Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study of the Oxidative Stbaility of the Oil Mixture in the Presence or Absence of the OLE
2.2.1. Oil Mixture Preparation
2.2.2. Oxidative Stability Conditions
2.2.3. Fatty Acid Composition
2.2.4. Oxidation Indexes
2.2.5. Analysis of Phenolic Compounds by HPLC
Extraction of Phenolic Compounds
HPLC Determination of Phenolic Fraction
2.3. In Vivo Study
2.3.1. Animals
2.3.2. Treatment
2.3.3. Measurement of Mean Arterial Pressure in Conscious Rats by Tail-Cuff System
2.3.4. Serum Measurements
2.3.5. Serum Lipid Extraction and Fatty Acid Analysis
2.3.6. Experiments of Vascular Reactivity
2.3.7. Incubation of Gastrocnemius Muscle and Epididymal Adipose Tissue Explants and Aorta Segments in Presence/Absence of Insulin (10−7 M)
2.3.8. Nitrite and Nitrate Concentrations in the Culture Medium
2.3.9. Protein Quantification by Western Blot
2.3.10. RNA Extraction and Purification
2.3.11. Quantitative Real-Time PCR
2.3.12. Isolation and qRT-PCR of Micro-RNAs from Serum
2.3.13. Immunohistochemistry
2.3.14. Statistical Analysis
3. Results
3.1. Study of the Oxidative Stability of the Oil Mixture in the Presence or Absence of the OLE
3.1.1. Changes in Oxidation Parameters
3.1.2. Changes in Fatty Acid Composition
3.1.3. Changes in Polyphenolic Content
3.2. In Vivo Study
3.2.1. Body Weight and Food Intake
3.2.2. Organ Weights
3.2.3. Blood Pressure
3.2.4. Lipid Profile, Serum Levels of Metabolic Hormones and HOMA-IR Index
3.2.5. Serum Inflammatory Parameters
3.2.6. Serum Micro-RNA Levels
3.2.7. Serum Fatty Acids Percentage
3.2.8. Hepatic Gene Expression of Metabolic, Inflammatory and Oxidative Stress Markers and PGC-1α Levels
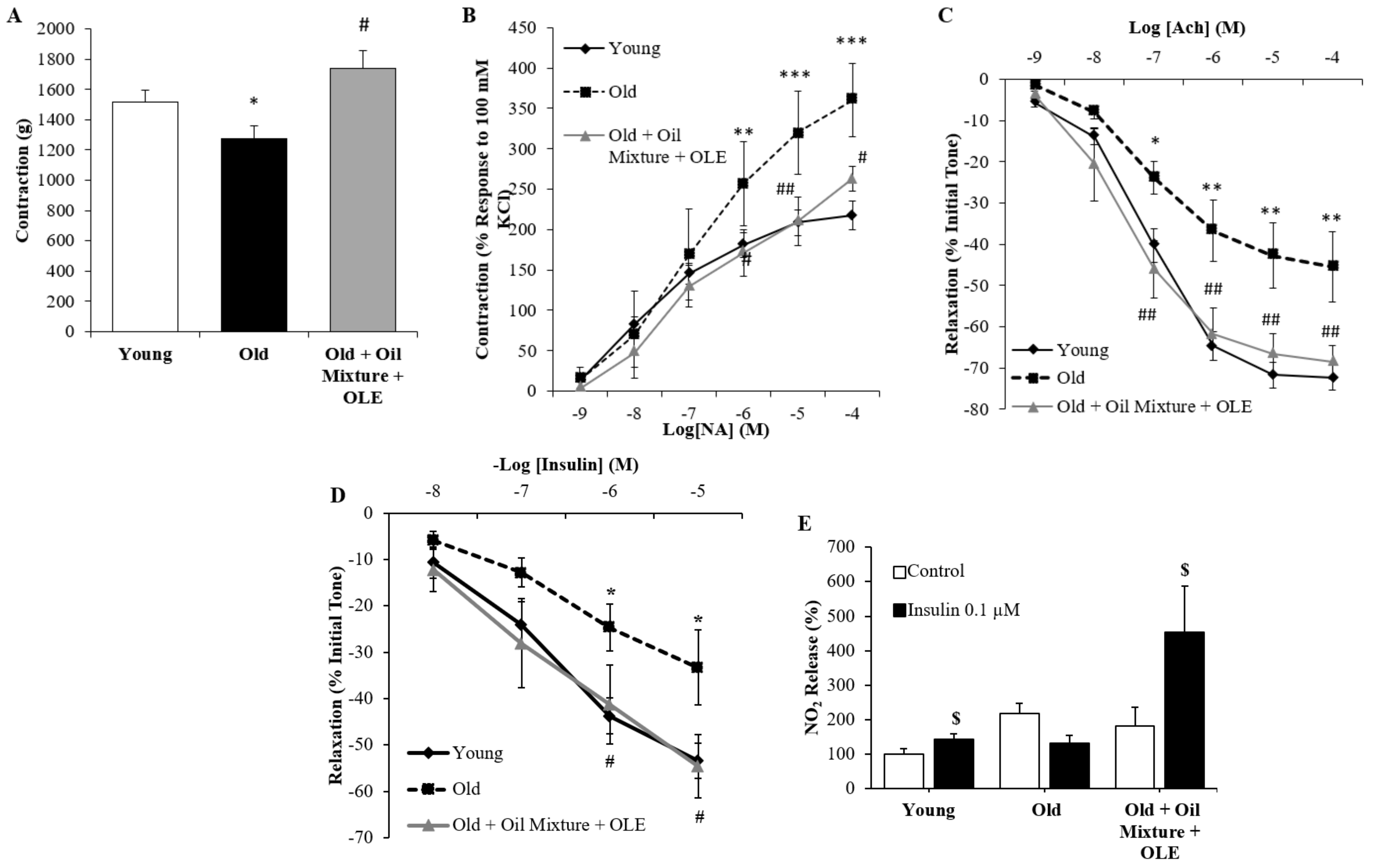
3.2.9. Peripheral Response to Insulin
3.2.10. Macrophage Infiltration in the Gastrocnemius and Epididymal Adipose Tissue
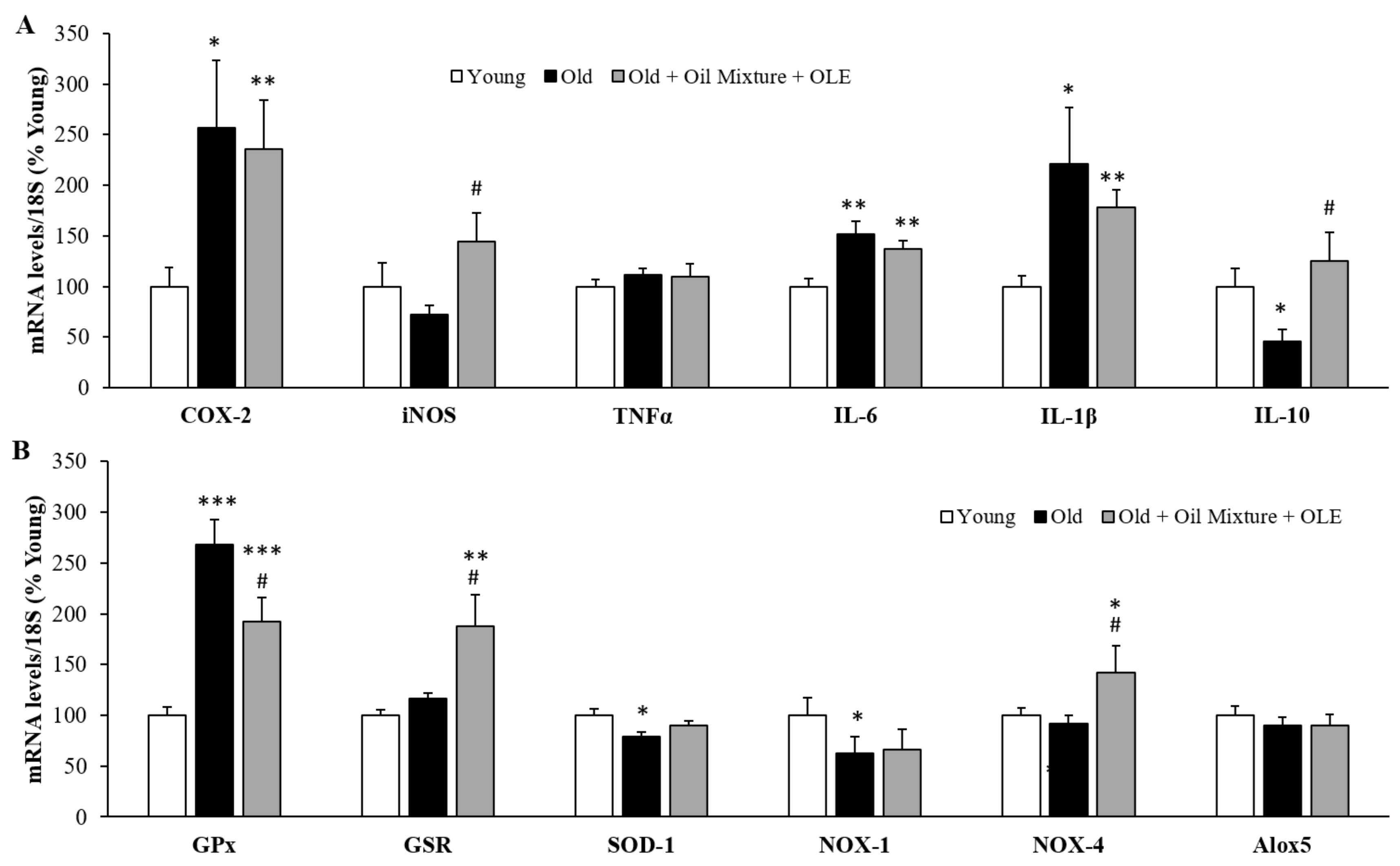
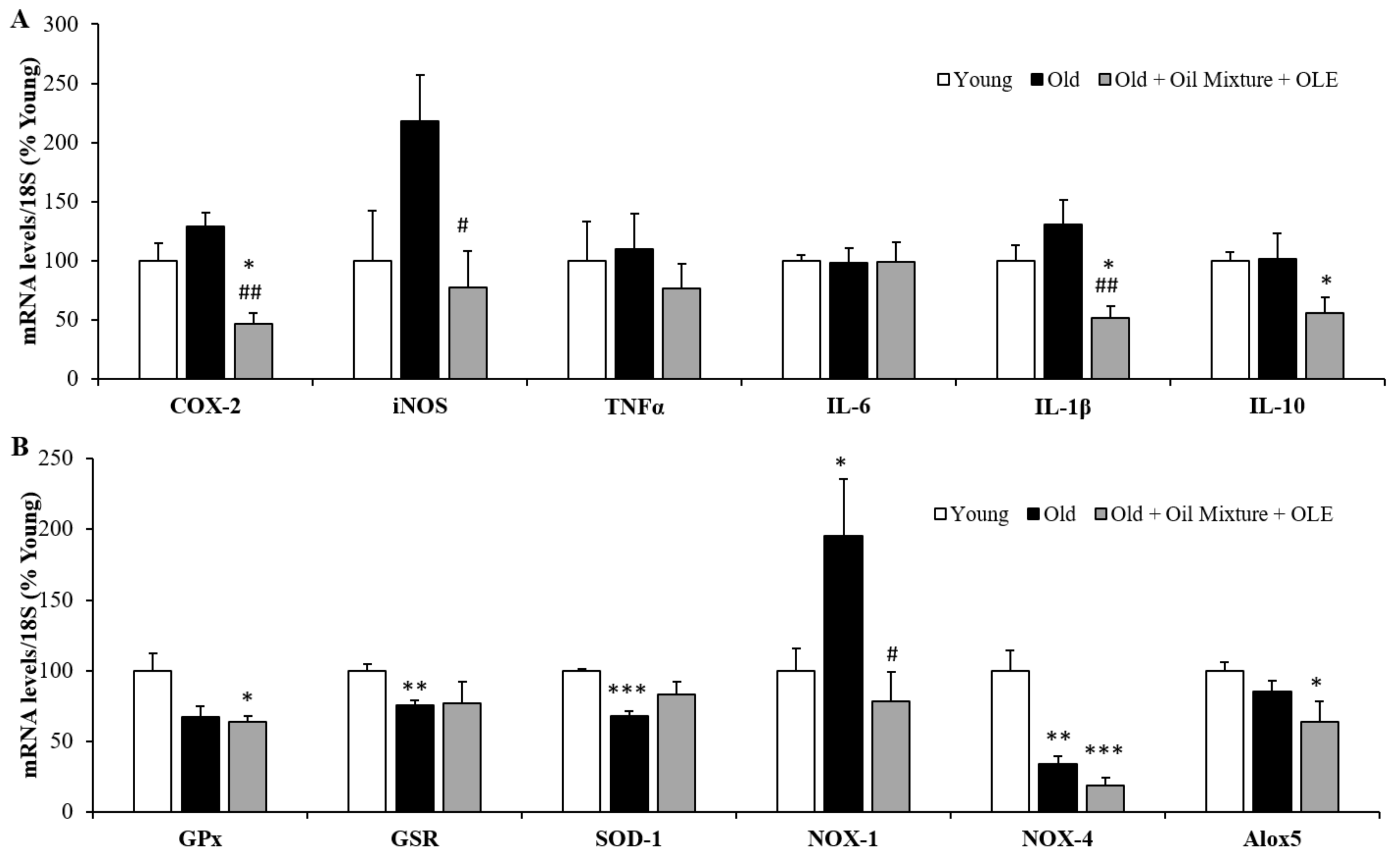
3.2.11. Gene Expression of Inflammatory and Oxidative Stress Markers in the Gastrocnemius Muscle
3.2.12. Gene Expression of Sarcopenia-Related Markers in Gastrocnemius Muscle
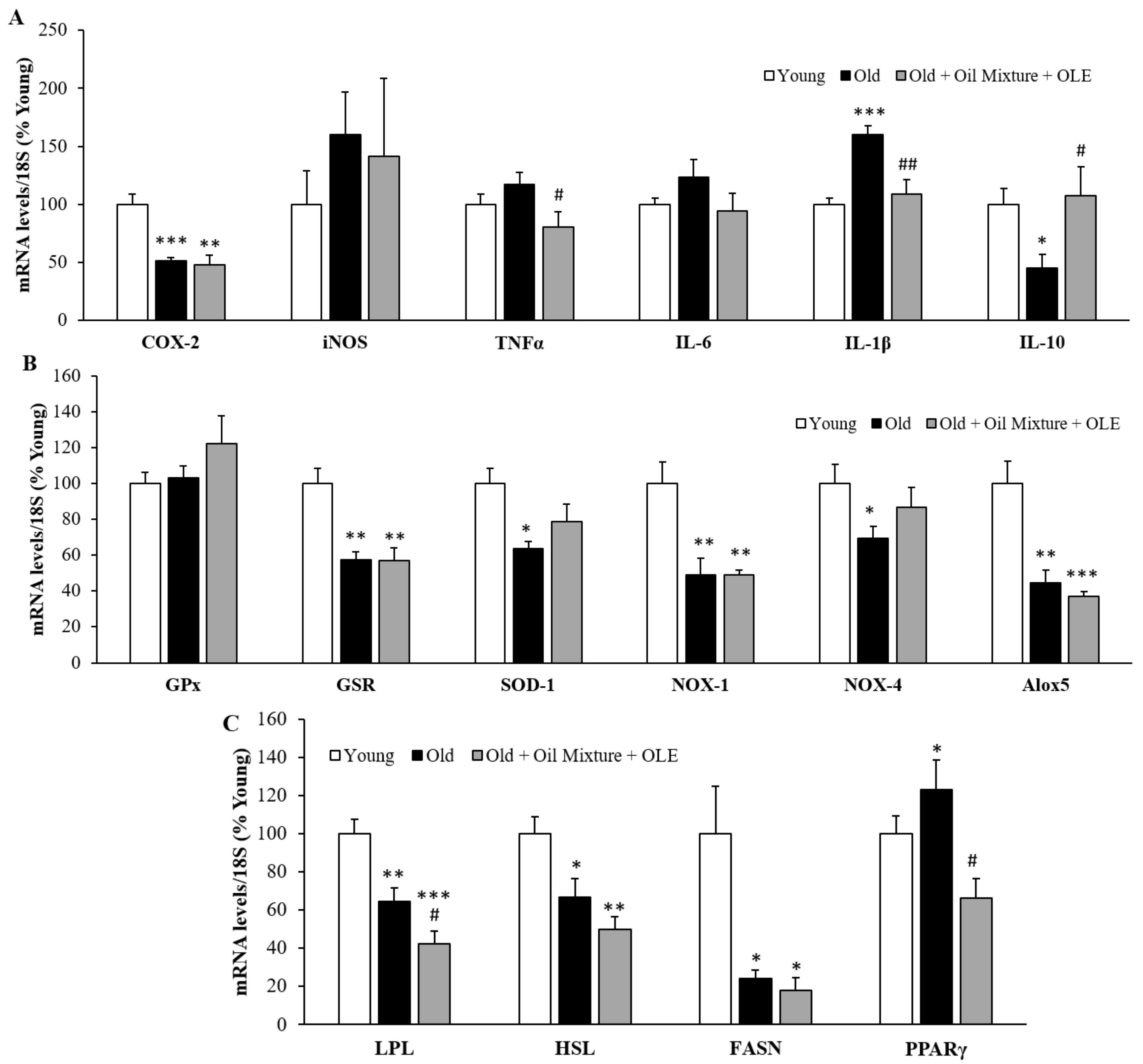
3.2.13. Gene Expression of Inflammatory, Oxidative Stress and Metabolic Markers in the Epididymal White Adipose Tissue
3.2.14. Aortic Vasoconstriction
3.2.15. Endothelium-Dependent and Independent Aortic Relaxation
3.2.16. Aortic Response to Insulin
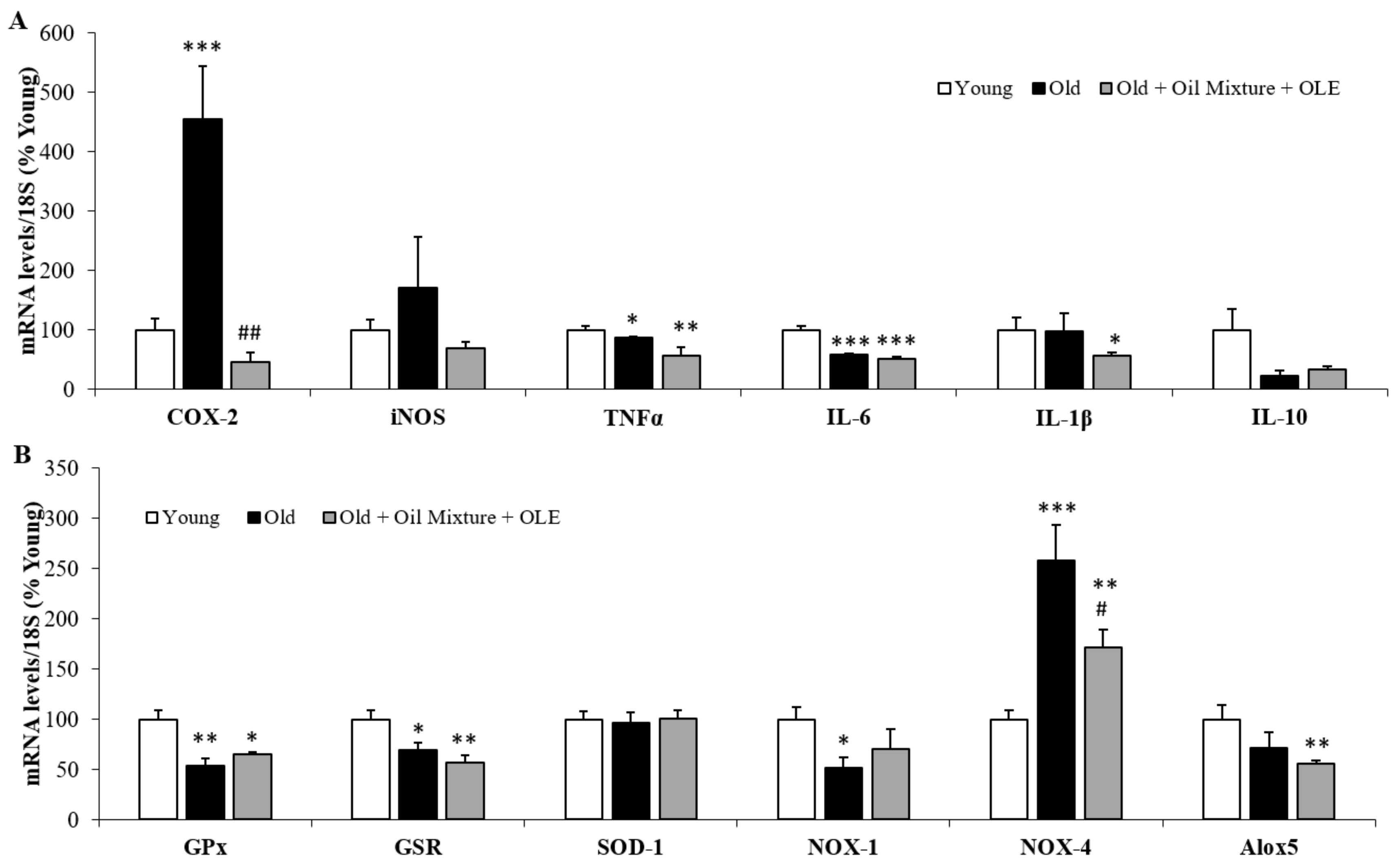
3.2.17. Aortic Gene Expression of Inflammatory and Oxidative Stress Markers
3.2.18. Cardiac Gene Expression of Inflammatory and Oxidative Stress Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.S. Insulin resistance with aging: Effects of diet and exercise. Sports Med. 2000, 30, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapsell, L.C. Foods and food components in the Mediterranean diet: Supporting overall effects. BMC Med. 2014, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Perez-Lopez, F.R.; Chedraui, P.; Haya, J.; Cuadros, J.L. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas 2009, 64, 67–79. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Bartekova, M.; Adameova, A.; Gorbe, A.; Ferenczyova, K.; Pechanova, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef]
- Barzi, F.; Woodward, M.; Marfisi, R.M.; Tavazzi, L.; Valagussa, F.; Marchioli, R.; Investigators, G.I.-P. Mediterranean diet and all-causes mortality after myocardial infarction: Results from the GISSI-Prevenzione trial. Eur. J. Clin. Nutr. 2003, 57, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, M.A.; Martin-Calvo, N. The major European dietary patterns and metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 265–271. [Google Scholar] [CrossRef]
- Rozati, M.; Barnett, J.; Wu, D.; Handelman, G.; Saltzman, E.; Wilson, T.; Li, L.; Wang, J.; Marcos, A.; Ordovas, J.M.; et al. Cardio-metabolic and immunological impacts of extra virgin olive oil consumption in overweight and obese older adults: A randomized controlled trial. Nutr. Metab. 2015, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Mora, J.J.; Cortes-Sierra, L.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A. Diet to Reduce the Metabolic Syndrome Associated with Menopause. The Logic for Olive Oil. Nutrients 2020, 12, 3184. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Raj, P.; Louis, X.L.; Perera, D.; Yamanagedara, P.; Zahradka, P.; Taylor, C.G.; Netticadan, T. Canola oil rich in oleic acid improves diastolic heart function in diet-induced obese rats. J. Physiol. Sci. 2017, 67, 425–430. [Google Scholar] [CrossRef]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, O.; Diaz-Castroverde, S.; Gomez-Hernandez, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols benefits of olive leaf (Olea europaea L) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.D.R.J.; del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Farag, R.S.; El-Baroty, G.S.; Basuny, A.M. The influence of phenolic extracts obtained from the olive plant (cvs. Picual and Kronakii), on the stability of sunflower oil. Int. J. Food sci. Technol. 2003, 38, 81–87. [Google Scholar] [CrossRef]
- Salta, F.N.; Mylona, A.; Chiou, A.; Boskou, G.; Andrikopoulos, N.K. Oxidative stability of edible vegetable oils enriched in polyphenols with olive leaf extract. Food Sci. Technol. Int. 2007, 13, 413–421. [Google Scholar] [CrossRef]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Elherik, K.; Bolton-Smith, C.; Barr, R.; Hill, A.; Murrie, I.; Belch, J.J. The effects of dietary fatty acid supplementation on endothelial function and vascular tone in healthy subjects. Cardiovasc. Res. 2003, 59, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, J.; Bellamy, M.F.; Ramsey, M.W.; Jones, C.J.; Lewis, M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 35, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Morgan, D.R.; Dixon, L.J.; Hanratty, C.G.; El-Sherbeeny, N.; Hamilton, P.B.; McGrath, L.T.; Leahey, W.J.; Johnston, G.D.; McVeigh, G.E. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am. J. Cardiol. 2006, 97, 547–551. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993, 36, 33–38. [Google Scholar] [CrossRef]
- Pauly, D.; Watson, R.; Alder, J. Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. n-3 Oil sources for use in aquaculture–alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.; McLean, C.H.; Silvers, K.M. Are the health benefits of fish oils limited by products of oxidation? Nutr. Res. Rev. 2006, 19, 53–62. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Diaz, T.; Lopez, O.; Fernandez-Bolanos, J.G.; Sanchez, J.; De Miguel, C.; Gil, M.V.; Martin-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Oliva, R.V.; Bakris, G.L. Management of hypertension in the elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V. Clinical practice. Isolated systolic hypertension in the elderly. N. Engl. J. Med. 2007, 357, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Kihara, Y.; Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012, 35, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Elahi, D.; Muller, D.C. Carbohydrate metabolism in the elderly. Eur. J. Clin. Nutr. 2000, 54 (Suppl. 3), S112–S120. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.M.; Halter, J.B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E7–E12. [Google Scholar] [CrossRef] [Green Version]
- Barzilai, N.; Ferrucci, L. Insulin resistance and aging: A cause or a protective response? J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1329–1331. [Google Scholar] [CrossRef]
- Priego, T.; Martin, A.I.; Gonzalez-Hedstrom, D.; Granado, M.; Lopez-Calderon, A. Role of hormones in sarcopenia. Vitam Horm. 2021, 115, 535–570. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Amor, S.; Martin-Carro, B.; Rubio, C.; Carrascosa, J.M.; Hu, W.; Huang, Y.; Garcia-Villalon, A.L.; Granado, M. Study of insulin vascular sensitivity in aortic rings and endothelial cells from aged rats subjected to caloric restriction: Role of perivascular adipose tissue. Exp. Gerontol. 2018, 109, 126–136. [Google Scholar] [CrossRef]
- Granado, M.; Amor, S.; Martin-Carro, B.; Guerra-Menendez, L.; Tejera-Munoz, A.; Gonzalez-Hedstrom, D.; Rubio, C.; Carrascosa, J.M.; Garcia-Villalon, A.L. Caloric restriction attenuates aging-induced cardiac insulin resistance in male Wistar rats through activation of PI3K/Akt pathway. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 97–105. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Muniyappa, R.; Quon, M.J. Insulin action and insulin resistance in vascular endothelium. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 523–530. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Hedström, D.; Amor, S.; de la Fuente-Fernandez, M.; Tejera-Munoz, A.; Priego, T.; Martin, A.I.; Lopez-Calderon, A.; Inarejos-Garcia, A.M.; Garcia-Villalon, A.L.; Granado, M. A Mixture of Algae and Extra Virgin Olive Oils Attenuates the Cardiometabolic Alterations Associated with Aging in Male Wistar Rats. Antioxidants 2020, 9, 483. [Google Scholar] [CrossRef]
- González-Hedström, D.; Granado, M.; Inarejos-García, A.M. Protective effects of extra virgin olive oil against storage-induced omega 3 fatty acid oxidation of algae oil. NFS J. 2020, 21, 9–15. [Google Scholar] [CrossRef]
- Wong, R.H.; Garg, M.L.; Wood, L.G.; Howe, P.R. Antihypertensive potential of combined extracts of olive leaf, green coffee bean and beetroot: A randomized, double-blind, placebo-controlled crossover trial. Nutrients 2014, 6, 4881–4894. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, I.L.; Hill, D.R.; Dias, D.A.; Jayasinghe, N.S.; Callahan, D.L.; Kentish, S.E.; Scales, P.J.; Martin, G.J. A quantitative analysis of microalgal lipids for optimization of biodiesel and omega-3 production. Biotechnol. Bioeng. 2013, 110, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-García, A.M.; Gómez-Rico, A.; Desamparados, S.M.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Rios, J.J.; Leon-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Fernandez, M.; Gonzalez-Hedstrom, D.; Amor, S.; Tejera-Munoz, A.; Fernandez, N.; Monge, L.; Almodovar, P.; Andres-Delgado, L.; Santamaria, L.; Prodanov, M.; et al. Supplementation with a Carob (Ceratonia siliqua L.) Fruit Extract Attenuates the Cardiometabolic Alterations Associated with Metabolic Syndrome in Mice. Antioxidants 2020, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Drews, B.; Milojevic, V.; Giller, K.; Ulbrich, S.E. Fatty acid profile of blood plasma and oviduct and uterine fluid during early and late luteal phase in the horse. Theriogenology 2018, 114, 258–265. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Trippa, D.; Siracusa, L.; Arena, E.; Restuccia, C.; Fallico, B. Addition of Olive Leaf Extract (OLE) for Producing Fortified Fresh Pasteurized Milk with An Extended Shelf Life. Antioxidants 2019, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Hedström, D.; Garcia-Villalon, A.L.; Amor, S.; de la Fuente-Fernandez, M.; Almodovar, P.; Prodanov, M.; Priego, T.; Martin, A.I.; Inarejos-Garcia, A.M.; Granado, M. Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef]
- Banares, C.; Martin, D.; Reglero, G.; Torres, C.F. Protective effect of hydroxytyrosol and rosemary extract in a comparative study of the oxidative stability of Echium oil. Food Chem. 2019, 290, 316–323. [Google Scholar] [CrossRef]
- Martinez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Hedström, D.; Priego, T.; Amor, S.; de la Fuente-Fernandez, M.; Martin, A.I.; Lopez-Calderon, A.; Inarejos-Garcia, A.M.; Garcia-Villalon, A.L.; Granado, M. Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle. Antioxidants 2021, 737. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hedström, D.; Priego, T.; Lopez-Calderon, A.; Amor, S.; de la Fuente-Fernandez, M.; Inarejos-Garcia, A.M.; Garcia-Villalon, A.L.; Martin, A.I.; Granado, M. Beneficial Effects of a Mixture of Algae and Extra Virgin Olive Oils on the Age-Induced Alterations of Rodent Skeletal Muscle: Role of HDAC-4. Nutrients 2020, 13, 44. [Google Scholar] [CrossRef]
- Palmeri, R.; Monteleone, J.I.; Spagna, G.; Restuccia, C.; Raffaele, M.; Vanella, L.; Li Volti, G.; Barbagallo, I. Olive Leaf Extract from Sicilian Cultivar Reduced Lipid Accumulation by Inducing Thermogenic Pathway during Adipogenesis. Front. Pharmacol. 2016, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid. Based Complement. Alternat. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Azuma, M.M.; Gomes-Filho, J.E.; Cardoso, C.B.M.; Pipa, C.B.; Narciso, L.G.; Bomfim, S.R.M.; Jacinto, R.C.; Cintra, L.T.A. Omega 3 Fatty Acids Reduce the Triglyceride Levels in Rats with Apical Periodontitis. Braz. Dent. J. 2018, 29, 173–178. [Google Scholar] [CrossRef]
- Qi, K.; Fan, C.; Jiang, J.; Zhu, H.; Jiao, H.; Meng, Q.; Deckelbaum, R.J. Omega-3 fatty acid containing diets decrease plasma triglyceride concentrations in mice by reducing endogenous triglyceride synthesis and enhancing the blood clearance of triglyceride-rich particles. Clin. Nutr. 2008, 27, 424–430. [Google Scholar] [CrossRef]
- Visioli, F.; Rise, P.; Plasmati, E.; Pazzucconi, F.; Sirtori, C.R.; Galli, C. Very low intakes of N-3 fatty acids incorporated into bovine milk reduce plasma triacylglycerol and increase HDL-cholesterol concentrations in healthy subjects. Pharmacol. Res. 2000, 41, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Moore, F.; Morgan, L.; Wright, J. Effects of n-3 fatty acids on postprandial triacylglycerol and hormone concentrations in normal subjects. Br. J. Nutr. 1992, 68, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, L.; Liu, Y.N.; Park, H.; Kim, H.S. Olive Leaf Extract Elevates Hepatic PPAR alpha mRNA Expression and Improves Serum Lipid Profiles in Ovariectomized Rats. J. Med. Food 2015, 18, 738–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppedisano, F.; Macri, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Hivert, M.F.; Sullivan, L.M.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B.S.; Wilson, P.W.; Meigs, J.B. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J. Clin. Endocrinol. Metab. 2008, 93, 3165–3172. [Google Scholar] [CrossRef]
- Coban, J.; Oztezcan, S.; Dogru-Abbasoglu, S.; Bingul, I.; Yesil-Mizrak, K.; Uysal, M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr. Gerontol. Int. 2014, 14, 996–1002. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Castillero, E.; Martin, A.I.; Lopez-Menduina, M.; Granado, M.; Villanua, M.A.; Lopez-Calderon, A. IGF-I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol. Cell Endocrinol. 2009, 309, 8–16. [Google Scholar] [CrossRef]
- Hyatt, J.P.; Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Nerve activity-independent regulation of skeletal muscle atrophy: Role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol. Cell Physiol. 2003, 285, C1161–C1173. [Google Scholar] [CrossRef] [Green Version]
- Aparecida Silveira, E.; Danesio de Souza, J.; Dos Santos Rodrigues, A.P.; Lima, R.M.; de Souza Cardoso, C.K.; de Oliveira, C. Effects of Extra Virgin Olive Oil (EVOO) and the Traditional Brazilian Diet on Sarcopenia in Severe Obesity: A Randomized Clinical Trial. Nutrients 2020, 12, 1498. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef]
- Villani, A.; Wright, H.; Slater, G.; Buckley, J. A randomised controlled intervention study investigating the efficacy of carotenoid-rich fruits and vegetables and extra-virgin olive oil on attenuating sarcopenic symptomology in overweight and obese older adults during energy intake restriction: Protocol paper. BMC Geriatr. 2018, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Fielding, R.A. Metabolites related to gut bacterial metabolism, peroxisome proliferator-activated receptor-alpha activation, and insulin sensitivity are associated with physical function in functionally-limited older adults. Aging Cell 2014, 13, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Castillero, E.; Nieto-Bona, M.P.; Fernandez-Galaz, C.; Martin, A.I.; Lopez-Menduina, M.; Granado, M.; Villanua, M.A.; Lopez-Calderon, A. Fenofibrate, a PPAR{alpha} agonist, decreases atrogenes and myostatin expression and improves arthritis-induced skeletal muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E790–E799. [Google Scholar] [CrossRef]
- Dai, J.; Xiang, Y.; Fu, D.; Xu, L.; Jiang, J.; Xu, J. Ficus carica L. Attenuates Denervated Skeletal Muscle Atrophy via PPARalpha/NF-kappaB Pathway. Front. Physiol. 2020, 11, 580223. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Tian, H.C.; Fan, Z.; Chen, Q.N.; Yang, Y.F.; Yu, J.; Wu, Y.X.; Xiao, Q. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-alpha pathway. J. Cell Mol. Med. 2021, 25, 3585–3600. [Google Scholar] [CrossRef]
- Camporez, J.P.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef] [Green Version]
- Dziegielewska-Gesiak, S.; Stoltny, D.; Brozek, A.; Muc-Wierzgon, M.; Wysocka, E. Are insulin-resistance and oxidative stress cause or consequence of aging. Exp. Biol. Med. 2020, 245, 1260–1267. [Google Scholar] [CrossRef]
- Cabo, J.; Alonso, R.; Mata, P. Omega-3 fatty acids and blood pressure. Br. J. Nutr. 2012, 107 (Suppl. 2), S195–S200. [Google Scholar] [CrossRef] [Green Version]
- Bercea, C.I.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 polyunsaturated fatty acids and hypertension: A review of vasodilatory mechanisms of docosahexaenoic acid and eicosapentaenoic acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Khayyal, M.T.; el-Ghazaly, M.A.; Abdallah, D.M.; Nassar, N.N.; Okpanyi, S.N.; Kreuter, M.H. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung 2002, 52, 797–802. [Google Scholar] [CrossRef]
- Teres, S.; Barcelo-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.P.; Lempereur, P.; Salembier, J.P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- Perona, J.S.; Canizares, J.; Montero, E.; Sanchez-Dominguez, J.M.; Catala, A.; Ruiz-Gutierrez, V. Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin. Nutr. 2004, 23, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Zhao, W.; Fu, G.; Li, Q.; Min, X.; Guo, Y. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci. Rep. 2020, 10, 4894. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, E.; Milanesi, E.; Ansari, A.; Zanetti, O.; Galluzzi, S.; Geroldi, C.; Gennarelli, M.; Bocchio-Chiavetto, L. miR-146a Plasma Levels Are Not Altered in Alzheimer’s Disease but Correlate with Age and Illness Severity. Front. Aging Neurosci. 2019, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Saferding, V.; Hofmann, M.; Brunner, J.S.; Niederreiter, B.; Timmen, M.; Magilnick, N.; Hayer, S.; Heller, G.; Steiner, G.; Stange, R.; et al. microRNA-146a controls age-related bone loss. Aging Cell 2020, 19, e13244. [Google Scholar] [CrossRef]
- Liu, H.C.; Han, D.S.; Hsu, C.C.; Wang, J.S. Circulating MicroRNA-486 and MicroRNA-146a serve as potential biomarkers of sarcopenia in the older adults. BMC Geriatr. 2021, 21, 86. [Google Scholar] [CrossRef]




| AA:EVOO | AA:EVOO + OLE | |
|---|---|---|
| Simple secoiridoids | 3.57 ± 0.2 | 400.1 ± 5.2 ** |
| Hydroxytyrosol derivatives | 11.7 ± 0.1 | 48.1 ± 1.9 * |
| Tyrosol derivatives | 19.2 ± 0.2 | 58.5 ± 2.0 * |
| Young | Old | Old + Oil Mixture + OLE | |
|---|---|---|---|
| Body weight change (g) | 18.5 ± 1.8 | −30.4 ± 5.7 ** | −37.9 ± 4.5 * |
| Food intake (g/rat/day) | 20.9 ± 0.7 | 17.1 ± 1.6 | 7.8 ± 0.9 |
| Heart | 314.6 ± 10.8 | 291.0 ± 14.4 | 274.0 ± 10.5 |
| Epidydimal visceral adipose tissue | 2107.1 ± 183.7 | 3201.8 ± 139.6 *** | 2381.3 ± 317.9 *# |
| Lumbar subcutaneous adipose tissue | 1063.1 ± 139.6 | 3974.2 ± 661.4 *** | 4029.1 ± 161.8 *** |
| Interscapular brown adipose tissue | 105.6 ± 9.8 | 111.1 ± 12.9 | 151.9 ± 11.2 ***# |
| Periaortic adipose tissue | 39.3 ± 5.9 | 47.8 ± 7.5 | 49.9 ± 3.6 * |
| Kidneys | 550.6 ± 5.9 | 512.1 ± 49.6 | 447.5 ± 21.2 *** |
| Suprarenal glands | 13.5 ± 0.7 | 12.4 ± 1.2 | 10.4 ± 0.7 ** |
| Liver | 2932.8 ± 103.6 | 2204.2 ± 138.6 *** | 2310.8 ± 119.5 *** |
| Spleen | 151.6 ± 3.9 | 175.5 ± 14.6 * | 142.3 ± 6.1 # |
| Soleus | 39.2 ± 2.1 | 26.0 ± 1.6 *** | 30.2 ± 1.2 ***# |
| Gastrocnemius | 534.4 ± 12.1 | 329.4 ± 15.8 *** | 367.2 ± 13.8 ***# |
| Young | Old | Old + Oil Mixture + OLE | |
|---|---|---|---|
| MAP (mmHg) | 123.6 ± 3.0 | 140.1 ± 6.1 * | 114.8 ± 1.8 # |
| Glycemia (mg/dL) | 90.7 ± 3.8 | 72.4 ± 11.1 | 55.0 ± 7.9 *** |
| Total Lipids (mg/dL) | 853.1 ± 63.9 | 1051 ± 37.4 * | 793.3 ± 56.7 ## |
| Triglycerides (mg/dL) | 97.6 ± 13.5 | 158.5 ± 36.4 * | 63.5 ± 15.4 # |
| Total Cholesterol (mg/dL) | 135.1 ± 15.3 | 199.3 ± 13.9 * | 114.3 ± 13.6 ## |
| LDL-cholesterol (mg/dL) | 28.8 ± 2.7 | 47.8 ± 2.6 ** | 17.82 ± 3.0 *### |
| HDL-cholesterol (mg/dL) | 15.7 ± 0.6 | 13.4 ± 2.2 | 13.0 ± 1.5 * |
| Insulin (ng/mL) | 17.7 ± 5.2 | 81.3 ± 32.2 ** | 19.9 ± 3.7 *# |
| HOMA-Index | 1.75 ± 0.3 | 13.1 ± 5.4 * | 2.59 ± 0.7 # |
| Leptin (ng/mL) | 11.82 ± 1.4 | 30.2 ± 5.9 ** | 35.8 ± 7.6 ** |
| Adiponectin (mg/dL) | 67.1 ± 6.7 | 108.9 ± 4.3 *** | 145.6 ± 7.5 ***## |
| Interleukin-6 (pg/mL) | 135.6 ± 7.1 | 188.7 ± 24.1 * | 130.1 ± 34.1 # |
| TNFα (pg/mL) | 0.1 ± 0.1 | 1.8 ± 0.9 * | 0.3 ± 0.2 # |
| miRNA-21/U6 (%) | 100.0 ± 39.6 | 447.3 ± 151.3 * | 155.6 ± 48.7 # |
| miRNA-34a/U6 (%) | 100.0 ± 18.7 | 325.4 ± 96.3 * | 304.8 ± 140.2 |
| miRNA-146a/U6 (%) | 100.0 ± 16.6 | 184.3 ± 43.8 * | 51.4 ± 16.0 ## |
| miRNA-204/U6 (%) | 100.0 ± 33.8 | 105.2 ± 38.3 | 101.7 ± 34.5 |
| Young | Old | Old + Oil Mixture + OLE | |
|---|---|---|---|
| SFA | 25.0 ± 0.8 | 28.4 ± 0.4 ** | 27.1 ± 1.2 |
| MUFA | 49.6 ± 0.6 | 42.0 ± 2.2 ** | 41.6 ± 1.3 *** |
| PUFA | 15.2 ± 2.5 | 16.1 ± 1.2 | 27.4 ± 2.5 **## |
| Palmitic Acid (C16:0) | 8.28 ± 0.3 | 9.92 ± 0.7 * | 8.11 ± 0.1 # |
| Palmitoleic Acid (C16:1) | 3.52 ± 0.5 | 5.47 ± 0.5 * | 7.65 ± 1.5 * |
| Stearic Acid (C18:0) | 13.2 ± 0.6 | 13.0 ± 0.5 | 11.4 ± 0.3 *# |
| Oleic Acid (C18:1) | 47.7 ± 0.7 | 40.8 ± 1.8 ** | 40.6 ± 1.6 ** |
| Linoleic Acid (C18:2) | 1.84 ± 0.2 | 1.15 ± 0.4 | 1.07 ± 0.6 |
| ALA (C18:3) | 3.42 ± 0.2 | 2.68 ± 0.1 ** | 2.67 ± 0.5 |
| EPA (C20:5n-3) | 4.68 ± 1.2 | 6.47 ± 0.5 | 13.7 ± 1.4 *## |
| DHA (C22:6n-3) | 7.12 ± 1.5 | 6.95 ± 0.7 | 11.3 ± 1.3 **## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hedström, D.; de la Fuente-Fernández, M.; Priego, T.; Martín, A.I.; Amor, S.; López-Calderón, A.; Inarejos-García, A.M.; García-Villalón, Á.L.; Granado, M. Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats. Antioxidants 2021, 10, 1066. https://doi.org/10.3390/antiox10071066
González-Hedström D, de la Fuente-Fernández M, Priego T, Martín AI, Amor S, López-Calderón A, Inarejos-García AM, García-Villalón ÁL, Granado M. Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats. Antioxidants. 2021; 10(7):1066. https://doi.org/10.3390/antiox10071066
Chicago/Turabian StyleGonzález-Hedström, Daniel, María de la Fuente-Fernández, Teresa Priego, Ana Isabel Martín, Sara Amor, Asunción López-Calderón, Antonio Manuel Inarejos-García, Ángel Luís García-Villalón, and Miriam Granado. 2021. "Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats" Antioxidants 10, no. 7: 1066. https://doi.org/10.3390/antiox10071066
APA StyleGonzález-Hedström, D., de la Fuente-Fernández, M., Priego, T., Martín, A. I., Amor, S., López-Calderón, A., Inarejos-García, A. M., García-Villalón, Á. L., & Granado, M. (2021). Addition of Olive Leaf Extract to a Mixture of Algae and Extra Virgin Olive Oils Decreases Fatty Acid Oxidation and Synergically Attenuates Age-Induced Hypertension, Sarcopenia and Insulin Resistance in Rats. Antioxidants, 10(7), 1066. https://doi.org/10.3390/antiox10071066







