Serum Vitamin D Is Associated with Antioxidant Potential in Peri-Parturient Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Vitamin Analysis
2.4. AOP Analysis
2.5. ROS Analysis
2.6. LC-MS/MS Analysis
2.7. Cell Culture and Treatments
2.8. mRNA Quantification in BAEC
2.9. Endothelial Cell-Substrate Impedance Sensing (ECIS)
2.10. Data Analyses
3. Results
3.1. Correlations between Vitamins and Oxylipids
3.2. Vitamins and AOP
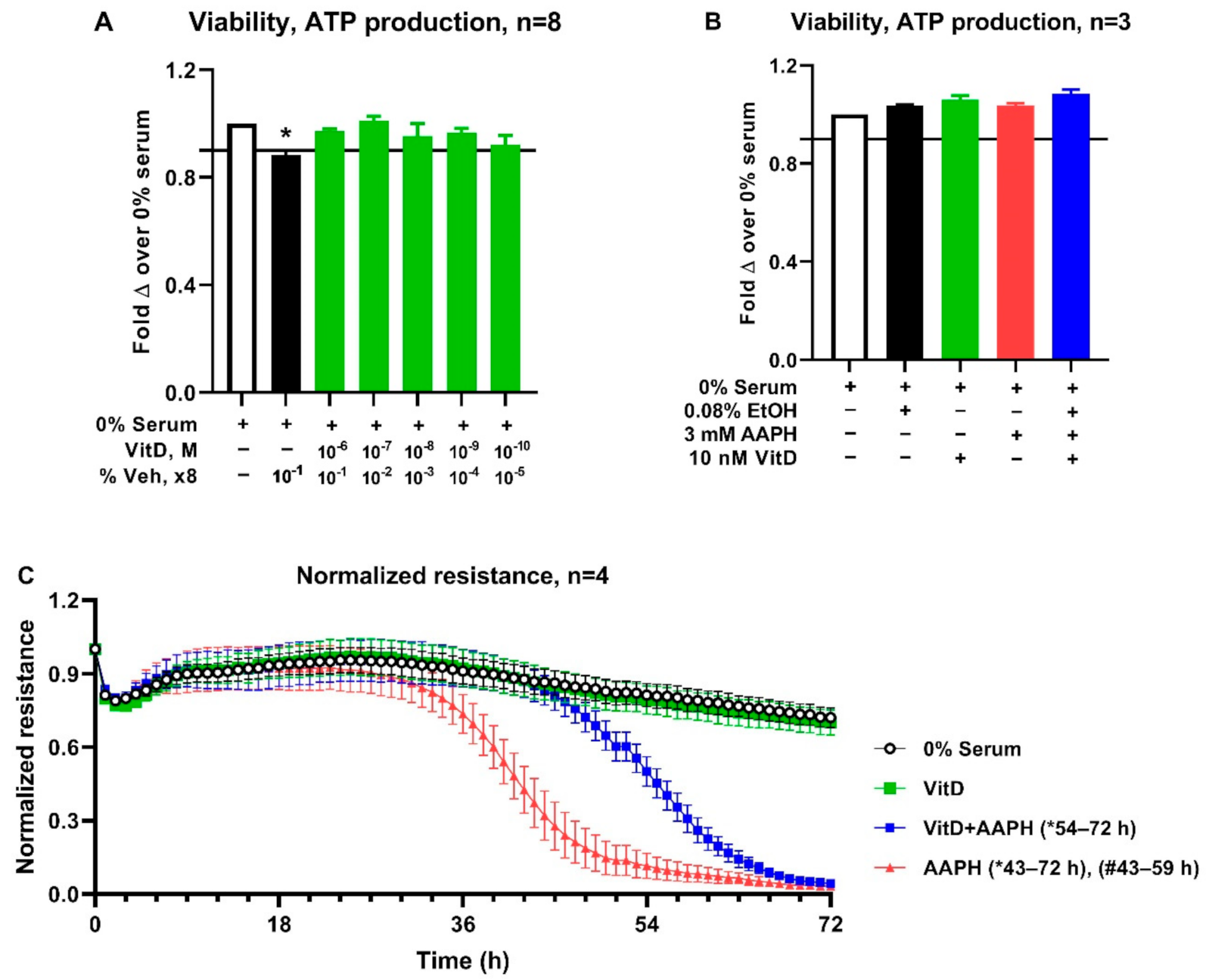
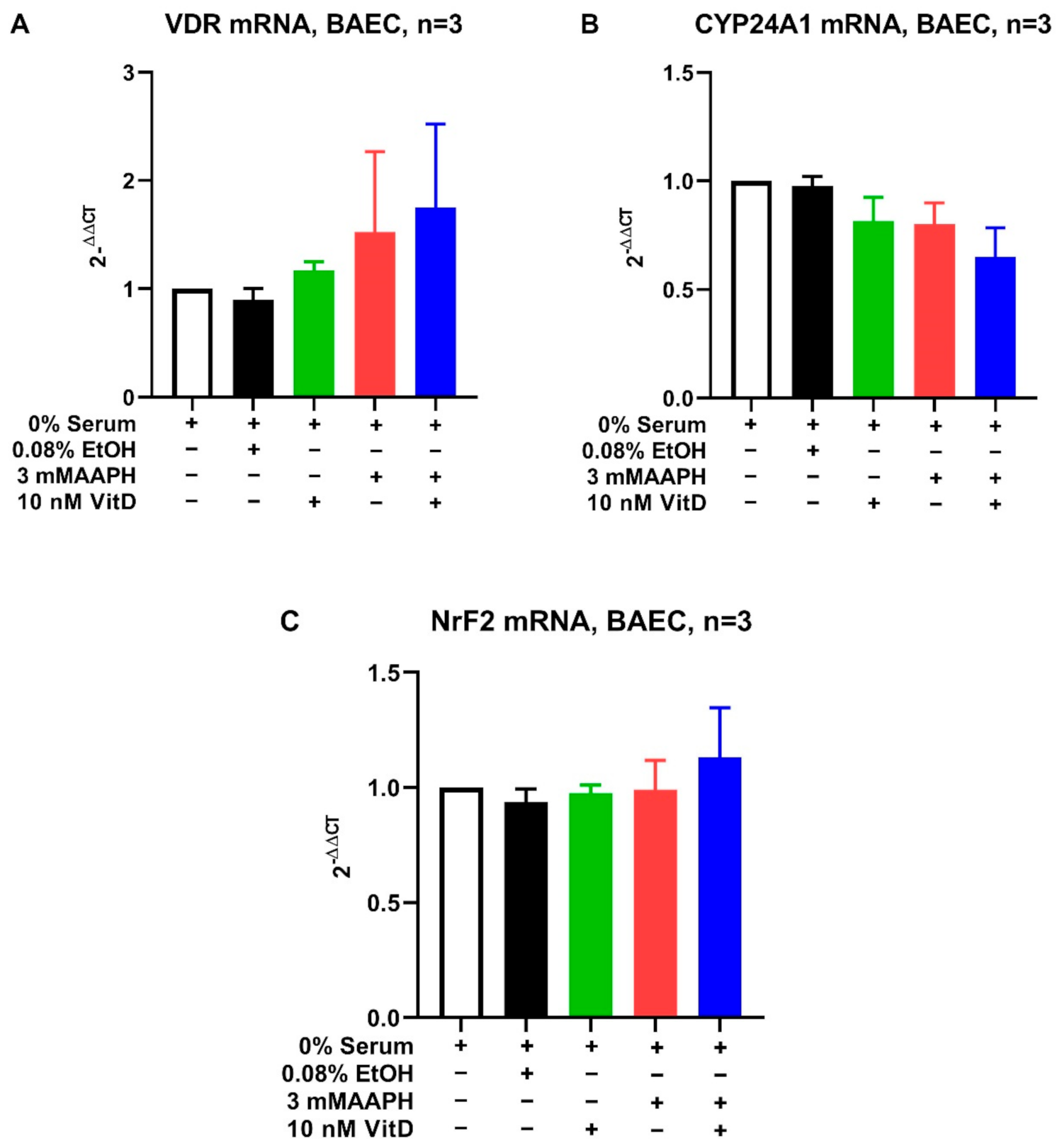
3.3. Cell Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.; Becker, T.; Gandy, J.; Sordillo, L. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev. Vet. Med. 2019, 163, 68–78. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Kawahara, N.; Kim, Y.-H.; Ichijo, T.; Sato, S. Changes in oxidative stress parameters in healthy and diseased Holstein cows during the transition period in Yamagata Prefecture, Japan. J. Vet. Med. Sci. 2020, 82, 955–961. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-Dependent Regulation of Oxidative Stress and Immunity in Periparturient Dairy Cattle. Vet. Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef] [Green Version]
- Weiss, W.P. A 100-Year Review: From ascorbic acid to zinc—Mineral and vitamin nutrition of dairy cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef]
- Brzezinska-Slebodzinska, E.; Miller, J.; Quigley, J., III; Moore, J.; Madsen, F. Antioxidant status of dairy cows supplemented prepartum with vitamin E and selenium. J. Dairy Sci. 1994, 77, 3087–3095. [Google Scholar] [CrossRef]
- Hemingway, R. The influences of dietary intakes and supplementation with selenium and vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet. Res. Commun. 2003, 27, 159–174. [Google Scholar] [CrossRef]
- Weiss, W.; Hogan, J.; Todhunter, D.; Smith, K. Effect of vitamin E supplementation in diets with a low concentration of selenium on mammary gland health of dairy cows. J. Dairy Sci. 1997, 80, 1728–1737. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.; Weiss, W.P.; Todhunter, D.; Schockey, W. Relationships among vitamin E, selenium, and bovine blood neutrophils. J. Dairy Sci. 1990, 73, 2372–2378. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Sordillo, L.M. Vitamin E analogs limit in vitro oxidant damage to bovine mammary endothelial cells. J. Dairy Sci. 2021. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Streicher, K.L.; Mullarky, I.K.; Gandy, J.C.; Trigona, W.; Corl, C.M. Selenium inhibits 15-hydroperoxyoctadecadienoic acid-induced intracellular adhesion molecule expression in aortic endothelial cells. Free Radic. Biol. Med. 2008, 44, 34–43. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001; p. 408. [Google Scholar]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L.M. Production of 15-F2t-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major Advances in Disease Prevention in Dairy Cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- Lopes, F.; Rosa, G.; Pinedo, P.; Santos, J.P.; Chebel, R.; Galvao, K.; Schuenemann, G.; Bicalho, R.; Gilbert, R.; Rodrigez-Zas, S. Genome-enable prediction for health traits using high-density SNP panel in US Holstein cattle. Anim. Genet. 2020, 51, 192–199. [Google Scholar] [CrossRef]
- Vieira-Neto, A.; Lima, I.; Lopes, F., Jr.; Lopera, C.; Zimpel, R.; Sinedino, L.; Jeong, K.; Galvão, K.; Thatcher, W.; Nelson, C. Use of calcitriol to maintain postpartum blood calcium and improve immune function in dairy cows. J. Dairy Sci. 2017, 100, 5805–5823. [Google Scholar] [CrossRef]
- Jin, L.; Yan, S.; Shi, B.; Bao, H.; Gong, J.; Guo, X.; Li, J. Effects of vitamin A on the milk performance, antioxidant functions and immune functions of dairy cows. Anim. Feed Sci. Technol. 2014, 192, 15–23. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Guerreiro, B.M.; Morais Junior, N.N.; Araujo, R.L.; Pereira, R.A.; Pereira, M.N. Supplementation of prepartum dairy cows with beta-carotene. J. Dairy Sci. 2015, 98, 6304–6314. [Google Scholar] [CrossRef]
- Sowers, M.; Lachance, L. Vitamins and arthritis. The roles of vitamins A, C, D, and E. Rheum. Dis. Clin. N. Am. 1999, 25, 315–332. [Google Scholar] [CrossRef]
- Goff, J.P.; Kimura, K.; Horst, R.L. Effect of mastectomy on milk fever, energy, and vitamins A, E, and beta-carotene status at parturition. J. Dairy Sci. 2002, 85, 1427–1436. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Wisnieski, L.; Gandy, J.; Norby, B.; Sordillo, L.M. Reduced serum vitamin D concentrations in healthy early-lactation dairy cattle. J. Dairy Sci. 2018, 101, 1488–1494. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Herdt, T.H.; Seymour, W.M.; Duffield, T.F.; Leslie, K.E. Peripartum serum vitamin E, retinol, and beta-carotene in dairy cattle and their associations with disease. J. Dairy Sci. 2004, 87, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Strickland, J.M.; Wisnieski, L.; Herdt, T.H.; Sordillo, L.M. Serum retinol, β-carotene, and α-tocopherol as biomarkers for disease risk and milk production in periparturient dairy cows. J. Dairy Sci. 2021, 104, 915–927. [Google Scholar] [CrossRef]
- Michal, J.J.; Heirman, L.R.; Wong, T.S.; Chew, B.P.; Frigg, M.; Volker, L. Modulatory effects of dietary beta-carotene on blood and mammary leukocyte function in periparturient dairy cows. J. Dairy Sci. 1994, 77, 1408–1421. [Google Scholar] [CrossRef]
- Wisnieski, L.; Brown, J.; Holcombe, S.; Gandy, J.; Sordillo, L. Serum vitamin D concentrations at dry-off and close-up predict increased postpartum urine ketone concentrations in dairy cattle. J. Dairy Sci. 2020, 103, 1795–1806. [Google Scholar] [CrossRef]
- Rodney, R.M.; Celi, P.; McGrath, H.M.; Anderson, S.T.; McNeill, D.M.; Fraser, D.R.; Lean, I.J. Metabolic and production responses to calcidiol treatment in mid-lactation dairy cows. Anim. Prod. Sci. 2018, 59, 449–460. [Google Scholar] [CrossRef]
- Sprecher, D.E.A.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Mavangira, V.; Gandy, J.C.; Zhang, C.; Ryman, V.E.; Jones, A.D.; Sordillo, L.M. Polyunsaturated fatty acids influence differential biosynthesis of oxylipids and other lipid mediators during bovine coliform mastitis. J. Dairy Sci. 2015, 98, 6202–6215. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Alberg, A.J.; Helzlsouer, K.J. Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin. Chem. 1993, 39, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Fortis, I.; Blachier, S.; Kia, D.; Favier, A. Simultaneous determination of retinol, alpha-tocopherol and beta-carotene in serum by isocratic high-performance liquid chromatography. J. Chromatogr. 1991, 572, 103–116. [Google Scholar] [CrossRef]
- Farrell, C.-J.L.; Martin, S.; McWhinney, B.; Straub, I.; Williams, P.; Herrmann, M. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography–tandem mass spectrometry methods. Clin. Chem. 2012, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N, N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Koch, E.; Mainka, M.; Dalle, C.; Ostermann, A.I.; Rund, K.M.; Kutzner, L.; Froehlich, L.-F.; Bertrand-Michel, J.; Gladine, C.; Schebb, N.H. Stability of oxylipins during plasma generation and long-term storage. Talanta 2020, 217, 121074. [Google Scholar] [CrossRef]
- Mavangira, V.; Brown, J.; Gandy, J.C.; Sordillo, L.M. 20-hydroxyeicosatetraenoic acid alters endothelial cell barrier integrity independent of oxidative stress and cell death. Prostaglandins Other Lipid Mediat. 2020, 149, 106425. [Google Scholar] [CrossRef]
- Aherne, K.; Davis, M.; Sordillo, L. Isolation and characterization of bovine mammary endothelial cells. Methods Cell Sci. 1995, 17, 41–46. [Google Scholar] [CrossRef]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Pro-inflammatory and pro-apoptotic responses of TNF-alpha stimulated bovine mammary endothelial cells. Vet. Immunol. Immunopathol. 2011, 140, 282–290. [Google Scholar] [CrossRef]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L.M. Apoptosis of Endothelial Cells by 13-HPODE Contributes to Impairment of Endothelial Barrier Integrity. Mediat. Inflamm. 2016, 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Bouwstra, R.J.; Nielen, M.; Newbold, J.R.; Jansen, E.H.J.M.; Jelinek, H.F.; van Werven, T. Vitamin E supplementation during the dry period in dairy cattle. Part II: Oxidative stress following vitamin E supplementation may increase clinical mastitis incidence postpartum. J. Dairy Sci. 2010, 93, 5696–5706. [Google Scholar] [CrossRef]
- Herdt, T.H.; Stowe, H.D. Fat-soluble vitamin nutrition for dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 1991, 7, 391–415. [Google Scholar] [CrossRef]
- Kweh, M.F.; Merriman, K.E.; Wells, T.L.; Nelson, C.D. Vitamin D signaling increases nitric oxide and antioxidant defenses of bovine monocytes. JDS Commun. 2021, 2, 73–79. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Zhang, C.; Jones, A.D.; Sordillo, L.M. Differences in the Oxylipid Profiles of Bovine Milk and Plasma at Different Stages of Lactation. J. Agric. Food Chem. 2017, 65, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, H.; Tang, H.; Li, X.; Tan, J.; Zeng, Q.; Sun, C. 20-Hydroxyeicosatetraenoic acid mediates isolated heart ischemia/reperfusion injury by increasing NADPH oxidase-derived reactive oxygen species production. Circ. J. 2013, 77, 1807–1816. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.J.; Sordillo, L.M. Inhibition of 20-hydroxyeicosatetraenoic acid biosynthesis by vitamin E analogs in human and bovine cytochrome P450 microsomes. J. Anim. Physiol. Anim. Nutr. 2021. [Google Scholar] [CrossRef]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L.M. Role of endothelial cells in bovine mammary gland health and disease. Anim. Health Res. Rev. 2015, 16, 135–149. [Google Scholar] [CrossRef]
- Schröder-Heurich, B.; von Hardenberg, S.; Brodowski, L.; Kipke, B.; von Meyer, N.; Borns, K.; Kaisenberg, C.S.; Brinkmann, H.; Claus, P.; von Versen-Höynck, F. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. FASEB J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-k B signaling pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef] [Green Version]
| Gene | NCBI Reference Sequence 1 | TaqMan Assay ID |
|---|---|---|
| CYP24A1 | NM_001191417.1 | Bt04306544_m1 |
| NFE2L2 | NM_001011678.2 | Bt03251878_m1 |
| VDR | NM_001167932.2 | Bt04301663_m1 |
| ACTB | NM_173979.3 | Bt03279174_g1 |
| GUSB | NM_001083436.1 | Bt03256165_m1 |
| RPS9 | NM_001101152.2 | Bt03272017_m1 |
| Biomarker | Time Point | N | Mean | SEM | Min | 25th Percentile | Median | 75th Percentile | Max |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin E (μg/mL) | DO | 239 | 2.66 | 0.06 | 0.71 | 1.99 | 2.60 | 3.29 | 5.28 |
| CU | 214 | 3.12 | 0.10 | 0.61 | 2.02 | 2.78 | 4.06 | 9.44 | |
| DIM 2-10 | 198 | 1.79 | 0.06 | 0.001 | 1.15 | 1.81 | 2.37 | 4.57 | |
| AOP (TE/μL) | DO | 240 | 5.08 | 0.13 | 1.31 | 3.57 | 4.69 | 6.43 | 10.80 |
| CU | 222 | 5.05 | 0.13 | 2.38 | 3.59 | 4.57 | 5.83 | 10.80 | |
| DIM 2-10 | 207 | 4.36 | 0.10 | 1.05 | 3.38 | 4.27 | 5.20 | 8.63 | |
| β-carotene (μg/mL) | DO | 238 | 5.00 | 0.25 | 0.21 | 2.62 | 4.07 | 6.00 | 23.62 |
| CU | 221 | 3.35 | 0.16 | 0.10 | 1.77 | 2.62 | 4.53 | 13.87 | |
| DIM 2-10 | 206 | 1.63 | 0.09 | 0.10 | 0.74 | 1.15 | 2.02 | 6.39 | |
| ROS (CarrU/μL) | DO | 35 | 169.40 | 10.44 | 16.3 | 130.7 | 155.2 | 212.3 | 310.3 |
| CU | 12 | 132.71 | 11.43 | 49.0 | 110.3 | 138.8 | 155.2 | 204.2 | |
| DIM 2-10 | 22 | 181.52 | 8.80 | 122.5 | 155.2 | 175.6 | 212.3 | 277.7 | |
| Vitamin A (ng/mL) | DO | 239 | 302.63 | 5.41 | 102 | 238 | 296 | 358 | 528 |
| CU | 221 | 277.15 | 5.88 | 112 | 213 | 278 | 331 | 561 | |
| DIM 2-10 | 205 | 256.81 | 8.33 | 53 | 177 | 241 | 316 | 764 | |
| Vitamin D (ng/mL) | DO | 186 | 99.12 | 2.02 | 25.40 | 81.0 | 97.60 | 112.50 | 198.60 |
| CU | 173 | 93.77 | 2.19 | 20.10 | 77.20 | 94.80 | 110.00 | 210.40 | |
| DIM 2-10 | 184 | 82.06 | 1.71 | 9.80 | 63.95 | 82.30 | 96.30 | 166.40 | |
| 5- iPF2alphaVI (ng/L) | DO | 7 | 0.07 | 0.01 | 0.05 | 0.05 | 0.05 | 0.10 | 0.10 |
| CU | 7 | 0.06 | 0.01 | 0.05 | 0.05 | 0.05 | 0.05 | 0.10 | |
| DIM 2-10 | 7 | 0.06 | 0.01 | 0..05 | 0.05 | 0.05 | 0.10 | 0.10 | |
| 8,12-isoprostane (ng/L) | DO | 7 | 0.26 | 0.05 | 0.1 | 0.10 | 0.30 | 0.40 | 0.40 |
| CU | 7 | 0.23 | 0.07 | 0 | 0.10 | 0.20 | 0.30 | 0.60 | |
| DIM 2-10 | 7 | 0.21 | 0.04 | 0.1 | 0.10 | 0.20 | 0.30 | 0.40 | |
| 8-isoprostane PGA2 (ng/L) | DO | 7 | 0.29 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 |
| CU | 7 | 0.73 | 0.13 | 0.40 | 0.50 | 0.60 | 0.90 | 1.4 | |
| DIM 2-10 | 7 | 0.16 | 0.04 | 0.10 | 0.10 | 0.10 | 0.20 | 0.40 | |
| 8-isoprostane PGF2alpha (ng/L) | DO | 7 | 0.26 | 0.15 | 0 | 0 | 0.10 | 0.30 | 1.1 |
| CU | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| DIM 2-10 | 7 | 0.73 | 0.17 | 0 | 0.50 | 0.70 | 1.0 | 1.5 | |
| 20-HETE (μg/L) | DO | 27 | 2.22 | 0.27 | 0.01 | 1.20 | 1.90 | 3.19 | 6.54 |
| CU | 24 | 5.28 | 1.66 | 0.28 | 1.71 | 3.60 | 5.75 | 41.41 | |
| DIM 2-10 | 27 | 9.31 | 2.31 | 0.50 | 2.78 | 4.80 | 8.50 | 51.95 |
| Dry Off | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin E | β-carotene | ROS | Vitamin A | Vitamin D | 5-iPF2alphaVI | 8,12-isoprostane | 8-isoprostane PGA2 | 8-isoprostane PGF2alpha | 20-HETE | |
| Vitamin E | 1 | |||||||||
| β-carotene | 0.04 | 1 | ||||||||
| ROS | 0.36 ** | 0.35 * | 1 | |||||||
| Vitamin A | 0.08 | 0.46 ** | 0.3 | 1 | ||||||
| Vitamin D | 0.08 | −0.002 | 0.20 ** | 1 | ||||||
| 5-iPF2alphaVI | 0.43 | −0.43 | 0.80 * | 0.43 | 1 | |||||
| 8,12-isoprostane | −0.13 | −0.77 * | 0 | 0.4 | 0.07 | 1 | ||||
| 8-isoprostane PGA2 | −0.13 | −0.80 * | −0.19 | 0.76 * | 0.15 | 0.74 | 1 | |||
| 8-isoprostane PGF2alpha | −0.07 | 0.15 | 0.2 | −0.3 | 0.15 | −0.56 | −0.28 | 1 | ||
| 20-HETE | −0.32 | 0.12 | 0.2 | 0.42 * | −0.04 | 0.29 | −0.78 * | −0.35 | 0.27 | 1 |
| Close up | ||||||||||
| Vitamin E | 1 | |||||||||
| β-carotene | 0.32 ** | 1 | ||||||||
| ROS | 0.16 | −0.03 | 1 | |||||||
| Vitamin A | 0.30 ** | 0.19 ** | 0.07 | 1 | ||||||
| Vitamin D | 0.19 * | −0.04 | 0.16 * | 1 | ||||||
| 5-iPF2alphaVI | 0.39 | −0.61 | 0.77 | 0 | 1 | |||||
| 8,12-isoprostane | 0.35 | −0.11 | 0.2 | −0.38 | 0.62 | 1 | ||||
| 8-isoprostane PGA2 | −0.17 | −0.67 | 0.95 | −0.04 | 0.62 | 0.33 | 1 | |||
| 8-isoprostane PGF2alpha | 0.37 * | 1 | ||||||||
| 20-HETE | −0.38 | 0.15 | 0.4 | −0.12 | 0.19 | −0.41 | −0.33 | 0.14 | 1 | |
| DIM2-10 | ||||||||||
| Vitamin E | 1 | |||||||||
| β-carotene | 0.23 ** | 1 | ||||||||
| ROS | 0.16 | −0.13 | 1 | |||||||
| Vitamin A | 0.18 * | 0.61 ** | 0.01 | 1 | ||||||
| Vitamin D | 0 | 0.07 | 0.29 ** | 1 | ||||||
| 5-iPF2alphaVI | −0.39 | −0.32 | 0.48 | 0.39 | 1 | |||||
| 8,12-isoprostane | −0.03 | −0.62 | 0.05 | 0.76 | 0.08 | 1 | ||||
| 8-isoprostane PGA2 | 0.39 | 0.18 | 0.23 | 0.65 | 0.39 | −0.19 | 1 | |||
| 8-isoprostane PGF2alpha | −0.26 | −0.54 | 0.73 | 0.66 | 0.79 * | 0.24 | 0.37 * | 1 | ||
| 20-HETE | 0.04 | −0.42 * | −0.02 | −0.57 ** | −0.4 | 0.79 * | −0.13 | 0.04 | 0.46 | 1 |
| All time points | ||||||||||
| Vitamin E | 1 | |||||||||
| β-carotene | 0.35 ** | 1 | ||||||||
| ROS | 0.03 | 0.14 | 1 | |||||||
| Vitamin A | 0.26 ** | 0.45 ** | 0.24 | 1 | ||||||
| Vitamin D | 0.16 ** | 0.16 ** | 0.26 ** | 1 | ||||||
| 5-iPF2alphaVI | 0.26 | −0.31 | 0.65 ** | 0.25 | 1 | |||||
| 8,12-isoprostane | 0.05 | −0.3 | 0.08 | 0.23 | 0.3 | 1 | ||||
| 8-isoprostane PGA2 | 0.42 | 0.09 | −0.23 | −0.05 | 0.05 | 0.13 | 1 | |||
| 8-isoprostane PGF2alpha | −0.45 | −0.38 | 0.42 | 0.21 | 0.24 | −0.1 | 0.37 * | 1 | ||
| 20-HETE | −0.23 * | −0.42 ** | 0.13 | −0.25 * | −0.19 | −0.02 | −0.4 | −0.07 | 0.23 | 1 |
| Variable | Coefficient | L95% CI | U95% CI | SE | z | p-Value |
|---|---|---|---|---|---|---|
| CU 1 (N = 173) | ||||||
| Vitamin D | 0.006 | 0.0001 | 0.01 | 0.003 | 2.01 | 0.04 |
| Intercept | 3.79 | 3.13 | 4.46 | 0.34 | 11.18 | <0.01 |
| DIM2-10 2 (N = 184) | ||||||
| Vitamin D | 0.008 | 0.003 | 0.01 | 0.003 | 2.96 | 0.01 |
| Intercept | 3.43 | 2.82 | 4.03 | 0.31 | 11.15 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strickland, J.M.; Wisnieski, L.; Mavangira, V.; Sordillo, L.M. Serum Vitamin D Is Associated with Antioxidant Potential in Peri-Parturient Cows. Antioxidants 2021, 10, 1420. https://doi.org/10.3390/antiox10091420
Strickland JM, Wisnieski L, Mavangira V, Sordillo LM. Serum Vitamin D Is Associated with Antioxidant Potential in Peri-Parturient Cows. Antioxidants. 2021; 10(9):1420. https://doi.org/10.3390/antiox10091420
Chicago/Turabian StyleStrickland, Jaimie M., Lauren Wisnieski, Vengai Mavangira, and Lorraine M. Sordillo. 2021. "Serum Vitamin D Is Associated with Antioxidant Potential in Peri-Parturient Cows" Antioxidants 10, no. 9: 1420. https://doi.org/10.3390/antiox10091420
APA StyleStrickland, J. M., Wisnieski, L., Mavangira, V., & Sordillo, L. M. (2021). Serum Vitamin D Is Associated with Antioxidant Potential in Peri-Parturient Cows. Antioxidants, 10(9), 1420. https://doi.org/10.3390/antiox10091420







