD, natural products have become candidates of choice. Plants are a rich source of bioactive and effective compounds used in treating numerous diseases. Various plant extracts are known to display neuroprotective activities by targeting different pathophysiological pathways in association with the diseases, such as inhibiting enzymes responsible for degrading neurotransmitters, reducing oxidative stress, neuroprotection, inhibiting amyloid plaque formation, and replenishing mitochondrial function. This review presented a comprehensive evaluation of the available scientific literature (in vivo, in vitro, and in silico) on the neuroprotective mechanisms displayed by the extracts/bioactive compounds from spices belonging to the Apiaceae family in ameliorating AD.
1. Introduction
Neurodegenerative disease is a broad term used to describe a range of conditions primarily affecting neurons, ultimately leading to the progressive loss of normal motor and cognitive functions. According to a recent report, around 50 million people suffer from such diseases worldwide [1]. Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative diseases that affect millions throughout the world. US Food and Drug Administration (FDA)-approved drugs for ameliorating AD symptoms include acetylcholinesterase inhibitors (rivastigmine, galantamine, tacrine, donepezil) and the NMDA receptor antagonist (memantine) [2,3]. A new drug, Aduhelm (antiamyloid antibody intravenous (IV) infusion therapy), was recently approved by the FDA, which can delay clinical degeneration, with benefits to both cognitive and motor functions in AD patients. However, all these treatments suffer from side effects ranging from headache, nausea, confusion, dizziness, fall, and amyloid-related imaging abnormalities (ARIAs, such as swelling in the brain and microhemorrhages/superficial siderosis). Therefore, it is of utmost importance to search for new strategies and drugs that can help delay the onset and progression of the disease. AD hallmarks include alterations in the levels of neurotransmitters, amyloid-beta (Aβ) plaque deposition, and atypical tau protein phosphorylation [4]. The vital role of oxidative stress in neurodegenerative disorders is also well recognized. The neurons are more susceptible to free-radical-mediated damage compared to the rest of the body [5]. Under normal physiological conditions, antioxidant enzymes help overcome the oxidative stress generated in the body, but in AD, these enzymes are unable to achieve this task [6]. Due to the accumulation of Aβ, the level of reactive oxygen species (ROS) increases, which severely affects the function of various proteins, enzymes, transporters, and ion channels due to oxidative damage [7,8]. Superoxide radicals also interact with nitric oxide produced by activated microglia, enhancing peroxynitrite and other RNS formation [9,10]. The ROS/RNS build-up with the activation of apoptosis and reduction of antioxidant enzymes has a catastrophic effect on cholinergic regions involved in cognitive performance [11]. Thus, controlling ROS levels can be an alternative approach in the pathophysiology of AD. Numerous reports suggest the protective role of phytochemicals against oxidative stress and neuroinflammation, which are the key hallmarks of neurodegenerative diseases (NDs).
In an epidemiological survey, the propensity of AD was found to be higher among European and American populations compared to Asia. The dietary pattern differences between Eastern and Western cultures may be among the most important reasons for the development of neurodegenerative disorders [1,12]. This was supported by the findings of Dodge et al. [13], who reported increased cases of AD in Japan after switching to a Western diet pattern. Most Asian countries consume plant-derived foods rich in antioxidants (grains, vegetables, beans, fruits, spices, herbs) as the main ingredients, followed by the consumption of seafood, poultry, and dairy products in moderation. Plants are a rich source of bioactive compounds and display vast therapeutic properties ranging from antioxidative, anti-inflammatory, cardioprotective, nephron-protective, antidiabetic, antihypertensive, antimicrobial, etc. [14,15,16]. They have also been reported to exert neuroprotective properties by affecting multiple signaling pathways [17].
Traditionally used Indian spices are a valuable collection of phytocompounds. A spice is the dried seed, fruit, root, or bark of a plant, primarily used for seasoning. As per Ayurveda, the Indian traditional system of medicine, the addition of spices not only enhances food taste but also “Agni” (digestive fire) and therefore helps in proper digestion. As cataloged by the Spice Board of India, important spices come from families such as Zingiberaceae (cardamom, turmeric), Solanaceae (chili), Fabaceae (fenugreek), Piperaceae (pepper), Myrtaceae (clove), Myristicaceae (nutmeg, mace), and Lauraceae (cinnamon). However, most spices used in Indian cuisine belong to the Apiaceae family (ajowan, asafoetida, cumin, coriander, caraway, dill, fennel).
Thus, considering their importance, Apiaceae family spices were studied to endorse the claims made in the complementary traditional system of medicine, with the main emphasis on the neuroprotective mechanism they display. This review is designed to discuss the most significant pathophysiological events linked with AD and enlists the available literature (PubMed, Google Scholar, Scopus databases) on in vivo, in vitro, and in silico studies demonstrating the pharmacological action and mechanism of important phytochemicals present in spices belonging to the Apiaceae family. Due to the presence of numerous bioactive constituents in the plants, they display a multitarget approach in ameliorating the disease symptoms, which may achieve more favorable clinical results, owing to complex AD etiology. Hence, this review can be a base for potential future complementary treatment in complex neurodegenerative diseases.
2. Traditional Spices and Their Neuroprotective Effect
The term “spice” is derived from the Latin word “Species aromatacea”, meaning aromatic species. Spices are dried, aromatic, or pungent edible plant parts (fruit, leaves, seed, root, bark, flower), whose primary purpose in food is seasoning rather than nutrition. The bioactive compounds present in spices such as alkaloids, phenols, terpenes, and flavonoids are responsible for their therapeutic potential. Family Apiaceae (also known as Umbelliferae) has mostly aromatic flowering plants. Numerous species of this family are reported to be rich in essential and vegetable oils and hence are used in the pharmaceutical, cosmetic, perfume, and food industries [18,19]. Reports suggest the role of terpenoids and phenylpropanoids in inhibiting acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity to a variable extent [20], which makes it important that cholinesterase inhibition by essential oils is elucidated more precisely with other chemical components. The vegetable oil obtained from umbelliferous seeds is a very rich source of a rare fatty acid, petroselinic acid (an isomer of oleic acid), and is used in chemical industries [21]. It has already been reported that fatty acids can cross the blood–brain barrier (BBB) via simple diffusion. Additionally, many transport proteins such as fatty acid binding protein 5 (FABP-5), fatty acid transport proteins-1 (FATP-1), FATP-4, and fatty acid translocase (CD36) also assist in the transport. In AD, decreased transport of many fatty acids (linoleic acid, myristic acid, palmitic acid, etc.) has been reported [22]. Moreover, expression of CD36, which is also a microglial receptor involved in the removal of Aβ, is downregulated in AD [23]. The neuroprotective mechanism of the spice active constituents and extracts belonging to the Apiaceae family is summarized in Table 1 and Table 2. The structures of important bioactive compounds from this family (Figure 1) and a diagrammatic representation for the multitarget approach by the spice extracts and phytocompounds are also depicted (Figure 2). The mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease are described below.

Table 1.
List of biologically active compounds identified from the Apiaceae species.

Table 2.
Neuroprotective activities in Alzheimer’s disease displayed by Apiaceae family spice extracts.
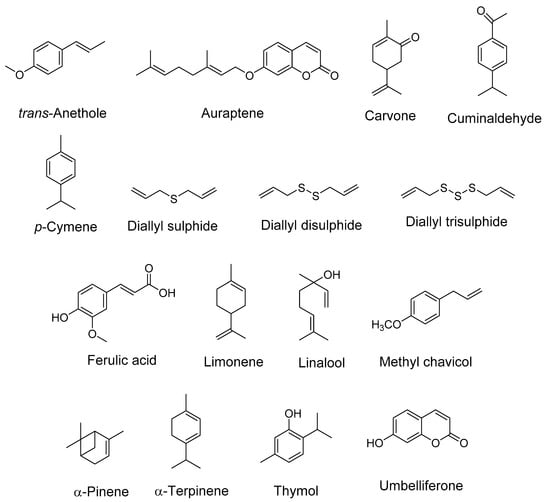
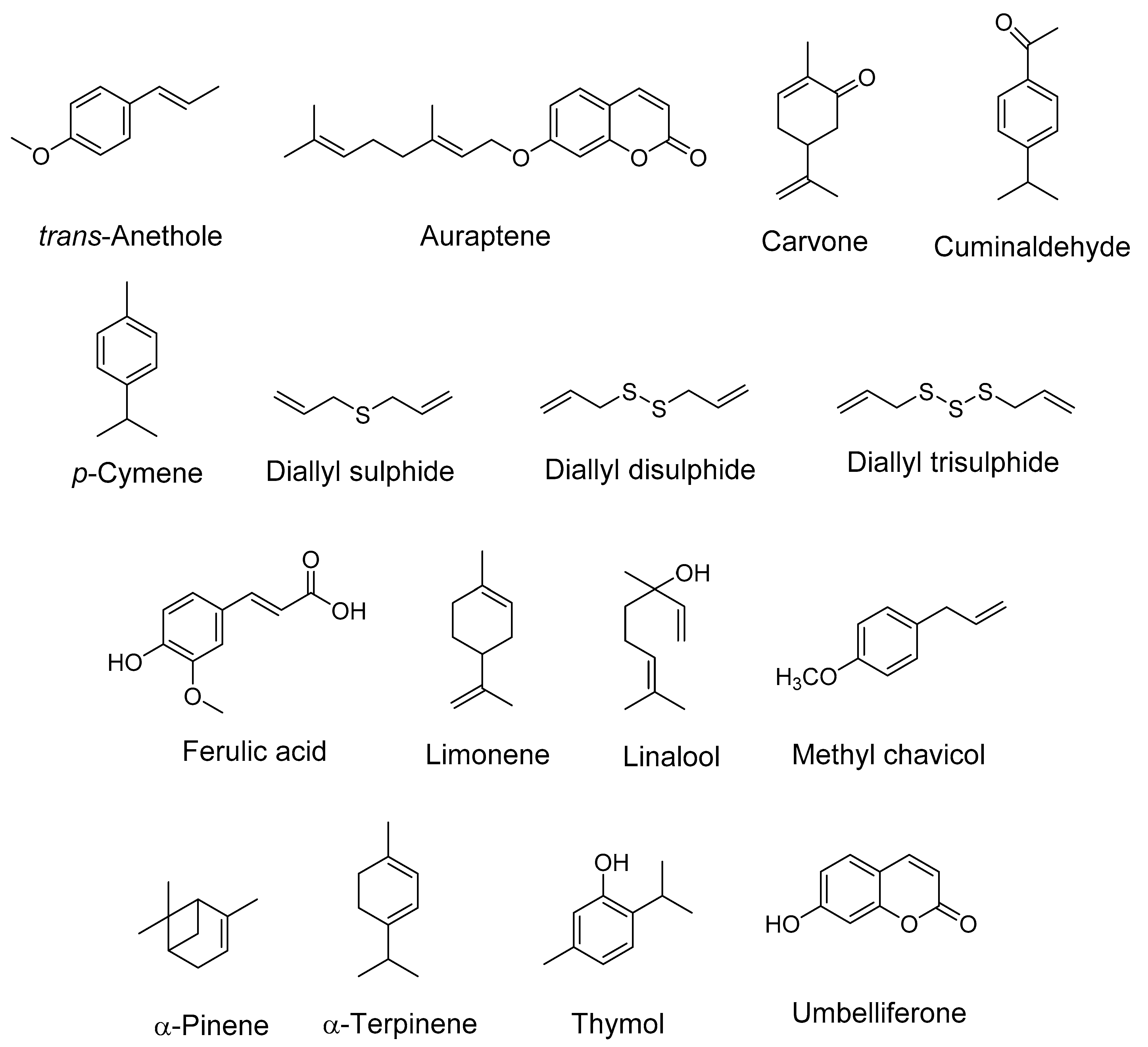
Figure 1.
Structures of some important bioactive compounds present in spices (Apiaceae family).
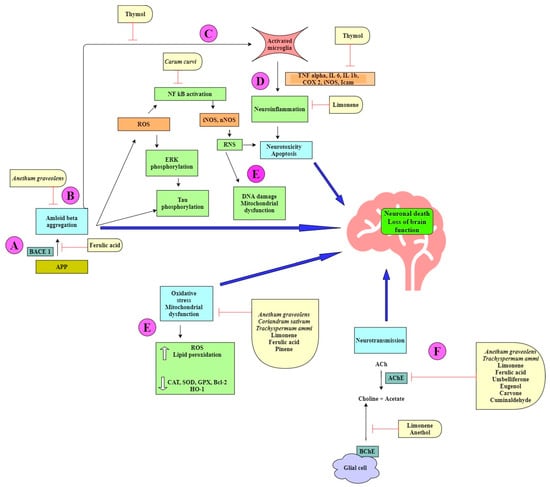
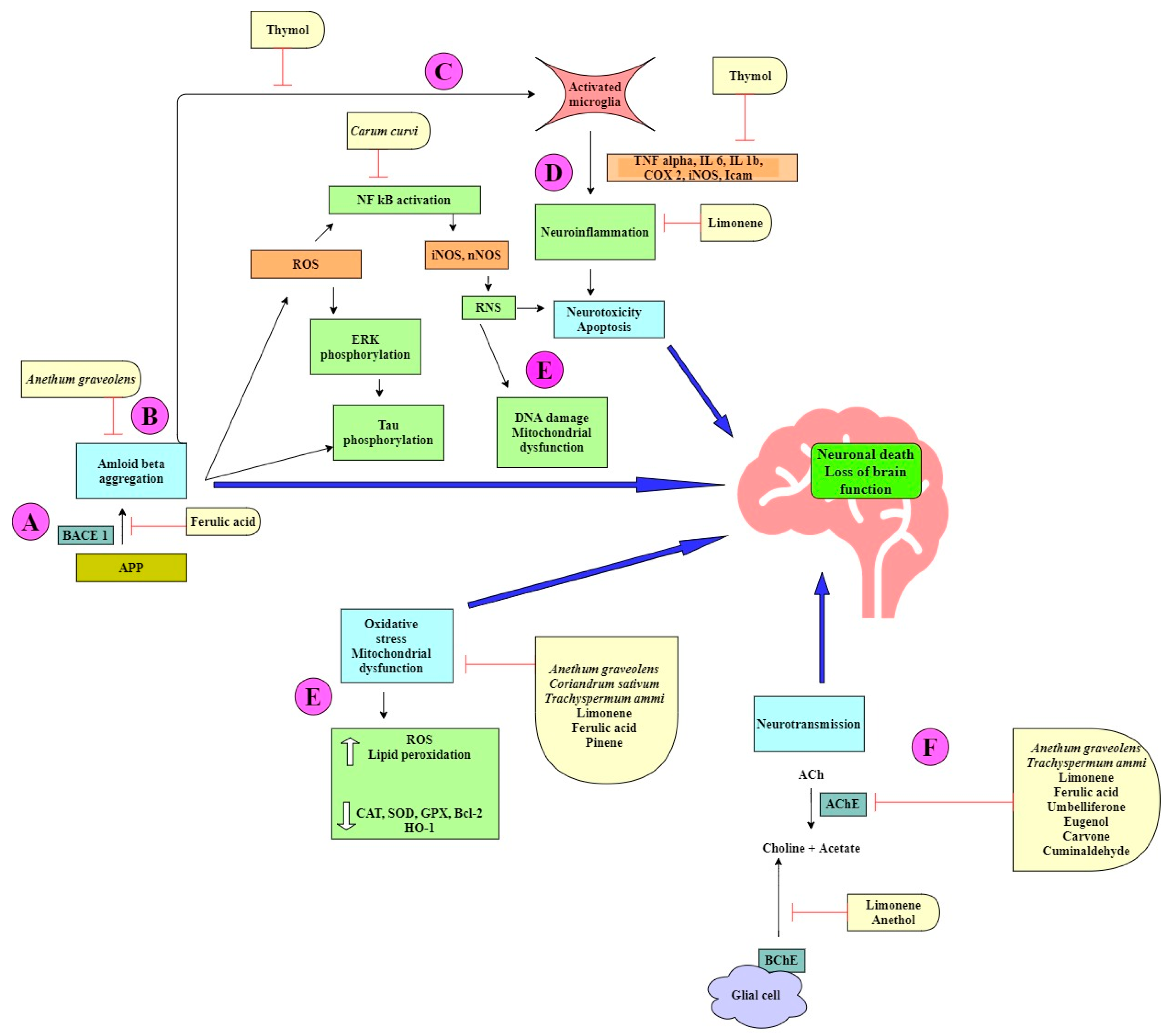
Figure 2.
Neuroprotective mechanisms in Alzheimer’s disease displayed by spice extracts and/or natural products belonging to the Apiaceae family.
The spice extracts and their phytochemicals exert a multitarget approach to ameliorate symptoms of AD (Figure 2). Some components prevent amyloid-beta aggregation by inhibiting the cleavage of the amyloid precursor protein (APP) by β-secretase (BACE-I) (Figure 2A). This causes a shift in the nonamyloidogenic pathway and reduces the levels of Aβ produced [24,53]. Aβ can self-aggregate to form oligomers and eventually amyloid plaques (Figure 2B). Some bioactive components are able to inhibit the formation of amyloid plaques by binding to Aβ, inhibiting aggregation, and thereby promoting the formation of nontoxic oligomers [45,61]. Toxic Aβ monomers and oligomers have been shown to induce microglial activation and proliferation (Figure 2C). Activated microglia secrete proinflammatory cytokines such as IL-1β and IL-6. Some natural products have been shown to reduce the levels of these cytokines [37,39,54]. Microglia also play a role in generating reactive nitrogen species (RNS), which further contribute to neurodegeneration [42,43] (Figure 2D). ROS and RNS irreversibly oxidize DNA and are important mediators of Aβ-induced neuronal cell death in the development of AD (Figure 2E). Many phytochemicals reduce oxidative stress by increasing the levels of antioxidant enzymes and reducing lipid peroxidation [30,36,37,38,39]. Acetylcholine (ACh), a neurotransmitter essential for processing memory and learning, is decreased in both concentration and function in AD (Figure 2F). Decreased levels of ACh can be restored by anticholinesterase activity of various bioactive compounds [24,25,44,63].
2.1. Anethum graveolens
Anethum graveolens (dill) seeds are generally used as a spice, flavoring, and seasoning agent in food such as pickles, salads, etc. Dill essence is rich in flavonoids, a subclass of phytoestrogens that may be accountable for having positive effects on memory enhancement, increasing levels of acetylcholine (Mesripour et al. 2016), and displaying potent antioxidant activity [64]. The most abundant constituents of essential oils in seeds were found to be carvotanacetone (21.76 ± 1.62%), dill apiole (18.65 ± 1.89%), limonene (9.01 ± 1.11%), dill ether (9.13 ± 1.12%), 4-isopropyltoluene (8.24 ± 0.89%), and myrcene (7.44 ± 0.68%) [64].
The methanolic extract of A. graveolens seeds demonstrated moderate neuroprotective effects in PC12 cells treated with Aβ (25–35) aggregates with an ED50 value of 18.8 µg/mL [45]. The administration of A. graveolens ethanolic leaves extract significantly improved the learning and memory damage induced by scopolamine in Morris water maze and elevated plus maze experiments. A substantial decrease in AChE activity, increased activity of brain antioxidant enzymes such as superoxide dismutase, and decreased lipid peroxidation were also observed in the study. The most potent action was seen at a dose of 400 mg/Kg body weight [46].
The memory-enhancing activity of A. graveolens aqueous extract (100, 200, and 300 mg/Kg body weight) was also appraised by the conditioned avoidance response (CAR) technique in rats using Cook’s pole climbing apparatus [48]. Changes in cognition, retention, and recovery in rats were dose dependent. The extract also inhibited lipid peroxidation in both liver and brain tissues, suggesting the role of extract in reducing oxidative stress. In another study, the protective effect of A. graveolens aqueous extract was studied on hypercholesterolemia-induced cognitive deficits (HCDs) and oxidative stress in hippocampus tissues of rats [47,49]. HCD considerably augmented serum cholesterol levels, induced Aβ deposition, transformed morphology of hippocampus, and impaired memory function. However, the changes were reversed by administration of A. graveolens extract, which acted by increasing antioxidant levels in the brain, lowering serum cholesterol, retarding Aβ deposition, and normalizing hippocampal morphology.
PM52, a combined extract of Cissampelos pareira and A. graveolens, was evaluated against age-related cognitive impairment in a rat model. The data proposed that the cognitive-enhancing effect of PM52 might be due to suppression of AchE, resulting in increased levels of acetylcholine, a neurotransmitter that plays an important role in learning and memory and enriching neuron density in hippocampus by reducing the oxidative stress [50].
Hence, A. graveolens extracts and active constituents improved cognitive function in AD brain mainly by inhibiting AChE, improving oxidative stress conditions, and retarding amyloid β aggregation.
2.2. Carum carvi
Carum carvi (caraway) is a biennial herb, the dried fruit of which is used as a spice due to its pleasing odor and sharp taste. The main components of essential oil are carvone (44.5–95.9%) and limonene (1.5–51.3%) and minor amounts of β-myrcene, trans-dihydrocarvone, trans-carveole (0–0.2%), α-pinene, sabinene, n-octanal, trans-β-ocimene, δ-terpinene, linalool, cis- and trans-limonene oxide, cis-dihydrocarvone, cis-carveol, perillaldehyde, trans-anethole, and trans-β-caryophyllene [65].
Microglia plays a dual role (neuroprotective or neurotoxic) in the progression of AD [66]. Microglia are regarded as initiators of neuroinflammation [67] and might play a role in the atypical networking in AD brain [68]. Various proinflammatory and neurotoxic substances, such as NO, iNOS, and COX-2, are produced by activated microglia. Therefore, reducing neuroinflammation by reducing microglia activation could be a promising therapeutic target in treatment of neuroinflammatory-mediated neurodegenerative diseases such as AD. It has already been reported that natural products with antineuroinflammatory activity are able to produce antiamyloid [69]. In an in vitro study, C. carvi aqueous extract was evaluated for its protective effects on LPS-activated neuroinflammation in BV-2 microglial cells. The extract inhibited LPS-induced phosphorylation/degradation of IκBα and translocation of NF-κB/p65 subunit in a concentration-dependent manner, which means the C. carvi extract plays an important role in regulating NF-κB signaling [51]. Carvone, the major component of C. carvi oil, is known to exhibit anti-inflammatory properties by inhibiting the synthesis of leukotrienes and prostaglandins [26]. Therefore, it is quite possible that carvone plays a role in modulation of the NF-κB pathway. Antioxidant, adaptogenic, and memory-enhancer activities of C. carvi aqueous extract were evaluated in rats using Cook’s pole climbing apparatus. The extract decreased lipid peroxidation in liver and brain homogenates [52]. Essential oil (EO) of C. carvi displayed strong in vitro anti-AChE activity (IC50 = 0.82 ± 0.05 mg/mL) compared to the reference drug galantamine (IC50 = 1.05 ± 0.05 mg/mL) [53]. Pharmacokinetics profiling of selected components of essential oils indicated their ability to penetrate the blood–brain barrier moderately (log BB values = 0.818–0.478). They also demonstrated high CaCO-2 permeability (log Papp values > 0.90 cm/s). All tested compounds were predicted neither substrates nor inhibitors of the human cytochrome P450 (CYP) isoforms. The boiled-egg graph (WLOGP vs. TPSA) prediction of GI absorption and BBB permeation, which helps in the calculation of polarity and lipophilicity, indicated that they possess a high probability of brain penetration [53].
Carvone, the main constituent of C. carvi, has been reported as an AChE inhibitor (IC50 = 2.9 ± 0.12 mM) compared to the reference drug galantamine (IC50 = 0.14 ± 0.005 mM) [27]. The inhibition is noncompetitive [70]. Additionally, docking studies revealed a putative H-bond interaction between the carvone and Tyr337 (2.92Å) of AChE, creating an anionic subsite. Other binding sites include Trp86, Tyr133, Tyr337, Phe338 (an anionic subsite), His447, Ser203 (an esteratic site), Gly121, Gly122 (oxyanion hole), Ile451, Gly448, Glu202, Gly120, and Ser125, all of which are most important portions of the AchE binding site [27]. In a recent study, (‒)-cis-carveol, a reduction product of carvone, improved Aβ1-42-induced memory deficits in an animal model, examined by Y-maze and radial arm maze in vivo tests [71]. The biochemical analyses of the hippocampus homogenates showed a reduction in oxidative stress parameters caused by Aβ1-42, suggesting the role of carveol in neuroprotection.
The second most important constituent in C. carvi is limonene, a monoterpene. Its protective role in spatial memory and anxiety has been established in a rat model exposed to immobilized stress [72]. Limonene also had a positive effect on scopolamine-induced amnesia, where it improved the modifications caused by scopolamine in a short-term memory test. It ameliorated the levels of oxidative stress markers (MDA, SOD, GSH) and inhibited AchE and BchE [20,37]. A study examined the effectiveness of limonene against Aβ1-42-induced neurotoxicity in a Drosophila model of AD. The results showed that limonene suppressed the neuronal cell death induced by Aβ42 and reduced oxidative stress, which prevented ERK phosphorylation [39]. In another study, the neuroprotective effect of limonene against neurotoxicity elicited by Aβ1-42 in Hoechst 33,258 cell lines was observed. Limonene decreased ROS production and prevented the upregulation of Kv3.4 (voltage-gated potassium channel) activity at 10 µg/mL. This channel is overexpressed in AD and other neurodegenerative diseases [36]. Downregulation of Kv3.4 expression by limonene prevented cell death in primary cortical neurons, thus confirming its neuroprotective function in AD [38]. Moreover, limonene displayed a specific activity almost comparable to galantamine, the reference drug used against AchE.
Hence, maintaining the levels of antioxidant enzymes, inhibiting AchE, suppressing neuroinflammation and downregulating voltage-gated potassium channels are the main roles of C. carvi extract and active components in combating AD.
2.3. Coriandrum sativum
Coriandrum (coriander) is a feathery annual plant, used as both an herb and a spice. The main constituents of coriander oil are linalool (64.2−79.9%), γ-terpinene (5.8−13.6%), neryl acetate (2.3−8.4%), α-pinene (2.8−7.1%), and p-cymene (1.1−3.6%) [73]. In the Aβ1-42 AD model, a test group of rats as made to inhale essential oil (1% and 3%) from C. sativum seed. Inhalation of essential oil considerably reduced levels of LDH and MDA, with an increase in glutathione peroxidase levels in the hippocampal region of rats. Additionally, there were fewer amyloid deposits in rats treated with EO. Specifically, linalool was found to be the active constituent in the EO; therefore, it can be speculated that linalool is responsible for cognitive-enhancing effects, along with antiapoptotic activities in Aβ1-42-treated rats. The antioxidant defense, along with a decrease in lipid peroxidation, could be correlated with involvement of linalool in neuroprotection [40]. C. sativum seed extract is reported to be nontoxic in up to 3000 mg/kg body weight (BW) and, thus, can be considered safe for intake [74]. In a recent experiment, C. sativum seed extract (200 mg/Kg BW) improved memory impairment in senescence-accelerated mouse-prone 8 (SAMP8) mice. The mRNA levels of nNOS were higher in diseased the frontal lobe of diseased animals, which decreased significantly with the treatment of extract, suggesting that C. sativum can reduce the production of RNS and ROS and thus improve oxidative stress conditions. Moreover, the mRNA level of neurofilament light (NF-L), an important protein in memory retention and synaptic plasticity, was found lower in the frontal lobe and hippocampus of untreated mice, indicating neuronal damage. However, the mRNA levels NF-L were elevated after extract administration [54], indicating the role of C. sativum in neuroprotection. Previous studies have shown that α-pinene, γ-terpinene, and many monoterpenoids have anti-AchE activity [27,75,76]. As these phytocompounds are present in C. sativum, it is very much likely that its extract will also show anti-AchE activity.
Therefore, as described above, C. sativum extract and its bioactive compounds play an imperative role in neuroprotection by reducing ROS/RNS, elevating the level of an important protein involved in synaptic plasticity, and suppressing AchE activity.
2.4. Cuminum cyminum
Cuminum cyminum (cumin) is the second most popular spice in the world after black pepper. It is a slender, annual, glabrous herb, and its seeds have been traditionally used in colic pain, abdominal discomfort, and deficient lactation. It also possesses antioxidant, anti-inflammatory, antibacterial, and antidiabetic activities [77,78,79]. Cumin seed is valued for its aroma, which is due to the presence of cuminaldehyde, cuminic alcohol, p-cymene, o-cymene, γ-terpinene, α-terpinene, p-menthadienol, and β-pinene as some of its chief components [80,81]. High levels of a rare, monounsaturated, omega-12 fatty acid, petroselinic fatty acid (C18:1), are also present in cumin [82].
Cuminaldehyde was displayed to play a role in neuroprotection and spatial learning and memory enhancement through the modulation of genes (Bdnf, Icam, ApoE, IL-6) coding for neurotrophic factors and/or those associated in damaging synaptic plasticity in both in vitro and in vivo experiments [28]. Memory-enhancing activity of C. cyminum water extract was reported in animal models of AD by maintaining the levels of antioxidant enzymes [55,56]. It also showed competitive inhibitory activity for AchE in vitro at low concentrations (12.5 μg/mL and 25 μg/mL) and mixed inhibition at higher concentrations (50 µg/mL) [57,83]. Additionally, cumin essential oil (cuminaldehyde) and its n-hexane fraction strongly inhibited α-synuclein (α-SN) aggregation in a concentration-dependent manner in PC12 cells by inhibiting the fibrillation process [59]. In a docking study [84], cuminaldehyde showed a docking score of −7.5, similar to that of selegiline (a known inhibitor), against monoamine oxidase (MAO-B), an enzyme that deactivates neurotransmitters such as dopamine, and other neuromodulatory amines such as polyamines [85]. The expression of MAO-B is enhanced in the hippocampus and cerebral cortex of AD brains compared to healthy brains [86]. Therefore, MAO-B inhibitors can be an alternative, Aβ-independent strategy to target AD [87].
To summarize, C. cyminum extract and important phytocompounds play a role in neuroprotection and cognitive enhancement by modulating the genes involved in preserving synaptic plasticity, repressing oxidative stress, and inhibiting enzymes involved in neurotransmission.
2.5. Ferula asafoetida
Ferula asafoetida (asafoetida) is a gum-like exudate from underground rhizomes or taproots of the plant. Asafoetida usually contains approximately 40–64% resin, 25% endogenous gum, and 10–17% volatile oil. The resin portion mainly contains asaresinotannols A and B, ferulic acid, and umbelliferone. The volatile oil is rich in various organosulfide compounds, such as 2-butyl-propenyl-disulfide, diallyl sulfide, diallyl disulfide, and dimethyl trisulfide [88,89]. The organosulfides are largely accountable for the peculiar smell and flavor of asafoetida.
In a previous report, in vitro and in vivo findings indicated that two natural components of some Ferula species, namely umbelliferone and ferulic acid, act as competitive AchE inhibitors. Umbelliferone markedly replenished nuclear factor erythroid-derived 2-like 2 (Nrf2) and heme oxygenase-1 (HO-1) levels in streptozotocin (STZ)-induced cognitive dysfunction in rats [44]. Oral administration of ferulic acid (30 mg/Kg) for 6 months decreased cleavage of the β-carboxyl-terminal APP fragment, BACE-I activity, neuroinflammation, and stabilized oxidative stress in a transgenic PS/APP mouse model of AD. As a result, significant cognitive improvement was observed in the animals [32]. It also inhibits BACE1 enzymatic action, as well as its mRNA expression level [90]. In addition, eugenol and limonene, which are among volatile constituents of some Ferula species, were shown to inhibit AchE [91]. Moreover, auraptene, which is a prenylated coumarin present in some Ferula species, displayed a neuroprotective effect with mild AchE-inhibitory activity [25]. Long-term administration of ferulic acid, another important component of Ferula, was reported to protect mice against Aβ-induced learning and memory deficits in vivo [31,35] and protect neurons against Aβ-induced oxidative stress and neurotoxicity in vitro [34] by modulating oxidative stress directly and by inducing protective genes such as heme oxygenase-1 (HO-1) and heat shock protein 72 (Hsp72). In another study, the same compound showed a protective role in trimethyltin-induced memory injury in a mouse model and decreased AchE activity [92].
Ferulic acid interferes with the apoptotic pathways induced by oxidative stress and inflammation due to Aβ aggregation [33]. Ferulic acid is also a potent antioxidant and has anti-inflammatory properties. Most importantly, it directly alters the kinetics of Aβ fibril formation and can directly inhibit plaque formation in vitro [93]. However, the oral administration of ferulic acid was unable to display any significant effect on Aβ oligomers or Aβ deposition in vivo [94], which could be due to poor absorption or difficulty in crossing BBB in the body. Ferulic acid has also been involved in enhancing the cell stress response by regulating heme oxygenase/Hsp70 [95], heme oxygenase/biliverdin reductase (HO/BVR) system [96,97], superoxide dismutase (SOD), catalase [98], ERK ½, and Akt [99] and inhibiting caspases [100].
Diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) are lipid soluble allyl sulfur compounds [101] and have potent antioxidant activity. They have the ability to trap trichloromethyl and trichloromethyl peroxyl free radicals. DADS also inhibited carbon tetrachloride-induced lipid peroxidation [30]. As lipid peroxidation increases in AD, these compounds might display antioxidant potential and reduce oxidative stress in the brain.
Thus, modulating oxidative stress, suppressing neuroinflammation, and inhibiting BACE-I and AchE activity are the modes of action of Ferula asafoetida in targeting AD.
2.6. Foeniculum vulgare
Foeniculum vulgare (fennel) is a perennial herb with seeds rich in essential oil, responsible for its aroma and taste. GC-MS analysis identified the main components of oil as trans-anethol (84.1–86.1%), fenchone (7.13–8.86%), limonene (3.0–3.3%), and methyl chavicol (2.5–2.7%) [102]. Fennel seeds also contain fatty acids such as petroselinic acid (62.08–66.71%), 10-nonadecanone (4.70–22.80%), and linoleic acid (1.32–7.59%) and minor amounts of oleic acid, stearic acid, eicosanoic acid, and linolenic acid [103].
In a scopolamine-induced rat model, fennel water extract potently inhibited lipid peroxidation in both rat liver and brain homogenates and improved memory, which can be correlated with the strong, antioxidant capacity of the extract [60]. Moreover, methanolic extract also ameliorated symptoms in a scopolamine-induced mouse model [104]. In a similar experiment, ethanolic extract (200 mg/Kg/d) displayed a neuroprotective effect in a lead-induced neurotoxicity mouse model by restoring the levels of oxidative stress markers and APP isoforms in cortex and hippocampus [61].
More recently, both fennel and trans-anethole were reported to prevent and treat stress-induced neurological disorders, such as memory and learning in isolated rats [105]. by strengthening the antioxidant system and reducing neuronal inflammation. Anethole also possesses both AchE and BchE inhibitory activity with IC50 values of 39.89 ± 0.32 μg/mL and 75.35 ± 1.47 μg/mL, respectively [24]. Methyl chavicol is a phenylpropene or estragole in nature, and these compounds are potent AchE inhibitors [106] with an IC50 of 0.337 µM [41]. Another compound, limonene (+), displayed a neuroprotective effect in Aβ1-42-induced neurotoxicity in a Drosophila model of AD by reducing ROS production, kinase phosphorylation, neuroinflammation, and cell death without affecting Aβ42 accumulation and aggregation [107].
Limonene also had a beneficial effect on scopolamine-induced amnesia by reducing oxidative stress markers and by inhibiting AchE and BchE activity (24.97% and 69.12%, respectively) [37]. The interaction of limonene with the hydrophobic choline esterase binding site could be the reason for inhibition [108].
In summary, F. vulgare extract and its bioactive components improve AD condition by inhibiting the enzymes involved in neurotransmission, suppressing oxidative stress, and neuroinflammation.
2.7. Trachyspermum ammi
Trachyspermum ammi (also known as ajowan, thymol seeds, bishop’s weed, or carom) is an annual herbaceous and aromatic plant. It primarily contains essential oils such as thymol, α- and γ-terpinene, p-cymene, and α- and β-pinenes [109].
In a recent experiment, alprazolam, scopolamine, and electroshock were used to induce amnesia in mice, and the effect of T. ammi seed (0.5%, 1.0%, and 2.0% w/w of normal diet) was studied on the learning and memory of mice using the passive avoidance paradigm and object recognition task (ORT) [62]. The supplementation of T. ammi not only enhanced step-down latency passive avoidance response and discrimination index of ORT animals but also considerably reduced brain AchE activity and oxidative damage by decreasing the levels of MDA and nitrite and increasing GSH. In a scopolamine-induced zebrafish model of memory impairments, T. vulgaris essential oil was reported to augment cognitive function through action on cholinergic neurons [63]. The essential oil decreased AChE activity and increased brain antioxidant capacity. The chief components detected by GC-MS were thymol (42%) and p-cymene (19%), which indicates the role of these two in neuroprotection. The activity of p-cymene (50 and 100 mg/Kg) was also assessed in a Aβ1–42-treated rat model of AD [29]. Results demonstrate that both doses have a positive effect on the learning and memory functions of the rats and reduce amyloid plaque deposition. Thymol possesses strong, antioxidant properties and inhibits β-amyloid in (Aβ)-induced cognitive-impaired rats [42,43], which suggests that thymol can attain the appropriate concentrations required to exert its therapeutic effects on neurons by crossing the BBB. Thymol treatment significantly decreased the number of activated astrocytes and microglia in the striatum region in ROT-injected animals. The reduction in the number of glial cells and reduced expression of COX-2 and iNOS following thymol treatment is suggestive of its anti-inflammatory effects [110].
In a study, α-pinene improved learning and memory in scopolamine-induced memory deficit in C57BL/6 mice by ameliorating the expression of proteins related to the synthesis of acetylcholine and antioxidant defense system [111]. However, α-terpinene displayed its neuroprotective action by altering the activity of enzymes responsible for neuronal plasticity and hydrolysis of ADP and ATP in female Wistar rats [112].
To conclude, T. ammi extract and active constituents act by inhibiting AChE, reducing amyloid aggregation, preventing neuroinflammation, and enhancing brain antioxidant activity.
3. Clinical Studies
A number of clinical trials have been conducted to study the effect of spices belonging to the Apiaceae family in several diseases such as diabetes [113,114], obesity [115,116,117], hyperlipidemia [118,119], metabolic syndrome [120,121], functional dyspepsia [122], neuropathic pain [123], arthritis [124], skin diseases [125], gynecological problems [126,127,128,129,130], and dental diseases [131]. All these studies ruled out any safety concerns with these spices. However, no preclinical or clinical study has been reported for the effectiveness of these spices in neurodegenerative diseases, even though in vivo and in vitro literature suggest their immense neuroprotective potential. Hence, there is a tremendous scope of preclinical and clinical trials on these spices.
4. Future Perspectives
The promising pharmacological effects of various plant extracts from the identified Apiaceae spices may pave the way for a deeper investigation of potential biologically active natural products. Aside from allyl sulfides, asaresinotannols A and B, umbelliferone, and ferulic acid, which were characterized in Ferula asafoetida, the reported components from the Indian spices were mostly monoterpenes concentrated in the essential oils. Over the years, the isolation of natural products possessing diverse structures and pharmacological activities was made possible using their crude extracts. Depending on the nature of the research, several approaches can be utilized in examining the potential active metabolites. These natural products have been used for further medicinal chemistry research, paving the way for compounds in the pipeline for clinical studies. The limited information on the potential bioactive natural products from the spices of the Apiaceae family has disclosed the need for in-depth phytochemical and pharmacological analyses. Currently, bioassay-guided isolation is our laboratory’s custom in the identification of neuroprotective natural products from plant sources. Aside from the essential oils, our interest is focused on examining neuroprotective compounds from the crude extracts, utilizing both chromatography and well-established bioassays in our laboratory.
5. Conclusions
AD is a predominant neurodegenerative disease with no effective drug treatment to date; therefore, the investigation of new phytochemicals from plant sources is utterly required. Plant secondary metabolites such as alkaloids, flavonoids, and phenolic acids play a key role in ameliorating disease conditions in AD. Essential oils also play an important role in AD pathogenesis, as they are a rich source of antioxidants, and most of them exhibit cholinesterase inhibitory potential. It is understood that as plant extracts contain several bioactive compounds, they can work additively or synergistically to display a variety of neuroprotective mechanisms that might be an effective tactic in AD drug discovery. Further research on ideal dosage, mode of action at both the molecular and cellular levels, in vivo effects, and pharmacokinetic profile of the discussed phytocompounds and plant extracts can lead to more effective new anti-AD product development in the future.
Author Contributions
Conceptualization, S.S.A.A. and N.S.; writing—original draft preparation, N.S. and M.A.T.; writing—review and editing, N.S., M.A.T. and S.S.A.A.; funding acquisition, S.S.A.A. All authors have read and agreed to the final version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea by the Korean Government (2020R1A2B5B01002463 and 2021R1A6A1A03038996).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alzheimer’s Association Report. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system—Too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing! Age Ageing 2006, 35, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Podtelezhnikov, A.A.; Tanis, K.Q.; Nebozhyn, M.; Ray, W.J.; Stone, D.J.; Loboda, A.P. Molecular insights into the pathogenesis of Alzheimer’s disease and its relationship to normal aging. PLoS ONE 2011, 6, e29610. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity. J. Exp. Neurosci. 2016, 10, JEN.S39887. [Google Scholar] [CrossRef]
- Sharma, J.; Chawla, R.; Kumar, R.; Sharma, A.; Sharma, R.; Arora, R. Camellia sinensis as a safe neuroprotective radiation counter measure agent. Int. J. Pharm. Sci. Invent. 2013, 2, 26–33. [Google Scholar]
- Bayer, T.A.; Schäfer, S.; Breyhan, H.; Wirths, O.; Treiber, C.; Multhaup, G. A vicious circle: Role of oxidative stress, intraneuronal Aβ and Cu in Alzheimer’s disease. Clin. Neuropathol. 2006, 25, 163–171. [Google Scholar]
- Ono, K.; Hamaguchi, T.; Naiki, H.; Yamada, M. Anti-amyloidogenic effects of antioxidants: Implications for the prevention and therapeutics of Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 575–586. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Reiter, C.D.; Teng, R.J.; Beckman, J.S. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J. Biol. Chem. 2000, 275, 32460–32466. [Google Scholar] [CrossRef]
- Grothe, M.; Zaborszky, L.; Atienza, M.; Gil-Neciga, E.; Rodriguez-Romero, R.; Teipel, S.J.; Amunts, K.; Suarez-Gonzalez, A.; Cantero, J.L. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer’s disease. Cereb. Cortex 2010, 20, 1685–1695. [Google Scholar] [CrossRef]
- Grant, W.B. Dietary links to Alzheimer’s disease. J. Alzheimers Dis. Rev. 1997, 2, 42–55. [Google Scholar]
- Dodge, H.H.; Buracchio, T.J.; Fisher, G.G.; Kiyohara, Y.; Meguro, K.; Tanizaki, Y.; Kaye, J.A. Trends in the prevalence of dementia in Japan. Int. J. Alzheimers Dis. 2012, 2012, 956354. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.M.; Vlad, A.T.; Oniga, I. Phytochemical profile, antioxidant, cardioprotective and nephro-protective activity of Romanian Chicory extract. Plants 2021, 10, 64. [Google Scholar] [CrossRef]
- Saravanan, S.; Parimelazhagan, T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.J.; Zhang, P.; Kang, S.G. Antioxidant, anti-inflammatory, and α-amylase inhibitory activities of Ulva lactuca extract. Korean J. Food Preserv. 2020, 27, 513–521. [Google Scholar] [CrossRef]
- Kannappan, R.; Gupta, S.C.; Kim, J.H.; Reuter, S.; Aggarwal, B.B. Neuro-protection by spice-derived nutraceuticals: You are what you eat! Mol. Neurobiol. 2011, 44, 142–159. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Chokr, A.; Kanaan, H.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crop. Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and phenylpropanoids as acetyl- and butyrylcholinesterase inhibitors: A comparative study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Talou, T.; Evon, P.; Cerny, M.; Merah, O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020, 326, 127034. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Dobri, A.M.; Dudău, M.; Enciu, A.M.; Hinescu, M.E. CD36 in Alzheimer’s disease: An overview of molecular mechanisms and therapeutic targeting. Neuroscience 2020, 453, 301–311. [Google Scholar] [CrossRef]
- Bhadra, S.; Mukherjee, P.K.; Kumar, N.S.; Bandyopadhyay, A. Anticholinesterase activity of standardized extract of Illicium verum Hook f fruits. Fitoterapia 2011, 82, 342–346. [Google Scholar] [CrossRef]
- Epifano, F.; Molinaro, G.; Genovese, S.; Ngomba, R.T.; Nicoletti, F.; Curini, M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci. Lett. 2008, 443, 57–60. [Google Scholar] [CrossRef]
- Agrahari, P.; Singh, D.K. A review on the pharmacological aspects of Carum carvi. J. Biol. Earth Sci. 2014, 4, M1–M13. [Google Scholar]
- Wojtunik-Kulesza, K.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Omari, Z.; Kazunori, S.; Sabti, M.; Bejaoui, M.; Hafidi, A.; Gadhi, C.; Isoda, H. Dietary administration of cumin-derived cuminaldehyde induce neuroprotective and learning and memory enhancement effects to aging mice. Aging 2021, 13, 1671–1685. [Google Scholar] [CrossRef]
- Nahavandi, B.S.; Yaghmaei, P.; Ahmadian, S.; Ebrahim-Habibi, A.; Ghobeh, M. Effects of terpinolene and physical activity on memory and learning in a model of Alzheimer’s disease among rats. Qom Univ. Med. Sci. J. 2020, 14, 25–33. [Google Scholar]
- Fanelli, S.L.; Castro, G.D.; de Toranzo, E.G.; Castro, J.A. Mechanisms of the preventive properties of some garlic components in the carbon tetrachloride-promoted oxidative stress. Diallyl sulfide, diallyl disulfide, allyl mercaptan and allyl methyl sulfide. Res. Commun. Mol. Pathol. Pharmacol. 1998, 102, 163–174. [Google Scholar]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long term administration of ferulic acid on microglial activation induced by intercerebroventricular injection of beta amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758. [Google Scholar] [CrossRef]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef]
- Angulo, E.; Noé, V.; Casadó, V.; Mallol, J.; Gomez-Isla, T.; Lluis, C.; Ferrer, I.; Ciudad, C.J.; Franco, R. Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer’s disease. J. Neurochem. 2004, 91, 547–557. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Orhan, I.E.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by Aβ1-42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Tramutola, A.; Triani, F.; Di Domenico, F.; Barone, E.; Cai, J.; Klein, J.B.; Perluigi, M.; Butterfield, D.A. Poly-ubiquitin profile in Alzheimer disease brain. Neurobiol. Dis. 2018, 118, 129–141. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β (1–42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Naturforsch. C 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Asadbegi, M.; Komaki, A.; Salehi, I.; Yaghmaei, P.; Ebrahim-Habibi, A.; Shahidi, S.; Sarihi, A.; Asl, S.S.; Golipoor, Z. Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res. Bull. 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Azizi, Z.; Ebrahimi, S.; Saadatfar, E.; Kamalinejad, M.; Majlessi, N. Cognitive enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 2012, 23, 241–249. [Google Scholar] [CrossRef]
- Hindam, M.O.; Sayed, R.H.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a strep-tozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytother. Res. 2020, 34, 2351–2365. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.S.; Hong, S.S.; Sul, D.; Hwang, K.W.; Lee, D. The neuroprotective effects of traditional oriental herbal medicines against β-amyloid-induced toxicity. Pharm. Biol. 2009, 47, 976–981. [Google Scholar] [CrossRef]
- Kumar, N.; Dhiman, C.; Kothiyal, P. Evaluation of Anethum graveolens extract on memory impaired mice. Indo Am. J. Pharm. Sci. 2017, 4, 1965–1975. [Google Scholar]
- Heshami, N.; Mohammadali, S.; Komaki, A.; Tayebinia, H.; Karimi, J.; Oshaghi, E.A.; Hashemnia, M.; Khodadadi, I. Favorable effects of dill tablets and Ocimum basilicum L. extract on learning, memory, and hippocampal fatty acid composition in hypercholesterolemic rats. Iran. J. Basic Med. Sci. 2021, 24, 300–311. [Google Scholar]
- Koppula, S.; Choi, D.K. Anethum graveolens Linn (Umbelliferae) extract attenuates stress-induced urinary biochemical changes and improves cognition in scopolamine induced amnesic rats. Trop. J. Pharm. Res. 2011, 10, 47–54. [Google Scholar] [CrossRef]
- Mohammadali, S.; Heshami, N.; Komaki, A.; Tayebinia, H.; Oshaghi, E.A.; Karimi, J.; Hashemnia, M.; Khodadadi, I. Dill tablet and Ocimum basilicum aqueous extract: Promising therapeutic agents for improving cognitive deficit in hypercholesterolemic rats. J. Food Biochem. 2020, 44, e13485. [Google Scholar] [CrossRef]
- Thukham-Mee, W.; Wattanathorn, J. Evaluation of safety and protective effect of combined extract of Cissampelos pareira and Anethum graveolens (PM52) against age-related cognitive impairment. Evid.-Based Complement. Altern. Med. 2012, 2012, 674101. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Koppula, S. Carum carvi Linn (Umbelliferae) attenuates lipopolysaccharide-induced neuroinflammatory responses via regulation of NF-κB signaling in BV-2 Microglia. Trop. J. Pharm. Res. 2015, 14, 1041–1047. [Google Scholar] [CrossRef]
- Koppula, S.; Kopalli, S.R.; Sreemantula, S. Adaptogenic and nootropic activities of aqueous extracts of Carum carvi Linn (Caraway) fruit: An experimental study in Wistar rats. Aust. J. Med. Herb. 2009, 21, 72–78. [Google Scholar]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Mima, Y.; Izumo, N.; Chen, J.-R.; Yang, S.-C.; Furukawa, M.; Watanabe, Y. Effects of Coriandrum sativum seed extract on aging-induced memory impairment in Samp8 mice. Nutrients 2020, 12, 455. [Google Scholar] [CrossRef]
- Kim, J.B.; Kopalli, S.R.; Koppula, S. Cuminum cyminum Linn (Apiaceae) extract attenuates MPTP-induced oxidative stress and behavioral impairments in mouse model of Parkinson’s disease. Trop. J. Pharm. Res. 2016, 15, 765–772. [Google Scholar] [CrossRef]
- Koppula, S.; Choi, D.K. Cuminum cyminum extract attenuates scopolamine-induced memory loss and stress-induced urinary biochemical changes in rats: A non-invasive biochemical approach. Pharm. Biol. 2011, 49, 702–708. [Google Scholar] [CrossRef]
- Kumar, S.; Brijeshlata, D.S.; Dixit, S. Screening of traditional Indian spices for inhibitory activity of acetylcholinesterase and butyrylcholinesterase enzymes. Int. J. Pharm. Bio. Sci. 2012, 3, 59–65. [Google Scholar]
- Fang, L.; Wang, X.; Guo, L.; Liu, Q. Antioxidant, antimicrobial properties and chemical composition of cumin essential oils extracted by three methods. Open Chem. 2018, 16, 291–297. [Google Scholar] [CrossRef]
- Morshedi, D.; Aliakbari, F.; Tayaranian-Marvian, A.; Fassihi, A.; Pan-Montojo, F.; Pérez-Sánchez, H. Cuminaldehyde as the major component Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food Sci. 2015, 80, H2336–H2345. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H. Foeniculum vulgare Mill (Umbelliferae) attenuates stress and improves memory in Wister rats. Trop. J. Pharm. Res. 2013, 12, 553–558. [Google Scholar] [CrossRef]
- Bhatti, S.; Ali Shah, S.A.; Ahmed, T.; Zahid, S. Neuroprotective effects of Foeniculum vulgare seeds extract on lead-induced neurotoxicity in mice brain. Drug Chem. Toxicol. 2018, 41, 399–407. [Google Scholar] [CrossRef]
- Soni, K.; Parle, M. Trachyspermum ammi seeds supplementation helps reverse scopolamine, alprazolam and electroshock induced amnesia. Neurochem. Res. 2017, 42, 1333–1344. [Google Scholar] [CrossRef]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris essential oil protects zebrafish against cognitive dysfunction by regulating cholinergic and antioxidants systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef]
- Ozliman, S.; Yaldiz, G.; Camlica, M.; Ozsoy, N. Chemical components of essential oils and biological activities of the aqueous extract of Anethum graveolens L. grown under inorganic and organic conditions. Chem. Biol. Technol. Agric. 2021, 8, 20. [Google Scholar] [CrossRef]
- Raal, A.; Arak, E.; Orav, A. The content and composition of the essential oil found in Carum carvi L. commercial fruits obtained from different countries. J. Essent. Oil Res. 2012, 24, 53–59. [Google Scholar] [CrossRef]
- Onuska, K.M. The dual role of microglia in the progression of Alzheimer’s disease. J. Neurosci. 2020, 40, 1608–1610. [Google Scholar] [CrossRef]
- Filipov, N.M. Overview of peripheral and central inflammatory responses and their contribution to neurotoxicity. Adv. Neurotoxicol. 2019, 3, 169–193. [Google Scholar]
- Passamonti, L.; Tsvetanov, K.A.; Jones, P.S.; Bevan-Jones, W.R.; Arnold, R.; Borchert, R.J.; Mak, E.; Su, L.; O’Brien, J.; Rowe, J. Neuroinflammation and functional connectivity in Alzheimer’s disease: Interactive influences on cognitive performance. J. Neurosci. 2019, 39, 7218–7226. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- López, M.D.; Campoy, F.J.; Pascual-Villalobos, M.J.; Muñoz-Delgado, E.; Vidal, C.J. Acetylcholinesterase activity of electric eel is increased or decreased by selected monoterpenoids and phenylpropanoids in a concentration-dependent manner. Chem. Biol. Interact. 2015, 229, 36–43. [Google Scholar] [CrossRef]
- Hritcu, L.; Boiangiu, R.S.; de Morais, M.C.; de Sousa, D.P. (‒)-cis-Carveol, a natural compound, improves β-amyloid-peptide 1-42-induced memory impairment and oxidative stress in the rat hippocampus. BioMed Res. Int. 2020, 2020, 8082560. [Google Scholar] [CrossRef]
- Bigdeli, Y.; Asle-Rousta, M.; Rahnema, M. Effects of limonene on chronic restraint stress-induced memory impairment and anxiety in male rats. Neurophysiology 2019, 51, 107–113. [Google Scholar] [CrossRef]
- Ebrahimi, S.N.; Hadian, J.; Ranjbar, H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Desai, S.; Devkar, R.; Ramachandran, A.V. Acute and sub-chronic toxicological evaluation of hydro-methanolic extract of Coriandrum sativum L. seeds. EXCLI J. 2012, 11, 566–575. [Google Scholar] [PubMed]
- Picollo, M.I.; Toloza, A.C.; Mougabure, C.G.; Zygadlo, J.; Zerba, E. Anticholinesterase and pediculicidal activities of monoterpenoids. Fitoterapia 2008, 79, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, B.; Yang, F.; Sun, Q.; Yang, Z.; Zhu, L. Chemical composition and anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stage. Iran. J. Pharm. Res. 2011, 10, 265–271. [Google Scholar] [PubMed]
- Allahghadri, T.; Rasooli, I.; Owlia, P.; Nadooshan, M.J.; Ghazanfari, T.; Taghizadeh, M.; Astaneh, S.D. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J. Food Sci. 2010, 75, H54–H61. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, L.; Pirbalouti, A.G.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) as a function of temperature. Int. J. Food Prop. 2017, 20, 1742–1750. [Google Scholar] [CrossRef]
- Willatgamuwa, S.A.; Platel, K.; Saraswathi, G.; Srinivasan, K. Antidiabetic influence of dietary cumin seeds (Cuminum cyminum) in streptozotocin induced diabetic rats. Nutr. Res. 1998, 18, 131–142. [Google Scholar] [CrossRef]
- Ali, A.; Jumma, H. Yield, quality and composition of cumin essential oil as affected by storage period. Int. J. Anal. Mass Spectrom. Chromatogr. 2019, 7, 9–17. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Z. Chemical composition of the essential oil of Cuminum cyminum L. from China. Flavour Fragr. J. 2004, 19, 311–313. [Google Scholar] [CrossRef]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical composition of cumin seeds, and biorefining study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Kumar, S.; Chowdhury, S. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Cuminum cyminum seeds. Int. J. Appl. Sci. Biotechnol. 2014, 2, 64–68. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, S. Inhibition of BACE1, MAO-B, cholinesterase enzymes, and anti-amyloidogenic potential of selected natural phytoconstituents: Multi-target-directed ligand approach. J. Food Biochem. 2021, 45, e13571. [Google Scholar] [CrossRef]
- Youdim, M.; Riederer, P. The relevance of glial monoamine Oxidase-B and polyamines to the action of selegiline in Parkinson’s disease. Mov. Disord. 1993, 8, S8–S13. [Google Scholar] [CrossRef]
- Emilsson, L.; Saetre, P.; Balciuniene, J.; Castensson, A.; Cairns, N.; Jazin, E.E. Increased monoamine oxidase messenger RNA expression levels in frontal cortex of Alzheimer’s disease patients. Neurosci. Lett. 2002, 326, 56–60. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alz-heimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Hassanabadi, M.; Ebrahimi, M.; Farajpour, M.; Dejahang, A. Variation in essential oil components among Iranian Ferula assa-foetida L. accessions. Ind. Crop. Prod. 2019, 140, 111598. [Google Scholar] [CrossRef]
- Mahendra, P.; Bisht, S. Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn. Rev. 2012, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, V.K.; Singh, D.K. Kinetics of enzyme inhibition by active molluscicidal agents ferulic acid, umbelliferone, eugenol and limonene in the nervous tissue of snail Lymnaea acuminata. Phytother. Res. 2009, 23, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Choi, S.J.; Lim, S.T.; Kim, H.K.; Heo, H.J.; Kim, E.K.; Jun, W.J.; Cho, H.Y.; Kim, Y.J.; Shin, D.-H. Ferulic acid supplementation prevents trimethyltin-induced cognitive deficits in mice. Biosci. Biotechnol. Biochem. 2007, 71, 1063–1068. [Google Scholar] [CrossRef]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamad, M. Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Joshi, G.; Sultana, R.; Calabrese, V.; De Marco, C.; Coccia, R.; Cini, C.; Butterfield, D.A. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1–42-induced oxidative stress. J. Neurosci. Res. 2006, 84, 418–426. [Google Scholar] [CrossRef]
- Joshi, G.; Perluigi, M.; Sultana, R.; Agrippino, R.; Calabrese, V.; Butterfield, D.A. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2, 2-azobis (2-amidino-propane) dihydrochloride (AAPH) or Fe2+/H2O2: Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem. Int. 2006, 48, 318–327. [Google Scholar]
- Scapagnini, G.; Butterfield, D.A.; Colombrita, C.; Sultana, R.; Pascale, A.; Calabrese, V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antiox. Redox Signal. 2004, 6, 811–818. [Google Scholar]
- Lenzi, J.; Rodriguez, A.F.; Rós Ade, S.; de Castro, A.B.; de Lima, D.D.; Magro, D.D.; Zeni, A.L. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef]
- Jin, Y.; Yan, E.; Fan, Y.; Zong, Z.; Qi, Z.; Li, Z. Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. Acta Pharmacol. Sin. 2005, 26, 943–951. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, Y.; Yan, E.; Liu, Z.; Zong, Z.; Qi, Z. Effects of sodium ferulate on amyloid-beta-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharmacol. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef]
- Thomson, M.; Ali, M. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Curr. Cancer Drug. Targets 2003, 3, 67–81. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Tarakeme, A. Essential oil content and composition of fennel (Foeniculum vulgare L.) fruits at different stages of development. J. Essent. Oil Bear. Plants 2011, 14, 605–609. [Google Scholar] [CrossRef]
- Agarwal, D.; Saxena, S.N.; Sharma, L.K.; Lal, G. Prevalence of essential and fatty oil constituents in fennel (Foeniculum vulgare Mill) genotypes grown in semi-arid regions of India. J. Essent. Oil Bear. Plants 2018, 21, 40–51. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Cholinergic basis of memory-strengthening effect of Foeniculum vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Asle-Rousta, M.; Rahnema, M. Protective effect of fennel, and its major component trans-anethole against social isolation induced behavioral deficits in rats. Physiol. Int. 2020, 107, 30–39. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, J.H.; Kim, H.Y.; Kim, M.J.; Kim, S.M. Chemical composition and biological activity of essential oil of Agastache rugosa (Fisch. & C. A. Mey.) O. Kuntze. Korean J. Med. Crop. Sci. 2020, 28, 95–110. [Google Scholar]
- Shin, M.; Liu, Q.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila Model of Alzheimer’s disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical constituents, antifungal and antioxidative effects of ajwain essential oil and its acetone extract. J. Agric. Food Chem. 2004, 52, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Azimullah, S.; Meeran, M.F.; Ansari, S.A.; Ojha, S. Neuroprotective effects of thymol, a dietary monoterpene against dopaminergic neurodegen-eration in rotenone-induced rat model of Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 1538. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 4926815. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Sagrillo, M.R.; De Brum, G.F.; Nascimento, K.; Peres, D.S.; Maciel, M.F.; Silveira, S.O.; Da Luz, S.C.A.; et al. Memory deficit, toxic effects and activity of Na+, K+-ATPase and NTPDase in brain of Wistar rats submitted to orally treatment with alpha-terpinene. Environ. Toxicol. Pharmacol. 2016, 46, 1–8. [Google Scholar] [CrossRef]
- Ghatreh Samani, K.; Gharib, M.H.; Momeni, A.; Hemati, Z.; Sedighin, R. A comparison between the effect of Cuminum cyminum and Vitamin E on the level of leptin, paraoxonase 1, HbA1c and oxidized LDL in diabetic patients. Int. J. Mol. Cell. Med. 2016, 5, 229–235. [Google Scholar]
- Haidari, F.; Zakerkish, M.; Borazjani, F.; Angali, K.A.; Foroushani, G.A. The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials 2020, 21, 483. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, J.; Choue, R.; Lim, H.F. Fennel (Forniculum vulgare) and fenugreek (Trigonella foenum-graecum) tea drinking suppresses subjective short-term appetite in overweight women. Clin. Nutr. Res. 2015, 4, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Radzi, C.; Hajifaraji, M.; Haerian, B.S.; Mosaddegh, M.H.; Cordell, G.A. Antiobesity effect of caraway extract on overweight and obese women: A randomized, triple-blind, placebo-controlled clinical trial. Evid.-Based Complement. Altern. Med. 2013, 2013, 928582. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Radzi, C.; Hajifaraji, M.; Cordell, G.A. Preliminary safety evaluation and biochemical efficacy of a Carum carvi extract: Results from a randomized, triple-blind, and placebo-controlled clinical trial. Phytother. Res. 2014, 28, 1456–1460. [Google Scholar] [CrossRef]
- Hadi, A.; Mohammadi, H.; Hadi, Z.; Roshanravan, N.; Kafeshani, M. Cumin (Cuminum cyminum L.) is a safe approach for management of lipid parameters: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2018, 32, 2146–2154. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Baradaran, A.; Rafieian-Kopaei, M. Anethum graveolens and hyperlipidemia: A randomized clinical trial. J. Res. Med. Sci. 2014, 19, 758–761. [Google Scholar]
- Mansouri, M.; Nayebi, N.; Keshtkar, A.; Hasani-Ranjbar, S.; Taheri, E.; Larijani, B. The effect of 12 weeks Anethum graveolens (dill) on metabolic markers in patients with metabolic syndrome; a randomized double blind controlled trial. DARU J. Pharm. Sci. 2012, 20, 47. [Google Scholar] [CrossRef]
- Morovati, A.; Gargari, B.P.; Sarbakhsh, P. Effects of cumin (Cuminum cyminum L.) essential oil supplementation on metabolic syndrome components: A randomized, triple-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 3261–3269. [Google Scholar] [CrossRef]
- Mala, K.N.; Thomas, J.; Syam, D.S.; Maliakel, B.; Krishnakumar, I.M. Safety and efficacy of Ferula asafoetida in functional dyspepsia: A randomized, double-blinded, placebo-controlled study. Evid.-Based Complement. Altern. Med. 2018, 2018, 4813601. [Google Scholar] [CrossRef]
- Petramfar, P.; Moein, M.; Samani, S.M.; Tabatabaei, S.H.; Zarshenas, M.M. Trachyspermum ammi 10% topical cream versus placebo on neuropathic pain, a randomized, double-blind, placebo-controlled trial. Neurol. Sci. 2016, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, C.U.; Siri, S.; Andallu, B. Antioxidant and antiarthritic potential of coriander (Coriandrum sativum L.) leaves. e-SPEN J. 2012, 7, e223–e228. [Google Scholar] [CrossRef]
- Talebi, Z.; Afshari, G.K.; Nasrollahi, S.A.; Firooz, A.; Ghovvati, M.; Samadi, A.; Karimi, M.; Kolahdooz, S.; Vazirian, M. Potential of Trachyspermum ammi (ajwain) gel for treatment of facial acne vulgaris: A pilot study with skin biophysical profile assessment and red fluorescence photography. Res. J. Pharmacogn. 2020, 7, 61–69. [Google Scholar]
- Afshar, S.; Afshar, F.; Rezazade, A.; Ardakani, Z.S.; Azar, Z.J.; Amin, G.; Shariat, M.; Haghollahi, F. Effects of a combination of Foeniculum vulgare, Melissa officinalis Extract, and Nigella saliva powder on healthy menopausal women with sexual dysfunction: A randomized clinical trial. Jundishapur J. Nat. Pharm. Prod. 2020, 15, e89925. [Google Scholar] [CrossRef]
- Akbari, M.; Javadnoori, M.; Siahpoosh, A.; Afshari, P.; Haghighi, M.; Lake, E. Comparison the effect of Anethum graveolens and oxytocin on induction of labor in term pregnancy: A randomized clinical trial. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e27876. [Google Scholar] [CrossRef]
- Asma, K.; Sultana, A.; Rahman, K. A single-blind randomized comparative study of Asafoetida vs. Mefenamic acid in dysmenorrhea, associated symptoms and health-related quality of life. J. Herb. Med. 2017, 9, 21–31. [Google Scholar]
- Ghazanfarpour, M.; Najafi, M.N.; Sharghi, N.B.; Mousavi, M.S.; Babakhanian, M.; Rakhshandeh, H. A double-blind, placebo-controlled trial of Fennel (Foeniculum vulgare) on menopausal symptoms: A high placebo response. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 122–127. [Google Scholar] [CrossRef]
- Motavalli, R.; Shahbazzadegan, S. Comparative study of the effects of fennel with Gelofen on the severity of primary dysmenorrhea: A randomized clinical trial. Iran. J. Obstet. Gynecol. Infertil. 2018, 21, 36–42. [Google Scholar]
- Hashemi, M.S.; Hashempur, M.H.; Lotfi, M.H.; Hemat, H.; Mousavi, Z.; Emtiazy, M.; Vaziri, F. The efficacy of asafoetida (Ferula assa-foetida oleo-gum resin) versus chlorhexidine gluconate mouthwash on dental plaque and gingivitis: A randomized double-blind controlled trial. Eur. J. Integr. Med. 2019, 29, 100929. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).