Redox Regulation of Lipid Mobilization in Adipose Tissues
Abstract
1. Introduction
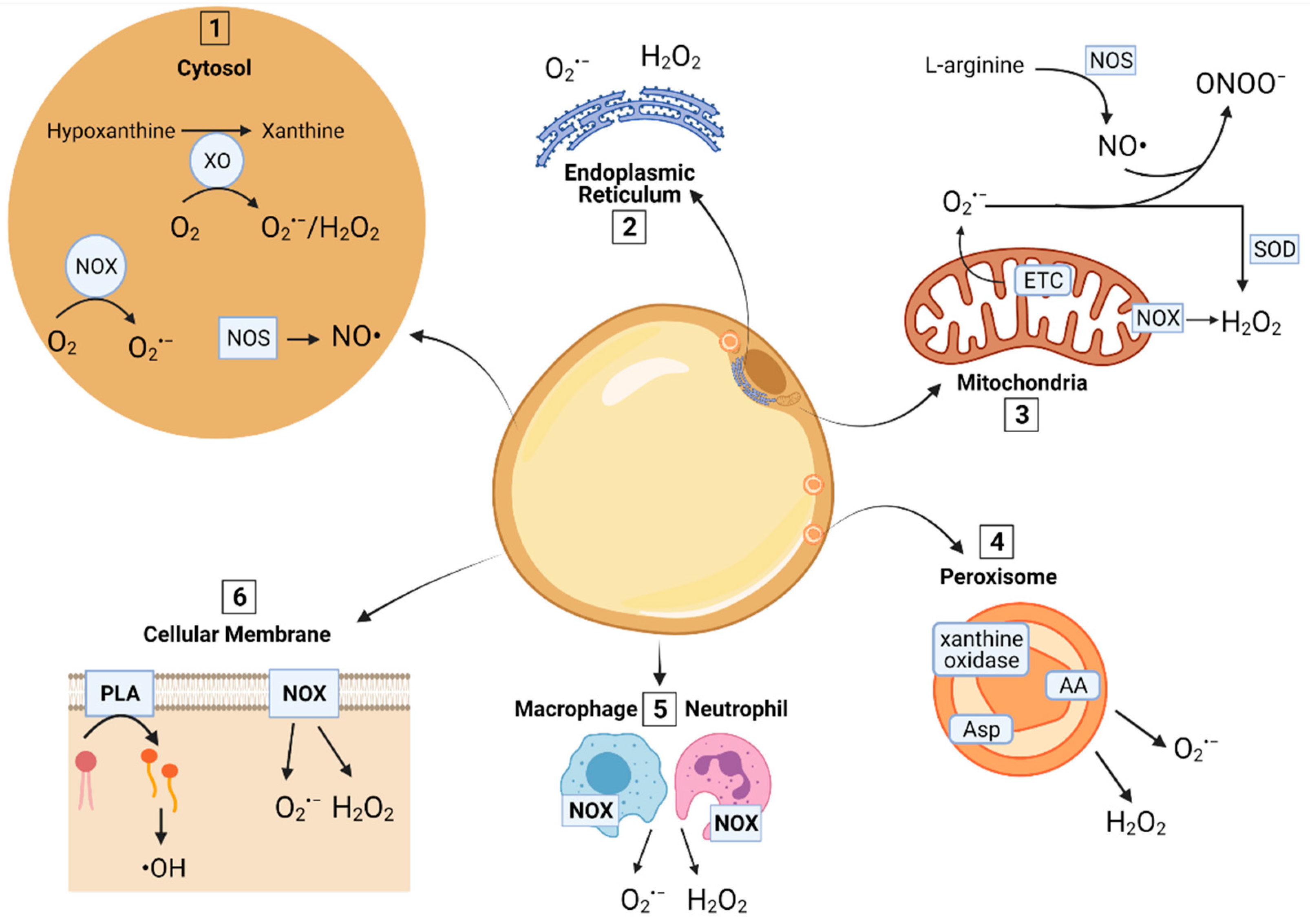
2. ROS and RNS Sources in AT
2.1. Mitochondria
2.2. Peroxisomes
2.3. Cytosol
2.4. Cellular Membrane
2.5. Endoplasmic Reticulum (ER)
2.6. Production of RNS
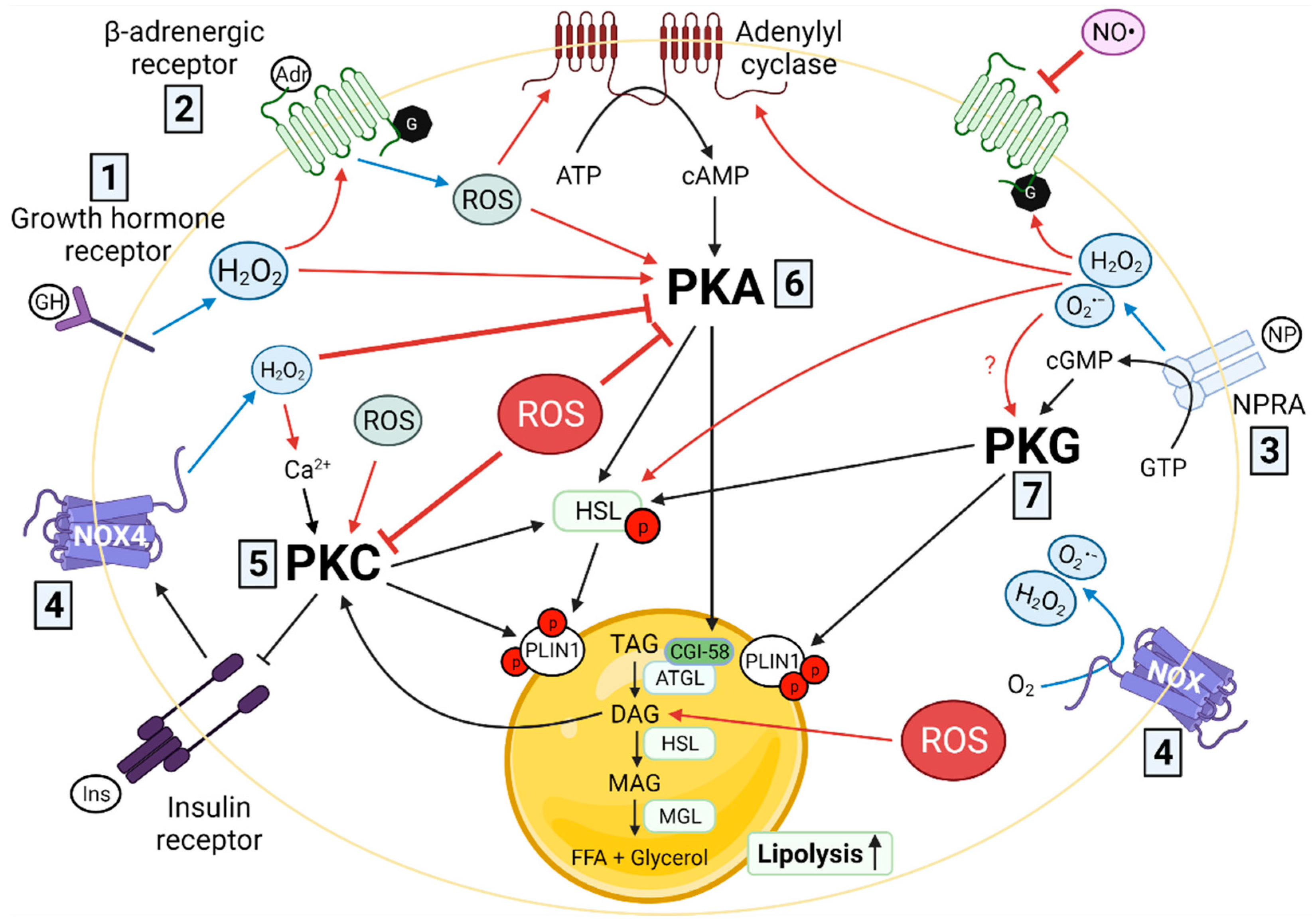
3. Redox Signaling and Lipolysis
3.1. Cell Membrane Receptors
3.1.1. β-Adrenergic Receptors
3.1.2. Growth Hormone Receptor
3.1.3. Natriuretic Peptide Receptors
3.2. Adenylyl and Guanylyl Cyclases and Their Cyclic Nucleotide Products (cAMP, cGMP)
3.3. Protein Kinases
3.3.1. cAMP-Dependent Protein Kinase A (PKA)
3.3.2. Protein Kinase C (PKC)
3.3.3. cGMP-Dependent Protein Kinase G (PKG)
3.4. Lipases
3.4.1. Hormone-Sensitive Lipase
3.4.2. Adipose Tissue Triglyceride Lipase
3.5. Redox Signaling Dysregulation and Lipolysis
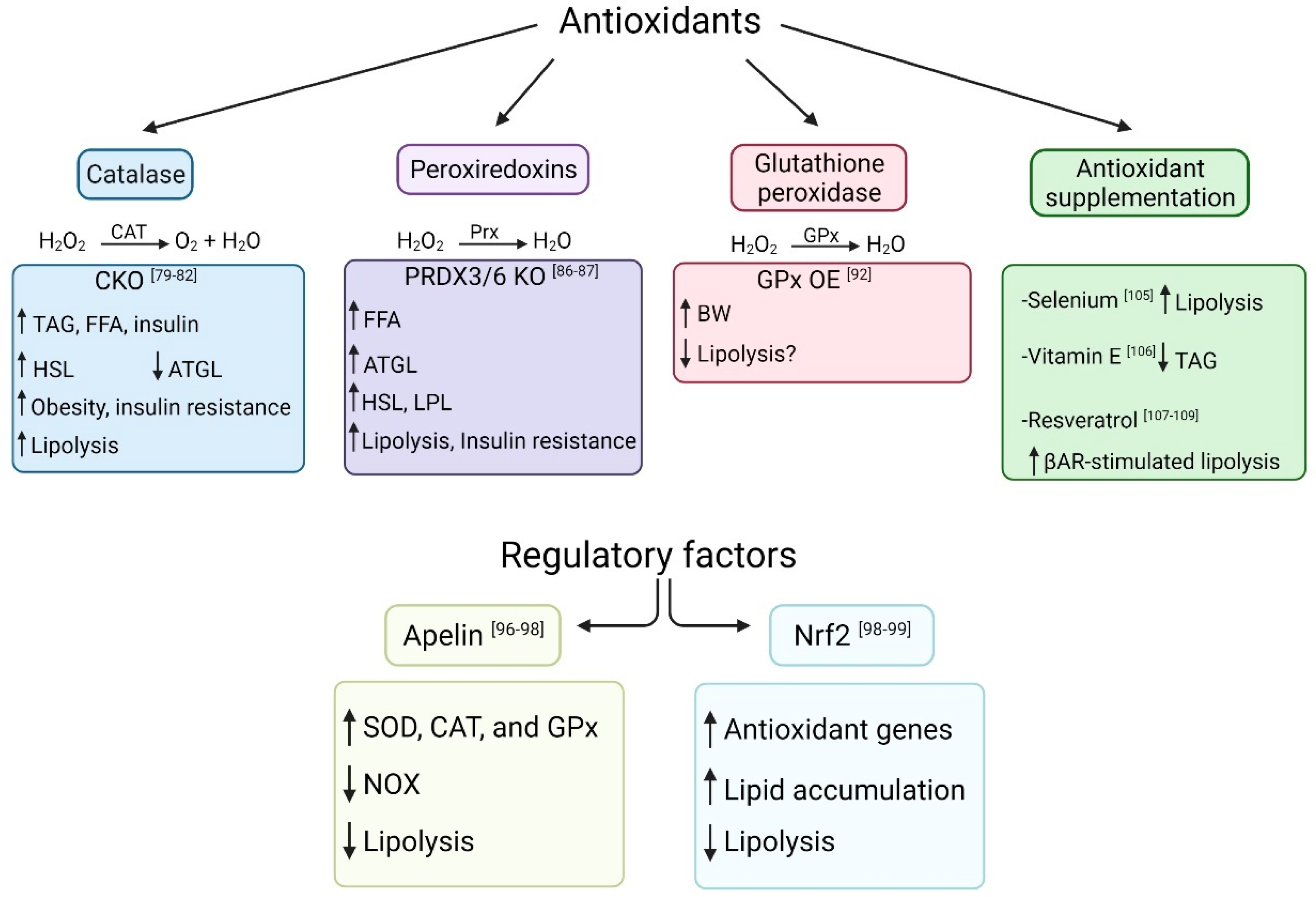
4. Antioxidants and Lipolysis
4.1. Catalase
4.2. Peroxiredoxins
4.3. Glutathione Peroxidase
4.4. Apelin
4.5. Nuclear Factor E2-Related Factor 2 (Nrf2)
4.6. Antioxidant Supplementation
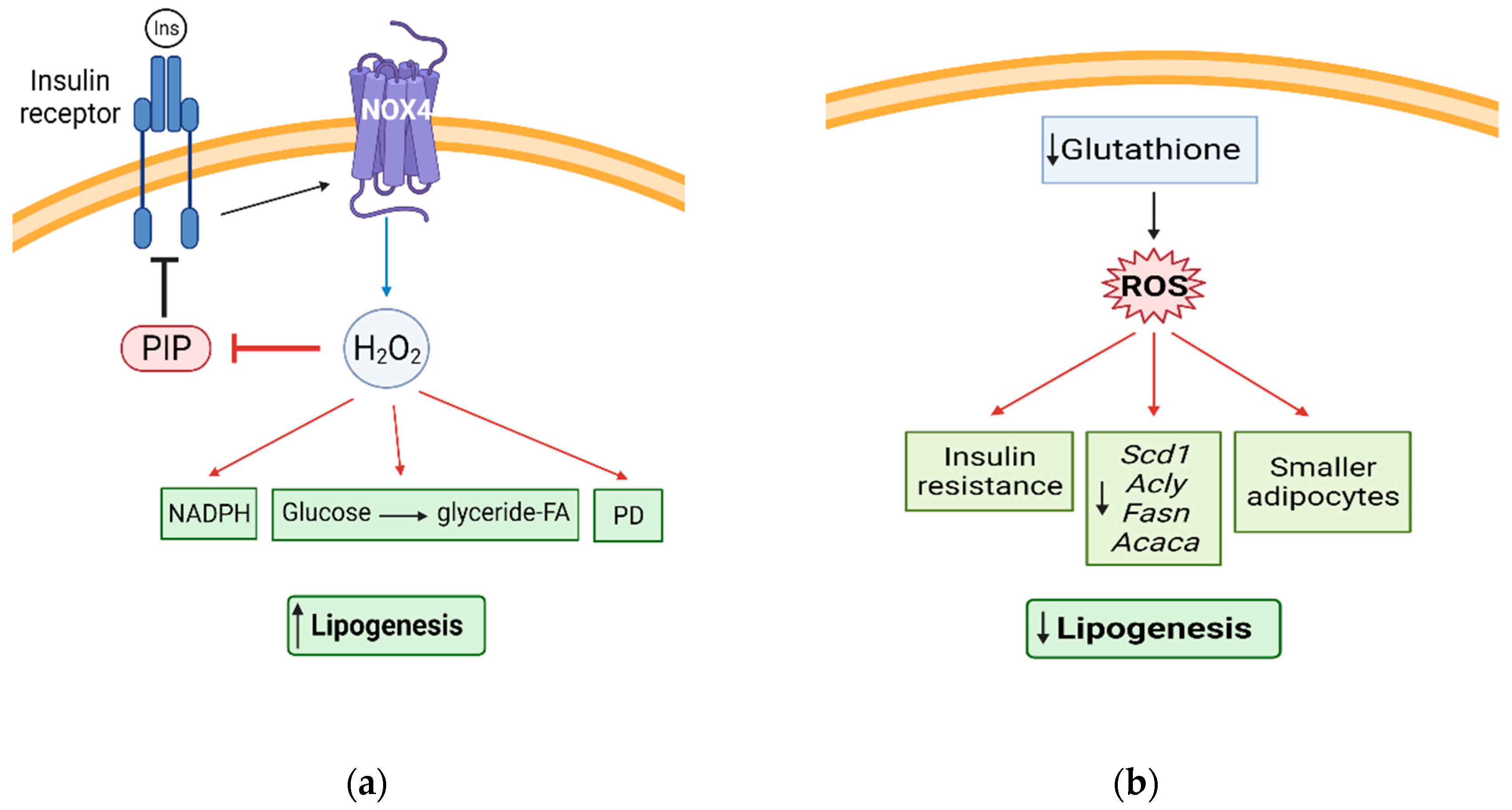
5. Redox Signaling and Lipogenesis
5.1. H2O2 and Lipogenesis
5.2. FA and TAG Synthesis
6. Redox Signaling and Dairy Cows’ Lipid Mobilization
7. Conclusions and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| AT | Adipose tissue |
| TAG | Triacylglycerol |
| FA | Fatty acid |
| OS | Oxidative stress |
| O2•− | Superoxide anion |
| H2O2 | Hydrogen peroxide |
| •OH | Hydroxyl radical |
| NO• | Nitric oxide |
| ONOO− | Peroxynitrite |
| NO2• | Nitrogen dioxide |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| NOS | Nitric oxide synthases |
| ATGL | Adipose tissue triglyceride lipase |
| HSL | Hormone sensitive lipase |
| DAG | Diacylglycerol |
| AC | Adenylyl cyclase |
| βAR | β-adrenergic receptor |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PKG | Protein kinase G |
| Prx | Peroxiredoxin |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| LPL | Lipoprotein lipase |
References
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant*. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perevoshchikova, I.V.; Quinlan, C.L.; Orr, A.L.; Gerencser, A.A.; Brand, M.D. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2013, 61, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Shi, B.; Klein, S.; Su, X. Peroxisomal regulation of redox homeostasis and adipocyte metabolism. Redox Biol. 2019, 24, 101167. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.E.; Khoo, N.K.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef]
- Nagao, H.; Nishizawa, H.; Tanaka, Y.; Fukata, T.; Mizushima, T.; Furuno, M.; Bamba, T.; Tsushima, Y.; Fujishima, Y.; Kita, S.; et al. Hypoxanthine Secretion from Human Adipose Tissue and its Increase in Hypoxia. Obesity 2018, 26, 1168–1178. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arter. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef]
- Han, C.Y.; Umemoto, T.; Omer, M.; Den Hartigh, L.J.; Chiba, T.; LeBoeuf, R.; Buller, C.L.; Sweet, I.R.; Pennathur, S.; Abel, E.D.; et al. NADPH Oxidase-derived Reactive Oxygen Species Increases Expression of Monocyte Chemotactic Factor Genes in Cultured Adipocytes*. J. Biol. Chem. 2012, 287, 10379–10393. [Google Scholar] [CrossRef]
- Vázquez-Meza, H.; de Piña, M.Z.; Pardo, J.P.; Riveros-Rosas, H.; Villalobos-Molina, R.; Piña, E. Non-steroidal anti-inflammatory drugs activate NADPH oxidase in adipocytes and raise the H2O2 pool to prevent cAMP-stimulated protein kinase a activation and inhibit lipolysis. BMC Biochem. 2013, 14, 13. [Google Scholar] [CrossRef][Green Version]
- Duncan, R.E.; Sarkadi-Nagy, E.; Jaworski, K.; Ahmadian, M.; Sul, H.S. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J. Biol. Chem. 2008, 283, 25428–25436. [Google Scholar] [CrossRef]
- Muralikrishna Adibhatla, R.; Hatcher, J.F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med. 2006, 40, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Robinson, J.M. Reactive oxygen species in phagocytic leukocytes. Histochem. Cell Biol. 2008, 130, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.; O’Brien-Simpson, N.M.; Holden, J.A.; Lenzo, J.C.; Fong, S.B.; Reynolds, E.C. Unprimed, M1 and M2 Macrophages Differentially Interact with Porphyromonas gingivalis. PLoS ONE 2016, 11, e0158629. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Kabara, E.; Brester, J.; Neuder, L.; Kiupel, M. Macrophage infiltration in the omental and subcutaneous adipose tissues of dairy cows with displaced abomasum. J. Dairy Sci. 2015, 98, 6176–6187. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Jia, H.; Loor, J.J.; Bucktrout, R.; Xu, Q.; Wang, Y.; Shu, X.; Dong, J.; Zuo, R.; et al. Effect of heat-shock protein B7 on oxidative stress in adipocytes from preruminant calves. J. Dairy Sci. 2019, 102, 5673–5685. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Liu, L.; Laflamme, C.J.; Karastergiou, K.; Meshulam, T.; Ding, S.-Y.; Wu, Y.; Lee, M.-J.; Gygi, S.P.; Fried, S.K.; et al. Adiporedoxin, an upstream regulator of ER oxidative folding and protein secretion in adipocytes. Mol. Metab. 2015, 4, 758–770. [Google Scholar] [CrossRef]
- Napier, J.A.; Michaelson, L.V.; Sayanova, O. The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 135–143. [Google Scholar] [CrossRef]
- Elizalde, M.; Rydén, M.; van Harmelen, V.; Eneroth, P.; Gyllenhammar, H.; Holm, C.; Ramel, S.; Olund, A.; Arner, P.; Andersson, K. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J. Lipid Res. 2000, 41, 1244–1251. [Google Scholar] [CrossRef]
- Ribiere, C.; Jaubert, A.M.; Gaudiot, N.; Sabourault, D.; Marcus, M.L.; Boucher, J.L.; Denis-Henriot, D.; Giudicelli, Y. White Adipose Tissue Nitric Oxide Synthase: A Potential Source for NO Production. Biochem. Biophys. Res. Commun. 1996, 222, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.R.; Wu, Y.; Shen, H.Q.; Zhu, J.S.; Baron, A.D. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 2000, 49, 684–687. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Zu, L.; He, J.; Jiang, H.; Xu, C.; Pu, S.; Xu, G. Bacterial endotoxin stimulates adipose lipolysis via toll-like receptor 4 and extracellular signal-regulated kinase pathway. J. Biol. Chem. 2009, 284, 5915–5926. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Collins, S. β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front. Endocrinol. 2012, 2. [Google Scholar] [CrossRef]
- Rambacher, K.M.; Moniri, N.H. The β2-adrenergic receptor-ROS signaling axis: An overlooked component of β2AR function? Biochem. Pharmacol. 2020, 171, 113690. [Google Scholar] [CrossRef]
- Burns, R.N.; Moniri, N.H. Agonist- and hydrogen peroxide-mediated oxidation of the β2 adrenergic receptor: Evidence of receptor s-sulfenation as detected by a modified biotin-switch assay. J. Pharmacol. Exp. Ther. 2011, 339, 914–921. [Google Scholar] [CrossRef]
- Andersson, K.; Gaudiot, N.; Ribiere, C.; Elizalde, M.; Giudicelli, Y.; Arner, P. A nitric oxide-mediated mechanism regulates lipolysis in human adipose tissue in vivo. Br. J. Pharmacol. 1999, 126, 1639–1645. [Google Scholar] [CrossRef]
- Gaudiot, N.; Jaubert, A.M.; Charbonnier, E.; Sabourault, D.; Lacasa, D.; Giudicelli, Y.; Ribière, C. Modulation of white adipose tissue lipolysis by nitric oxide. J. Biol. Chem. 1998, 273, 13475–13481. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Stoffels, M.; Franke, G.; Christensen, N.J.; Luft, F.C.; Boschmann, M. Interaction between beta-adrenergic receptor stimulation and nitric oxide release on tissue perfusion and metabolism. J. Clin. Endocrinol. Metab. 2001, 86, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Sakharova, A.A.; Horowitz, J.F.; Surya, S.; Goldenberg, N.; Harber, M.P.; Symons, K.; Barkan, A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 2008, 93, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mulder, H.; Holm, C.; Edén, S. Effects of Growth Hormone on the Function of β-Adrenoceptor Subtypes in Rat Adipocytes. Obes. Res. 2004, 12, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.G.; Goodman, H.M. Growth hormone and dexamethasone stimulate lipolysis and activate adenylyl cyclase in rat adipocytes by selectively shifting Gi alpha2 to lower density membrane fractions. Endocrinology 1999, 140, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- DeYulia, G.J., Jr.; Cárcamo, J.M.; Bórquez-Ojeda, O.; Shelton, C.C.; Golde, D.W. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 5044–5049. [Google Scholar] [CrossRef] [PubMed]
- Kovacova, Z.; Tharp, W.G.; Liu, D.; Wei, W.; Xie, H.; Collins, S.; Pratley, R.E. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity 2016, 24, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Sengenès, C.; Berlan, M.; de Glisezinski, I.; Lafontan, M.; Galitzky, J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000, 14, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Fürst, R.; Brueckl, C.; Kuebler, W.M.; Zahler, S.; Krötz, F.; Görlach, A.; Vollmar, A.M.; Kiemer, A.K. Atrial Natriuretic Peptide Induces Mitogen-Activated Protein Kinase Phosphatase-1 in Human Endothelial Cells via Rac1 and NAD(P)H Oxidase/Nox2-Activation. Circ. Res. 2005, 96, 43–53. [Google Scholar] [CrossRef]
- Serazin-Leroy, V.; Morot, M.; de Mazancourt, P.; Giudicelli, Y. Differences in type II, IV, V and VI adenylyl cyclase isoform expression between rat preadipocytes and adipocytes. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2001, 1550, 37–51. [Google Scholar] [CrossRef]
- Tan, C.M.; Xenoyannis, S.; Feldman, R.D. Oxidant Stress Enhances Adenylyl Cyclase Activation. Circ. Res. 1995, 77, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; Banchelli, G.; Sgromo, L.; Pirisino, R.; Ner, M.; Parini, A.; Cambon, C. Hydrogen peroxide generation by monoamine oxidases in rat white adipocytes: Role on cAMP production. Eur. J. Pharmacol. 2000, 395, 177–182. [Google Scholar] [CrossRef]
- Vatner, S.F.; Pachon, R.E.; Vatner, D.E. Inhibition of Adenylyl Cyclase Type 5 Increases Longevity and Healthful Aging through Oxidative Stress Protection. Oxidative Med. Cell. Longev. 2015, 2015, 250310. [Google Scholar] [CrossRef]
- Guers, J.J.; Zhang, J.; Campbell, S.C.; Oydanich, M.; Vatner, D.E.; Vatner, S.F. Disruption of adenylyl cyclase type 5 mimics exercise training. Basic Res. Cardiol. 2017, 112, 59. [Google Scholar] [CrossRef]
- Vatner, S.F.; Park, M.; Yan, L.; Lee, G.J.; Lai, L.; Iwatsubo, K.; Ishikawa, Y.; Pessin, J.; Vatner, D.E. Adenylyl cyclase type 5 in cardiac disease, metabolism, and aging. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1–H8. [Google Scholar] [CrossRef]
- Lafontan, M.; Moro, C.; Sengenes, C.; Galitzky, J.; Crampes, F.; Berlan, M. An Unsuspected Metabolic Role for Atrial Natriuretic Peptides. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2032–2042. [Google Scholar] [CrossRef]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule—Recent developments in cGMP research and development. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Pennypacker, J.K.; Taylor, S.S. Redox Regulation of cAMP-dependent Protein Kinase Signaling: Kinase versus phosphatase inactivation. J. Biol. Chem. 2007, 282, 22072–22079. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Juliano, C.; Taylor, S.S. Regulation of cAMP-dependent Protein Kinase Activity by Glutathionylation. J. Biol. Chem. 2002, 277, 43505–43511. [Google Scholar] [CrossRef]
- de Pina, M.Z.; Vazquez-Meza, H.; Pardo, J.P.; Rendon, J.L.; Villalobos-Molina, R.; Riveros-Rosas, H.; Pina, E. Signaling the signal, cyclic AMP-dependent protein kinase inhibition by insulin-formed H2O2 and reactivation by thioredoxin. J. Biol. Chem. 2008, 283, 12373–12386. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Mahadev, K.; Wu, X. Redox paradox: Insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 2005, 54, 311–321. [Google Scholar] [CrossRef]
- Steinberg, S.F. Mechanisms for redox-regulation of protein kinase C. Front. Pharm. 2015, 6, 128. [Google Scholar] [CrossRef]
- Fricke, K.; Heitland, A.; Maronde, E. Cooperative activation of lipolysis by protein kinase A and protein kinase C pathways in 3T3-L1 adipocytes. Endocrinology 2004, 145, 4940–4947. [Google Scholar] [CrossRef]
- Loegering, D.J.; Lennartz, M.R. Protein kinase C and toll-like receptor signaling. Enzym. Res 2011, 2011, 537821. [Google Scholar] [CrossRef]
- Wang, X.T.; McCullough, K.D.; Wang, X.J.; Carpenter, G.; Holbrook, N.J. Oxidative stress-induced phospholipase C-gamma 1 activation enhances cell survival. J. Biol. Chem. 2001, 276, 28364–28371. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Parhar, R.; Saleh, S.; Al-Hijailan, R.; Inglis, A.; Al-Jufan, M.; Bazzi, M.; Hashmi, S.; Conca, W.; Collison, K.; et al. Intracellular calcium and NF-kB regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Sci. 2018, 212, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Deal, M.S.; Taylor, S.S. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J. Biol. Chem. 2005, 280, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Sengenes, C.; Bouloumie, A.; Hauner, H.; Berlan, M.; Busse, R.; Lafontan, M.; Galitzky, J. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J. Biol. Chem. 2003, 278, 48617–48626. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, J.R.; Prysyazhna, O.; Rudyk, O.; Eaton, P. cGMP-dependent activation of protein kinase G precludes disulfide activation: Implications for blood pressure control. Hypertension 2012, 60, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, S.A.; Haller, J.F.; Ferrante, T.; Zoeller, R.A.; Corkey, B.E. Reactive oxygen species facilitate translocation of hormone sensitive lipase to the lipid droplet during lipolysis in human differentiated adipocytes. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Zhou, C.; Zaman, N.; Li, Y.; Martinez-Arguelles, D.B.; Papadopoulos, V.; Zirkin, B.; Traore, K. Redox regulation of hormone sensitive lipase: Potential role in the mechanism of MEHP-induced stimulation of basal steroid synthesis in MA-10 Leydig cells. Reprod. Toxicol. 2019, 85, 19–25. [Google Scholar] [CrossRef]
- Sahu-Osen, A.; Montero-Moran, G.; Schittmayer, M.; Fritz, K.; Dinh, A.; Chang, Y.-F.; McMahon, D.; Boeszoermenyi, A.; Cornaciu, I.; Russell, D.; et al. CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: Control of subcellular localization. J. Lipid Res. 2015, 56, 109–121. [Google Scholar] [CrossRef]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W., Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Issa, N.; Lachance, G.; Bellmann, K.; Laplante, M.; Stadler, K.; Marette, A. Cytokines promote lipolysis in 3T3-L1 adipocytes through induction of NADPH oxidase 3 expression and superoxide production. J. Lipid Res. 2018, 59, 2321–2328. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Le Lay, S.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative stress and metabolic pathologies: From an adipocentric point of view. Oxidative Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Bernlohr, D.A. Oxidative stress and protein carbonylation in adipose tissue — Implications for insulin resistance and diabetes mellitus. J. Proteom. 2013, 92, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Tacuma, D.; Parales-Giron, J.; Prom, C.; Chirivi, M.; Laguna, J.; Lock, A.L.; Contreras, G.A. Transcriptomic profiling of adipose tissue inflammation, remodeling, and lipid metabolism in periparturient dairy cows (Bos taurus). BMC Genom. 2020, 21, 824. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Uddin, M.J.; Pak, E.S.; Kang, H.; Jin, E.-J.; Jo, S.; Kang, D.; Lee, H.; Ha, H. The impaired redox balance in peroxisomes of catalase knockout mice accelerates nonalcoholic fatty liver disease through endoplasmic reticulum stress. Free Radic. Biol. Med. 2020, 148, 22–32. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, H.-W.; Song, S.-E.; Bae, J.-H.; Im, S.-S.; Hwang, I.; Ha, H.; Song, D.-K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflügers Arch. Eur. J. Physiol. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Shin, S.-K.; Cho, H.-W.; Song, S.-E.; Im, S.-S.; Bae, J.-H.; Song, D.-K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 101749. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Souza, V.; Dias-Júnior, N.M.; Eleutério-Silva, M.A.; Ferreira-Neves, V.P.; Moura, F.A.; Alenina, N.; Bader, M.; Rabelo, L.A. 3-Amino-1,2,4-Triazole Induces Quick and Strong Fat Loss in Mice with High Fat-Induced Metabolic Syndrome. Oxid Med. Cell. Longev. 2020, 2020, 3025361. [Google Scholar] [CrossRef] [PubMed]
- Prasad Mukherjee, S. Mediation of the antilipolytic and lipogenic effects of insulin in adipocytes by intracellular accumulation of hydrogen peroxide. Biochem. Pharmacol. 1980, 29, 1239–1246. [Google Scholar] [CrossRef]
- Abbas, K.; Riquier, S.; Drapier, J.-C. Chapter Six—Peroxiredoxins and Sulfiredoxin at the Crossroads of the NO and H2O2 Signaling Pathways. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 527, pp. 113–128. [Google Scholar]
- Arevalo, J.A.; Vázquez-Medina, J.P. The Role of Peroxiredoxin 6 in Cell Signaling. Antioxidants 2018, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Arriga, R.; Pacifici, F.; Capuani, B.; Coppola, A.; Orlandi, A.; Scioli, M.G.; Pastore, D.; Andreadi, A.; Sbraccia, P.; Tesauro, M.; et al. Peroxiredoxin 6 Is a Key Antioxidant Enzyme in Modulating the Link between Glycemic and Lipogenic Metabolism. Oxid Med. Cell. Longev. 2019, 2019, 9685607. [Google Scholar] [CrossRef]
- Huh, J.Y.; Kim, Y.; Jeong, J.; Park, J.; Kim, I.; Huh, K.H.; Kim, Y.S.; Woo, H.A.; Rhee, S.G.; Lee, K.J.; et al. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid. Redox Signal. 2012, 16, 229–243. [Google Scholar] [CrossRef]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; Mendoza-Carrera, F.; Ruiz-Vivanco, G.; Torres-Rasgado, E.; Gonzalez-Mejia, M.E.; Garcia-Zapien, A.; Flores-Martinez, S.E.; Perez-Fuentes, R. Serum levels of glutathione peroxidase 3 in overweight and obese subjects from central Mexico. Arch. Med. Res. 2012, 43, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Baez-Duarte, B.G.; Mendoza-Carrera, F.; García-Zapién, A.; Flores-Martínez, S.E.; Sánchez-Corona, J.; Zamora-Ginez, I.; Torres-Rasgado, E.; León-Chávez, B.A.; Pérez-Fuentes, R. Glutathione peroxidase 3 serum levels and GPX3 gene polymorphisms in subjects with metabolic syndrome. Arch. Med. Res. 2014, 45, 375–382. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, A.Y.; Choi, J.W.; Kim, M.; Yasue, S.; Son, H.J.; Masuzaki, H.; Park, K.S.; Kim, J.B. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008, 22, 2176–2189. [Google Scholar] [CrossRef]
- Langhardt, J.; Flehmig, G.; Klöting, N.; Lehmann, S.; Ebert, T.; Kern, M.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; et al. Effects of Weight Loss on Glutathione Peroxidase 3 Serum Concentrations and Adipose Tissue Expression in Human Obesity. Obes. Facts 2018, 11, 475–490. [Google Scholar] [CrossRef]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.V.; Shakhristova, E.V.; Stepovaya, E.A.; Zhavoronok, T.V.; Novitsky, V.V. Effect of alloxan on spontaneous lipolysis and glutathione system in isolated rat adipocytes. Bull. Exp. Biol. Med. 2011, 151, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Zhang, X.; Leow, M.K.; Poh, C.L.; Chong, S.K.; Chen, P. Apelin attenuates oxidative stress in human adipocytes. J. Biol. Chem. 2014, 289, 3763–3774. [Google Scholar] [CrossRef]
- Than, A.; Cheng, Y.; Foh, L.C.; Leow, M.K.; Lim, S.C.; Chuah, Y.J.; Kang, Y.; Chen, P. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 2012, 362, 227–241. [Google Scholar] [CrossRef]
- Yue, P.; Jin, H.; Xu, S.; Aillaud, M.; Deng, A.C.; Azuma, J.; Kundu, R.K.; Reaven, G.M.; Quertermous, T.; Tsao, P.S. Apelin Decreases Lipolysis via Gq, Gi, and AMPK-Dependent Mechanisms. Endocrinology 2011, 152, 59–68. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Jia, H.; Wang, H.; Shui, G.; Qin, Y.; Shu, X.; Wang, Y.; Dong, J.; Liu, G.; et al. Nuclear Factor E2-Related Factor 2 Mediates Oxidative Stress-Induced Lipid Accumulation in Adipocytes by Increasing Adipogenesis and Decreasing Lipolysis. Antioxid. Redox Signal. 2020, 32, 173–192. [Google Scholar] [CrossRef]
- Xu, J.; Kulkarni, S.R.; Donepudi, A.C.; More, V.R.; Slitt, A.L. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 2012, 61, 3208–3218. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.-A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.W.; Burnett, J.R.; Croft, K.D. Vitamin E in human health and disease. Crit. Rev. Clin. Lab. Sci. 2008, 45, 417–450. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Long, Z.; Sun, Q.; Liu, J.; Liu, Y.; Hai, C. Differential effect of Se on insulin resistance: Regulation of adipogenesis and lipolysis. Mol. Cell. Biochem. 2016, 415, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, M.; Sánchez-Vera, I.; Sevillano, J.; Herrero, L.; Serra, D.; Ramos, M.P.; Viana, M. Vitamin E reduces adipose tissue fibrosis, inflammation, and oxidative stress and improves metabolic profile in obesity. Obesity 2015, 23, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Treguer, K.; Mercader, J.; Carpene, C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef]
- Szkudelska, K.; Nogowski, L.; Szkudelski, T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J. Steroid Biochem. Mol. Biol. 2009, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Ølholm, J.; Paulsen, S.K.; Bennetzen, M.F.; Richelsen, B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int. J. Obes. 2008, 32, 1250–1255. [Google Scholar] [CrossRef]
- Merkel, M.; Eckel, R.H.; Goldberg, I.J. Lipoprotein lipase. J. Lipid Res. 2002, 43, 1997–2006. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Kahn, B.B. Glucose Transporters and Insulin Action—Implications for Insulin Resistance and Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Ciaraldi, T.P.; Olefsky, J.M. Comparison of the effects of insulin and H2O2 on adipocyte glucose transport. J. Cell. Physiol. 1982, 110, 323–328. [Google Scholar] [CrossRef]
- May, J.M.; de Haën, C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J. Biol. Chem. 1979, 254, 9017–9021. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Okuno, Y.; Fukuhara, A.; Hashimoto, E.; Kobayashi, H.; Kobayashi, S.; Otsuki, M.; Shimomura, I. Oxidative Stress Inhibits Healthy Adipose Expansion Through Suppression of SREBF1-Mediated Lipogenic Pathway. Diabetes 2018, 67, 1113–1127. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef]
- Guo, W.; Xie, W.; Han, J. Modulation of adipocyte lipogenesis by octanoate: Involvement of reactive oxygen species. Nutr. Metab. 2006, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; de Souza, J.; Gandy, J.; Mavangira, V.; Lock, A.L.; Sordillo, L.M. Periparturient lipolysis and oxylipid biosynthesis in bovine adipose tissues. PLoS ONE 2017, 12, e0188621. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of Body Condition Score on Relationships Between Metabolic Status and Oxidative Stress in Periparturient Dairy Cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Liang, Y.; Alharthi, A.S.; Bucktrout, R.; Elolimy, A.A.; Lopreiato, V.; Martinez-Cortés, I.; Xu, C.; Fernandez, C.; Trevisi, E.; Loor, J.J. Body condition alters glutathione and nuclear factor erythroid 2-like 2 (NFE2L2)-related antioxidant network abundance in subcutaneous adipose tissue of periparturient Holstein cows. J. Dairy Sci. 2020, 103, 6439–6453. [Google Scholar] [CrossRef] [PubMed]
- Andres Contreras, G.; De Koster, J.; de Souza, J.; Laguna, J.; Mavangira, V.; Nelli, R.K.; Gandy, J.; Lock, A.L.; Sordillo, L.M. Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows. J. Dairy Sci. 2020, 103, 1944–1955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Rjeileh, U.; Contreras, G.A. Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants 2021, 10, 1090. https://doi.org/10.3390/antiox10071090
Abou-Rjeileh U, Contreras GA. Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants. 2021; 10(7):1090. https://doi.org/10.3390/antiox10071090
Chicago/Turabian StyleAbou-Rjeileh, Ursula, and G. Andres Contreras. 2021. "Redox Regulation of Lipid Mobilization in Adipose Tissues" Antioxidants 10, no. 7: 1090. https://doi.org/10.3390/antiox10071090
APA StyleAbou-Rjeileh, U., & Contreras, G. A. (2021). Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants, 10(7), 1090. https://doi.org/10.3390/antiox10071090





