Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Pollen Samples
2.2. Extract Preparation
2.3. Determination of Total Phenolic Content
2.4. Determination of Antioxidant Activity using Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Determination of Antioxidant Activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.6. Statistical Analysis
3. Results
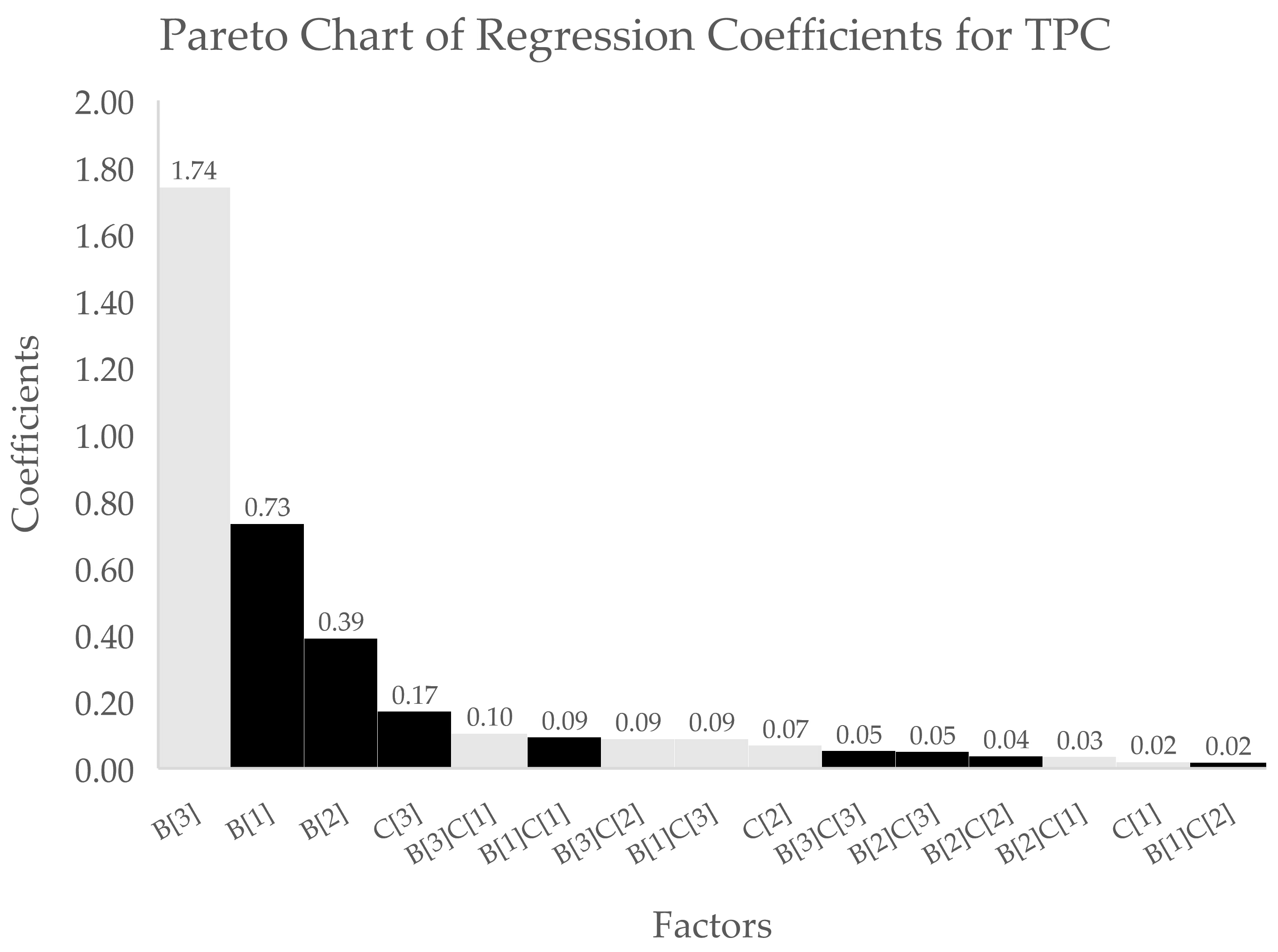
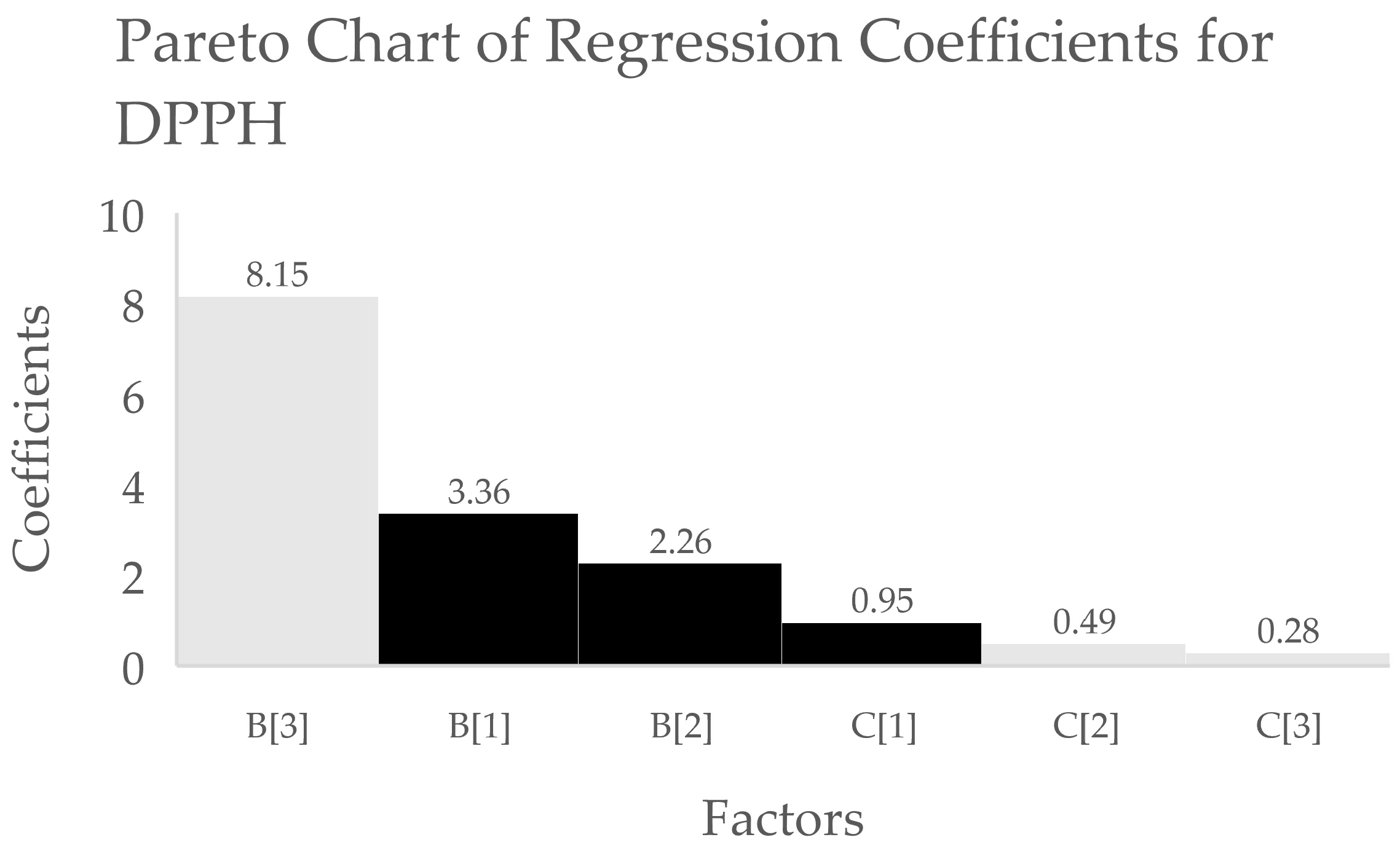
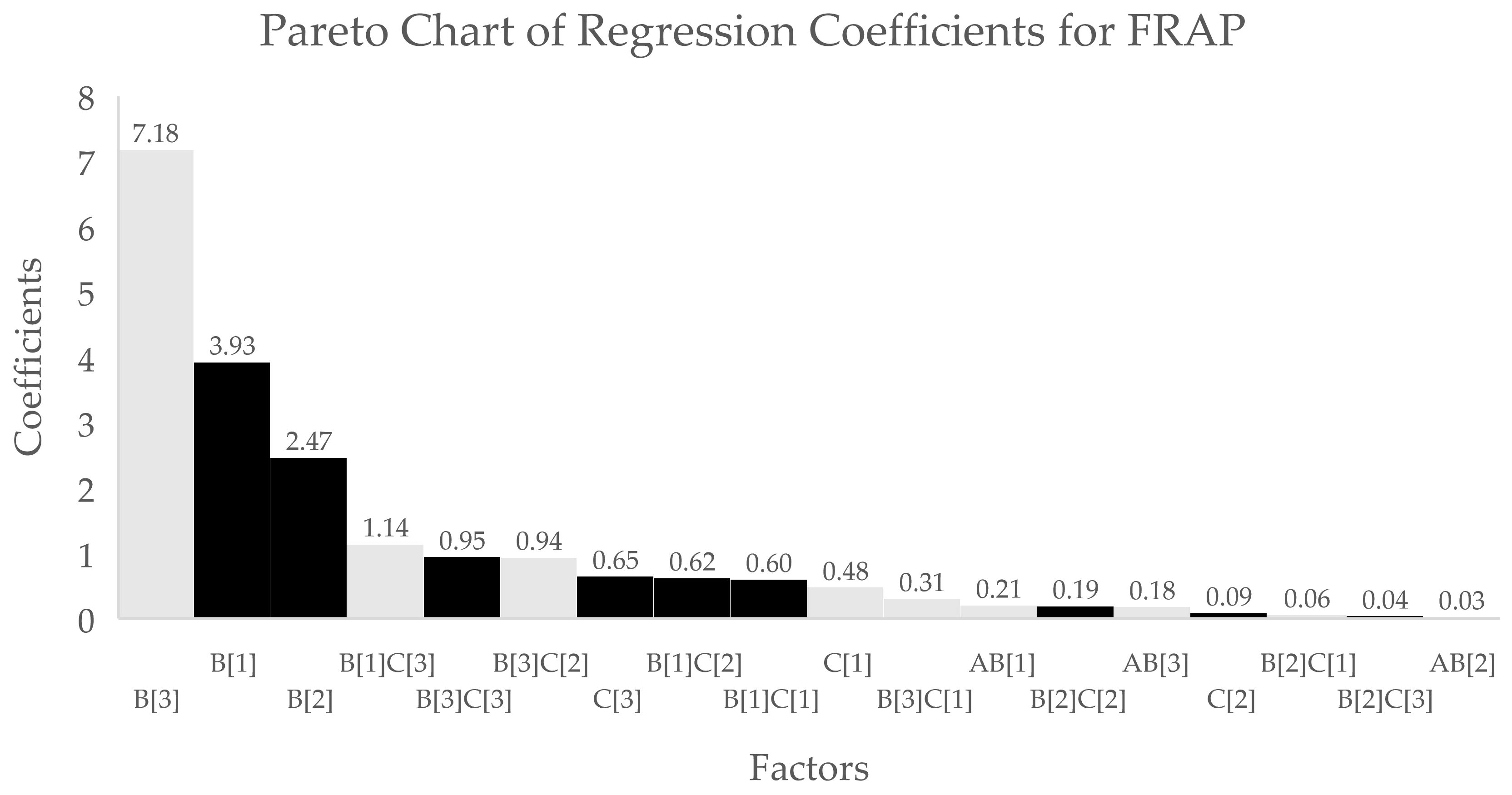
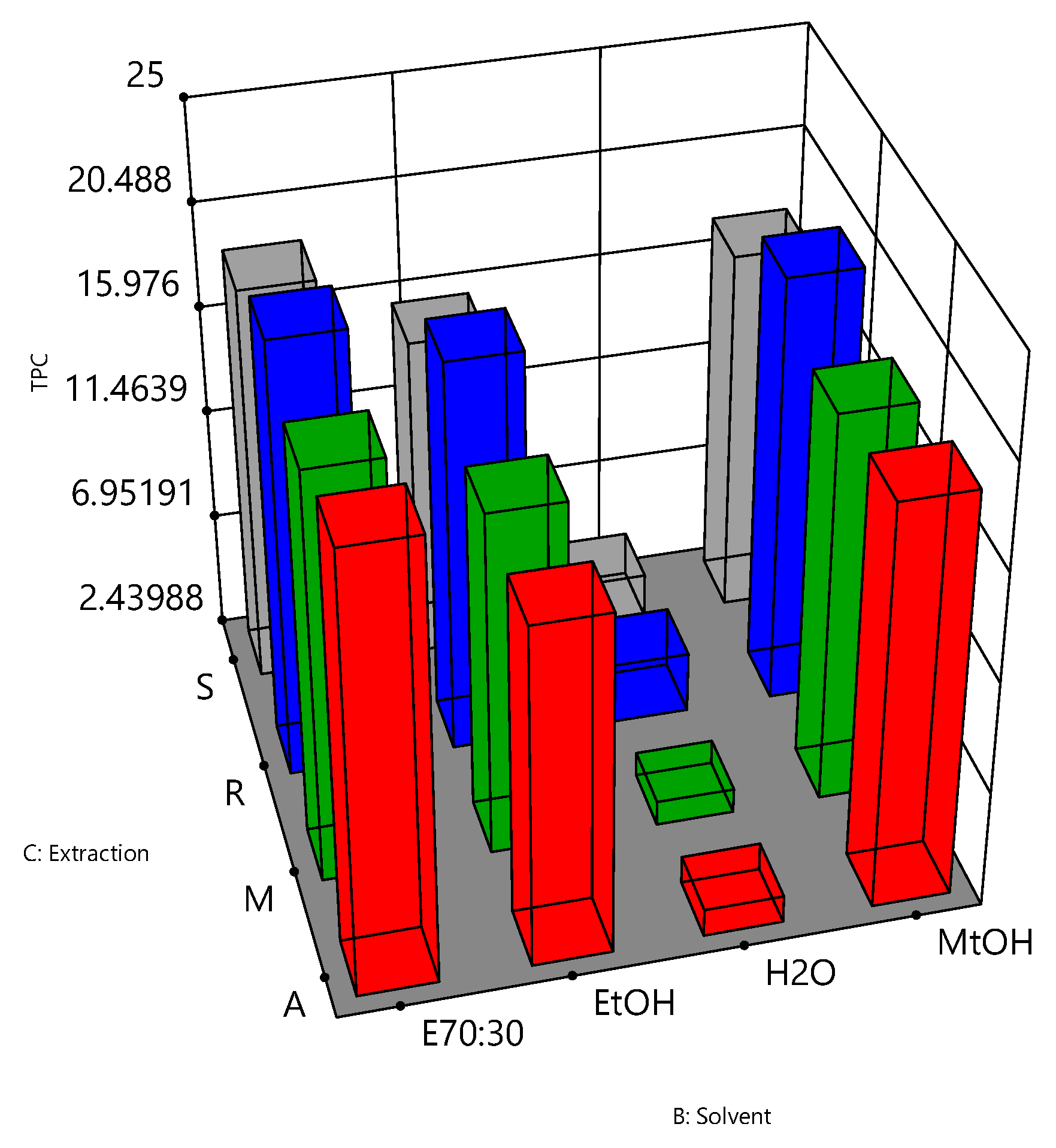
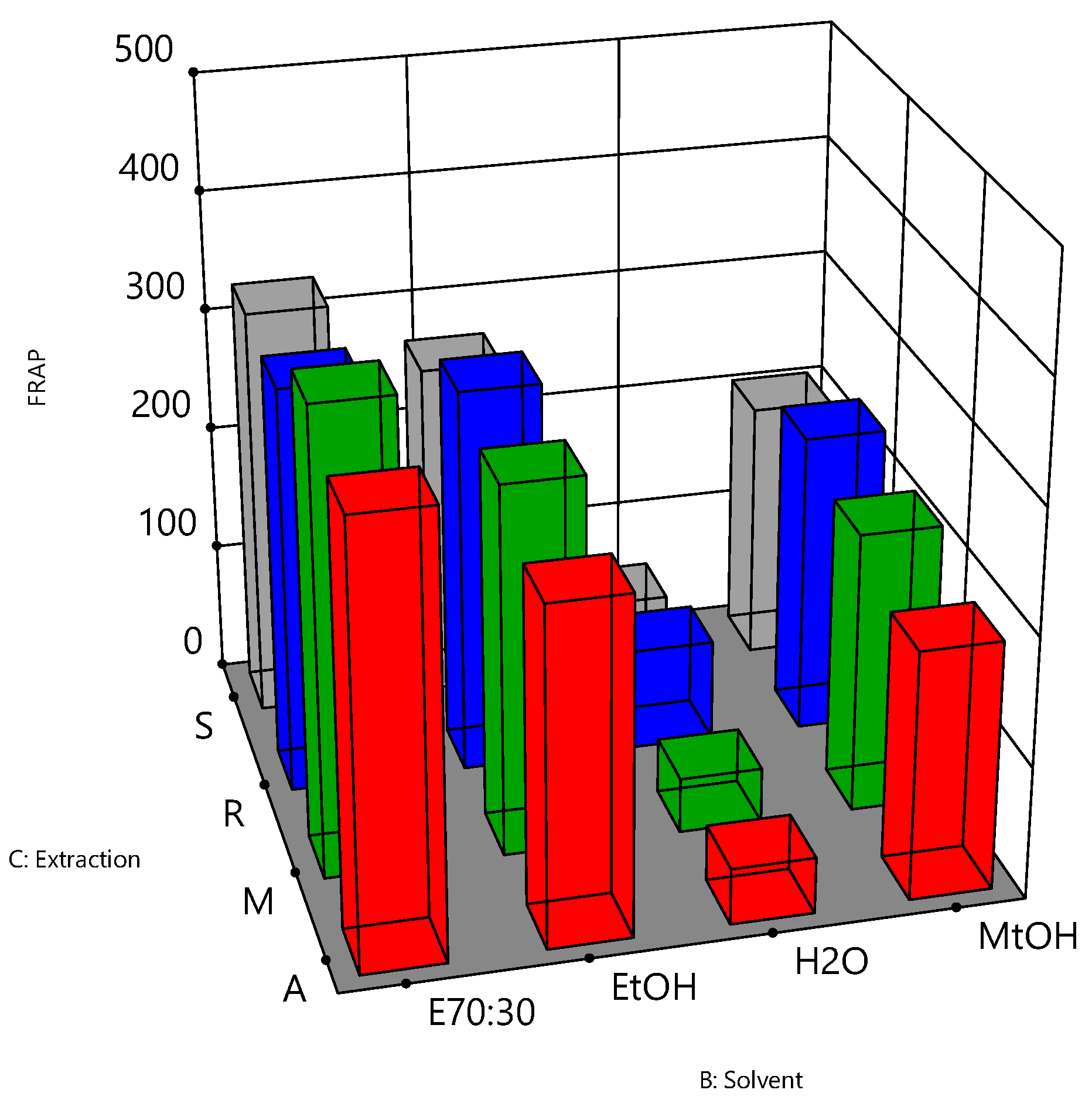
3.1. Analysis of the Optimisation Process
3.2. Correlation of TPC, DPPH and FRAP Antioxidant Activity
3.3. Choosing Optimum Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Dias, T.; Anjos, O. Influence of the storage conditions (frozen vs dried) in health-related lipid indexes and antioxidants of bee pollen. Eur. J. Lipid Sci. Technol. 2018, 121, 1–9. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C.; Lyoussi, B. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Féas, X.; Vázquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic Bee Pollen: Botanical Origin, Nutritional Value, Bioactive Compounds, Antioxidant Activity and Microbiological Quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolaylı, S.; Erim, F. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K.; Qiao, J.; Dong, J.; Li, Z.; Zhang, H. Improving nutrient release of wall-disrupted bee pollen with a combination of ultrasonication and high shear technique. J. Sci. Food Agric. 2019, 99, 564–575. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Borycka, K.; Grabek-Lejko, D.; Kasprzyk, I. Antioxidant and antibacterial properties of commercial bee pollen products. J. Apic. Res. 2015, 54, 491–502. [Google Scholar] [CrossRef]
- Ozkok, A. Determination of antioxidant capacities, chemical composition and melissopalynological characterization of Verbascum spp. and Euphorbia spp. honeys. Fresenius Environ. Bull. 2019, 28, 2644–2649. [Google Scholar]
- Čeksterytė, V.; Kurtinaitienė, B.; Venskutonis, P.; Pukalskas, A.; Kazernavičiūtė, R.; Balžekas, J. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT 2016, 74, 504–513. [Google Scholar] [CrossRef]
- Cinkmanis, I.; Dimins, F.; Mikelsone, V. Influence of Lyophilization and convective type drying on antioxidant properties, total phenols and flavonoids in pollens. In Proceedings of the 11th Baltic Conference on Food Science and Technology “Food Science and Technology in a Changing World, Jelgava, Latvia, 27–28 April 2017; pp. 201–203. [Google Scholar]
- Ozkan, K.; Sagcan, N.; Ozulku, G.; Sagdic, O.; Toker, O.S.; Muz, M.N. Bioactive and bioaccessibility characteristics of honeybee pollens collected from different regions of Turkey. J. Food Meas. Charact. 2017, 12, 581–587. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhou, Q.; Wang, L.; Huang, W.; Wang, R. Effect of ultrasonic and ball-milling treatment on cell wall, nutrients, and antioxidant capacity of rose (Rosa rugosa) bee pollen, and identification of bioactive components. J. Sci. Food Agric. 2019, 99, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Gorjanović, S.Ž.; Alvarez-Suarez, J.M.; Novaković, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Sužnjević, D.Ž. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Khalil, I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, A.; Islam, N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef]
- Hmidani, A.; Bouhlali, E.D.T.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Benlyas, M.; Alem, C. Effect of extraction methods on antioxidant and anticoagulant activities of Thymus atlanticus aerial part. Sci. Afr. 2019, 5, 5. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Azwanida, A. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 04, 04. [Google Scholar] [CrossRef]
- Khadra, L.; Kamel, M.; Hamama, B.; Ibrahim, D.; Daoud, H. Chemical composition, antioxidant and antimicrobial activities of algerian bee pollen (inula viscosa) methanolic extract. Int. J. Res. Pharm. Chem. 2019, 9, 9. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, M.L.M.F.; Sattler, J.A.G.; Souza, B.R.; Freitas, A.D.S.; Barth, O.M.; de Almeida-Muradian, L.B. Effect of processing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. LWT 2016, 65, 808–815. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobis, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Screening of Indian bee pollen based on antioxidant properties and polyphenolic composition using UHPLC-DAD-MS/MS: A multivariate analysis and ANN based approach. Food Res. Int. 2021, 140, 110041. [Google Scholar] [CrossRef] [PubMed]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.D.S.D.; Barth, O.M.; de Almeida-Muradian, L.B. A multivariate approach based on physicochemical parameters and biological potential for the botanical and geographical discrimination of Brazilian bee pollen. Food Biosci. 2018, 25, 91–110. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Omar, W.A.W.; Azhar, N.A.; Fadzilah, N.H.; Kamal, N.N.S.N.M. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 265–269. [Google Scholar] [CrossRef]
- Khongkarat, P.; Ramadhan, R.; Phuwapraisirisan, P.; Chanchao, C. Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon 2020, 6, e03638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Wang, K.; Li, C. Antioxidant and Tyrosinase Inhibitory Properties of Aqueous Ethanol Extracts from Monofloral Bee Pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Hemmami, H.; Ben Seghir, B.; Ben Ali, M.; Rebiai, A.; Zeghoud, S.; Brahmia, F. Phenolic profile and antioxidant activity of bee pollen extracts from different regions of Algeria. Ovidius Univ. Ann. Chem. 2020, 31, 93–98. [Google Scholar] [CrossRef]
- Izuta, H.; Narahara, Y.; Shimazawa, M.; Mishima, S.; Kondo, S.-I.; Hara, H. 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Activity of Bee Products and Their Constituents Determined by ESR. Biol. Pharm. Bull. 2009, 32, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga-Domínguez, C.; Serrato-Bermudez, J.; Quicazan, M.; Zuluaga, C.; Serrato, J. Influence of drying-related operations on microbiological, structural and physicochemical aspects for processing of bee-pollen. Eng. Agric. Environ. Food 2018, 11, 57–64. [Google Scholar] [CrossRef]
- Keskin, M.; Özkök, A. Effects of drying techniques on chemical composition and volatile constituents of bee pollen. Czech J. Food Sci. 2020, 38, 203–208. [Google Scholar] [CrossRef]
- Vasconcelos, M.R.D.S.; Duarte, A.; Gomes, E.P.; Da Silva, S.C.; López, A.M.Q. Physicochemical composition and antioxidant potential of bee pollen from different botanical sources in Alagoas, Brazil. Ciência Agrotecnol. 2017, 41, 447–458. [Google Scholar] [CrossRef]
- Predictive mathematical modeling for EC50 calculation of antioxidant activity and antibacterial ability of Thai bee products. J. Appl. Pharm. Sci. 2017, 7, 122–133. [CrossRef][Green Version]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Chemical Composition and Antioxidant Activity of Walnut Pollen Samples. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 361–365. [Google Scholar] [CrossRef][Green Version]
- Bridi, R.; Atala, E.; Pizarro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.; Silva, R.H.M.; Queiroz, P.F.D.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; Da Rocha, C.Q.; Lima, S.T.D.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Garcia, J.B.S.; Pinheiro, M.S.D.; Pereira, F.A.N.; Camelo, D.D.S.; De Morais, S.V.; Freitas, J.R.B.; Da Rocha, C.Q.; Ribeiro, M.N.D.S.; et al. Anti-Inflammatory and Antioxidant Activity of Pollen Extract Collected by Scaptotrigona affinis postica: In silico, in vitro, and in vivo Studies. Antioxidants 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Futui, W.; Thongwai, N. Antimicrobial and Antioxidant Activities, Total Phenolic and Flavonoid Contents of Bee Pollen Crude Extracts. Int. J. Biosci. Biochem. Bioinform. 2020, 10, 42–48. [Google Scholar] [CrossRef]
- Nurdianah, H.F.; Firdaus, A.A.H.; Azam, O.E.; Wan Adnan, W.O. Antioxidant activity of bee pollen ethanolic extracts from Malaysian stingless bee measured using DPPH-HPLC assay. Int. Food Res. J. 2016, 23, 403–405. [Google Scholar]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stricik, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and Anti-tyrosinase Activities of Phenolic Extracts from Rape Bee Pollen and Inhibitory Melanogenesis by cAMP/MITF/TYR Pathway in B16 Mouse Melanoma Cells. Front. Pharmacol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Su, J.; Yang, X.; Lu, Q.; Liu, R. Antioxidant and anti-tyrosinase activities of bee pollen and identification of active components. J. Apic. Res. 2021, 60, 297–307. [Google Scholar] [CrossRef]
- Uçar, M.; Barlak, Y.; Deger, O. Antioxidant properties of dmso and water extracts of turkish bee pollen. World J. Pharm. Pharm. Sci. 2017, 6647, 154–163. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-Assisted Extraction of Polyphenols from Crude Pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and Cardioprotective Effects of Schisandra chinensis Bee Pollen Extract on Isoprenaline-Induced Myocardial Infarction in Rats. Molecules 2019, 24, 1090. [Google Scholar] [CrossRef] [PubMed]
- Saral, Ö.; Yildiz, O.; Aliyazicioğlu, R.; Yuluğ, E.; Canpolat, S.; Öztürk, F.; Kolayli, S. Apitherapy products enhance the recovery of CCL4-induced hepatic damages in rats. Turk. J. Med. Sci. 2016, 46, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, E.M.; Vit, P.; Rivas, E.; Sciortino, R.; Sosa, A.; Tejada, D.; Rodríguez-Malaver, A.J. Antioxidant activity of four color fractions of bee pollen from Mérida, Venezuela. Arch. Latinoam. Nutr. 2012, 62, 375–380. [Google Scholar]
- Daudu, O.M. Bee Pollen Extracts as Potential Antioxidants and Inhibitors of α-Amylase and α-Glucosidase Enzymes In Vitro Assessment. J. Apic. Sci. 2019, 63, 315–325. [Google Scholar] [CrossRef]
- Stanciu, O.G.; Dezmirean, D.S.; Campos, M.G. Bee Pollen in Transylvania (Romania): Palynological Characterization and ORACFL Values of Lipophilic and Hydrophilic Extracts of Monofloral Pollen Pellets. J. Agric. Sci. Technol. A 2016, 6, 18–37. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Bayram, N.E.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Anjos, O.; Fernandes, R.; Cardoso, S.M.; Delgado, T.; Farinha, N.; Paula, V.; Estevinho, L.M.; Carpes, S.T. Bee pollen as a natural antioxidant source to prevent lipid oxidation in black pudding. LWT 2019, 111, 869–875. [Google Scholar] [CrossRef]
- Almeida, J.D.F.; dos Reis, A.S.; Heldt, L.F.S.; Pereira, D.; Bianchin, M.; de Moura, C.; Plata-Oviedo, M.V.; Haminiuk, C.; Ribeiro, I.S.; Luz, C.F.P.; et al. Lyophilized bee pollen extract: A natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT 2017, 76, 299–305. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically active antimicrobial and antioxidant substances in theHelianthus annuusL. bee pollen. J. Environ. Sci. Health Part B 2016, 51, 176–181. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- El Ghouizi, A.; El Menyiy, N.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical composition, antioxidant activity, and diuretic effect of Moroccan fresh bee pollen in rats. Veter. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Rebiai, A.; Lane, T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from Northwest Algeria. J. Fundam. Appl. Sci. 2012, 4, 26–35. [Google Scholar] [CrossRef]
- Araújo, J.S.; Chambó, E.D.; Costa, M.A.P.D.C.; Da Silva, S.M.P.C.; De Carvalho, C.A.L.; Estevinho, L.M. Chemical Composition and Biological Activities of Mono- and Heterofloral Bee Pollen of Different Geographical Origins. Int. J. Mol. Sci. 2017, 18, 921. [Google Scholar] [CrossRef]
- Bárbara, M.S.; Machado, C.S.; Sodré, G.D.S.; Dias, L.G.; Estevinho, L.M.; De Carvalho, C.A.L. Microbiological Assessment, Nutritional Characterization and Phenolic Compounds of Bee Pollen from Mellipona mandacaia Smith, 1983. Molecules 2015, 20, 12525–12544. [Google Scholar] [CrossRef]
- Carpes, S.T. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz. J. Food Technol. 2009, 12, 220–229. [Google Scholar] [CrossRef]
- Sartini, S.; Djide, M.N.; Nainu, F. Correlation Phenolic Concentration to Antioxidant and Antibacterial Activities of Several Ethanolic extracts from Indonesia. J. Phys. Conf. Ser. 2019, 1341, 072009. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Babiker, E.E.; Uslu, N.; Ceylan, D.A.; Ghafoor, K.; Özcan, M.M.; Dursun, N.; Ahmed, I.M.; Jamiu, F.G.; et al. Determination of Antioxidant Activity, Phenolic Compound, Mineral Contents and Fatty Acid Compositions of Bee Pollen Grains Collected from Different Locations. J. Apic. Sci. 2019, 63, 69–79. [Google Scholar] [CrossRef]
- Zuluaga-Dominguez, C.M.; Quicazan, M. Effect of Fermentation on Structural Characteristics and Bioactive Compounds of Bee-Pollen Based Food. J. Apic. Sci. 2019, 63, 209–222. [Google Scholar] [CrossRef]
- Duran, A.; Quicazan, M.; Zuluaga-Dominguez, C. Effect of Solar Drying Process on Bioactive Compounds and Antioxidant Activity in Vitro of High Andean Region Bee Pollen. Chem. Eng. Trans. 2019, 75, 91–96. [Google Scholar] [CrossRef]
- Aleksieva, K.I.; Mladenova, R.B.; Solakov, N.Y.; Loginovska, K.K. EPR analysis of free radical components and antioxidant activity of bee pollen before and after gamma-irradiation. J. Radioanal. Nucl. Chem. 2021, 327, 713–719. [Google Scholar] [CrossRef]
- Atsalakis, E.; Chinou, I.; Makropoulou, M.; Karabournioti, S.; Graikou, K. Evaluation of Phenolic Compounds in Cistus creticus Bee Pollen from Greece. Antioxidant and Antimicrobial Properties. Nat. Prod. Commun. 2017, 12, 1813–1816. [Google Scholar] [CrossRef]
- Saral, Ö.; Kiliçarslan, M.; Şahin, H.; Yildiz, O.; Dinçer, B. Evaluation of antioxidant activity of bee products of different bee races in Turkey. Turk. J. Veter. Anim. Sci. 2019, 43, 441–447. [Google Scholar] [CrossRef]
- Al-Salem, H.S.; Al-Yousef, H.M.; Ashour, A.E.; Ahmed, A.F.; Amina, M.; Issa, I.S.; Bhat, R.S. Antioxidant and hepatorenal protective effects of bee pollen fractions against propionic acid-induced autistic feature in rats. Food Sci. Nutr. 2020, 8, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Yıldız, O.; Can, Z.; Saral, O.; Yuluğ, E.; Oztürk, F.; Aliyazıcıoğlu, R.; Canpolat, S.; Kolaylı, S. Hepatoprotective Potential of Chestnut Bee Pollen on Carbon Tetrachloride-Induced Hepatic Damages in Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2018, 54, 335–346. [Google Scholar] [CrossRef]
- Yan, S.; Li, Q.; Xue, X.; Wang, K.; Zhao, L.; Wu, L. Analysis of improved nutritional composition of bee pollen (Brassica campestris L.) after different fermentation treatments. Int. J. Food Sci. Technol. 2019, 54, 2169–2181. [Google Scholar] [CrossRef]
- Rebiai, A.; Lanez, T. A Facile Electrochemical Analysis to Determine Antioxidant Activity of Bee Pollen. Int. Lett. Chem. Phys. Astron. 2013, 14, 31–38. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Muñoz, E.; Velásquez, P.; Rodriguez, K.; Montenegro, G.; Giordano, A. Influence of Brassica campestris and Galega officinalis on Antioxidant Activity of Bee Pollen. Rev. Bras. Farm. 2020, 30, 444–449. [Google Scholar] [CrossRef]
- Yesiltas, B.; Capanoglu, E.; Fıratlıgil-Durmuş, E.; Sunay, A.E.; Samanci, T.; Boyacioglu, D. Investigating the in-vitro bioaccessibility of propolis and pollen using a simulated gastrointestinal digestion System. J. Apic. Res. 2014, 53, 101–108. [Google Scholar] [CrossRef]
- Mejías, E.; Montenegro, G. The Antioxidant Activity of Chilean Honey and Bee Pollen Produced in the Llaima Volcano’s Zones. J. Food Qual. 2012, 35, 315–322. [Google Scholar] [CrossRef]
- Negri, G.; Teixeira, É.W.; Alves, M.L.T.M.F.; Moreti, A.C.D.C.C.; Otsuk, I.P.; Borguini, R.G.; Salatino, A. Hydroxycinnamic Acid Amide Derivatives, Phenolic Compounds and Antioxidant Activities of Extracts of Pollen Samples from Southeast Brazil. J. Agric. Food Chem. 2011, 59, 5516–5522. [Google Scholar] [CrossRef]
- Campos, M.G.; Webby, R.F.; Markham, K.R.; Mitchell, K.A.; Da Cunha, A.P. Age-Induced Diminution of Free Radical Scavenging Capacity in Bee Pollens and the Contribution of Constituent Flavonoids. J. Agric. Food Chem. 2003, 51, 742–745. [Google Scholar] [CrossRef]
- Ulusoy, E.; Kolayli, S. Phenolic Composition and Antioxidant Properties of Anzer Bee Pollen. J. Food Biochem. 2013, 38, 73–82. [Google Scholar] [CrossRef]
- Mohdaly, A.A.; Mahmoud, A.A.; Roby, M.; Smetanska, I.; Ramadan, M.F. Phenolic Extract from Propolis and Bee Pollen: Composition, Antioxidant and Antibacterial Activities. J. Food Biochem. 2015, 39, 538–547. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.D.S.D.; Barth, O.M.; de Almeida-Muradian, L.B. Phenolic profile by HPLC-MS, biological potential, and nutritional value of a promising food: Monofloral bee pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Şahin, S.; Karkar, B. The antioxidant properties of the chestnut bee pollen extract and its preventive action against oxidatively induced damage in DNA bases. J. Food Biochem. 2019, 43, e12888. [Google Scholar] [CrossRef] [PubMed]
- Belina-Aldemita, M.D.; Schreiner, M.; D’Amico, S. Characterization of phenolic compounds and antioxidative potential of pot-pollen produced by stingless bees (Tetragonula biroiFriese) from the Philippines. J. Food Biochem. 2020, 44, e13102. [Google Scholar] [CrossRef]
- Zuluaga-Domínguez, C.; Castro-Mercado, L.; Quicazán, M.C. Effect of enzymatic hydrolysis on structural characteristics and bioactive composition of bee-pollen. J. Food Process. Preserv. 2019, 43, e13983. [Google Scholar] [CrossRef]
- Bujang, J.S.; Zakaria, M.H.; Ramaiya, S.D. Chemical constituents and phytochemical properties of floral maize pollen. PLoS ONE 2021, 16, e0247327. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Katilevičiūtė, A.; Kaškonas, P.; Maruska, A. The impact of solid-state fermentation on bee pollen phenolic compounds and radical scavenging capacity. Chem. Pap. 2018, 72, 2115–2120. [Google Scholar] [CrossRef]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A.A. Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci. Biotechnol. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Castagna, A.; Sgherri, C.; Poli, P.; Serra, A.; Mele, M.; Ranieri, A.; Signorini, F.; Bientinesi, M.; et al. Microwave-Assisted Drying for the Conservation of Honeybee Pollen. Materials 2016, 9, 363. [Google Scholar] [CrossRef]
- Freire, K.R.L.; Lins, A.C.S.; Dórea, M.C.; Santos, F.A.R.; Camara, C.A.; Silva, T.M.S. Palynological Origin, Phenolic Content, and Antioxidant Properties of Honeybee-Collected Pollen from Bahia, Brazil. Molecules 2012, 17, 1652–1664. [Google Scholar] [CrossRef]
- Kim, S.B.; Jo, Y.H.; Liu, Q.; Ahn, J.H.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Optimization of Extraction Condition of Bee Pollen Using Response Surface Methodology: Correlation between Anti-Melanogenesis, Antioxidant Activity, and Phenolic Content. Molecules 2015, 20, 19764–19774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, F.; Jamali, M.A.; Peng, Z. Antioxidant Enzyme Activities and Lipid Oxidation in Rape (Brassica campestris L.) Bee Pollen Added to Salami during Processing. Molecules 2016, 21, 1439. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Castagna, A.; Benelli, G.; Conte, G.; Sgherri, C.; Signorini, F.; Nicolella, C.; Ranieri, A.; Canale, A. Drying Techniques and Storage: Do They Affect the Nutritional Value of Bee-Collected Pollen? Molecules 2020, 25, 4925. [Google Scholar] [CrossRef] [PubMed]
- Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Pizarro, P.N.; Núñez, G.; Montenegro, G.; Bridi, R. Honeybee Pollen Extracts Reduce Oxidative Stress and Steatosis in Hepatic Cells. Molecules 2021, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Asmae, E.G.; Nawal, E.M.; Bakour, M.; Lyoussi, B. Moroccan Monofloral Bee Pollen: Botanical Origin, Physicochemical Characterization, and Antioxidant Activities. J. Food Qual. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, I.; Ortega-Toro, R.; Diaz, C. Influence of microwave- and ultrasound-assisted extraction on bioactive compounds from pollen. Contemp. Eng. Sci. 2018, 11, 1669–1676. [Google Scholar] [CrossRef]
- Velasquez, P.; Rodriguez, K.; Retamal, M.; Giordano, A.; Valenzuela, L.M.; Montenegro, G. Relation between composition, antioxidant and antibacterial activities and botanical origin of multi-floral bee pollen. J. Appl. Bot. Food Qual. 2017, 90, 306–314. [Google Scholar] [CrossRef]
- Carpes, S.; De Alencar, S.; Cabral, I.; Oldoni, T.; Mourão, G.; Haminiuk, C.; Da Luz, C.; Masson, M.L. Polyphenols and palynological origin of bee pollen ofApis melliferaL. from Brazil. Characterization of polyphenols of bee pollen. CyTA J. Food 2013, 11, 150–161. [Google Scholar] [CrossRef]
- Bárbara, M.F.S.; Moreira, M.M.; Machado, C.S.; Chambó, E.D.; Pascoal, A.; De Carvalho, C.A.L.; Sodré, G.D.S.; Delerue-Matos, C.; Estevinho, L.M. Storage methods, phenolic composition, and bioactive properties of Apis mellifera and Trigona spinipes pollen. J. Apic. Res. 2020, 60, 1–9. [Google Scholar] [CrossRef]
- Carpes, S.T.; Begnini, R.; De Alencar, S.M.; Masson, M.L. Study of preparations of bee pollen extracts, antioxidant and antibacterial activity. Ciência Agrotecnol. 2007, 31, 1818–1825. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, J.; Huang, W.; Zhu, L.; Shao, M.; Dordoe, C.; Ahn, Y.-J.; Wang, D.; Zhao, Y.; Xiong, Y.; et al. The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea. BMC Complement. Med. Ther. 2020, 20, 236. [Google Scholar] [CrossRef]
- Paradowska, K.; Zielińska, A.; Kuras, M.; Wawer, I. The composition of bee pollen color fractions evaluated by solid-state 1H and 13C NMR: Their macroelement content and antioxidant properties. J. Apic. Res. 2017, 56, 523–532. [Google Scholar] [CrossRef]
- Yildiz, O.; Karahalil, F.Y.; Can, Z.; Sahin, H.; Kolayli, S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and propolis. J. Enzym. Inhib. Med. Chem. 2014, 29, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Camara, C.; Lins, A.C.; Agra, M.D.F.; Silva, E.M.; Reis, I.T.; Freitas, B. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). An. Acad. Bras. Ciências 2009, 81, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ding, Z.; Lei, Y.; Yufen, B. Polyphenol Content in Chinese Bee Products and Their Antioxidant Activity. Asian J. Chem. 2013, 25, 8019–8021. [Google Scholar] [CrossRef]
- Amalia, E.; Diantini, A.; Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.D.C.T.C.; De Morais, S.M.; Siqueira, S.M.C.; De Menezes, J.E.S.A.; Ramos, D.N.; Machado, L.K.A.; Magalhães, I.L. Phenolic Content and Antioxidant and Antiacetylcholinesterase Properties of Honeys from Different Floral Origins. J. Med. Food 2011, 14, 658–663. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]




| Author | Reference | Country | Botanical Origin | Pre-Extraction | Pulve-Risation | Extraction Method | Solvent | Volume of Solvent | Extraction Tempe-Rature | Mixing/ Power | Extraction Time | No. of Extractions | Post-Extraction | Antioxidant Assays |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daoud, Ibrahim et al., 2019 | [22] | Algeria | Inula viscosa | Stored at 4 °C | None | Maceration | 80% Methanol (Aqueous) | 10 g w/100 mL | N.I. | N.I. | 24 h | 2 | Dried in vacuo | TPC, TFC, β-carotene bleaching |
| Machado De-Melo, Estevinho et al., 2016 | [23] | Brazil | multifloral | Stored at −20 °C, some batches were oven dried at 42 °C, another was vacuum lyophilised | Crushed and sieved through a 0.595 mm sieve | Agitation | 70% Ethanol (Aqueous) | 2 g w/ 25 mL | 70 °C | 105 rpm | 30 min | N.I. | Volume was adjusted to 25 mL | TPC, DPPH, ORAC |
| Kalaycıoğlu, Kaygusuz et al., 2017 | [7] | Turkey | Chestnuts, Buckwheat, Oak, multifloral | N.I. | Ground in mortar and pestle | Maceration | Water | 0.1 g w/5 mL | 80 °C | None | 15 min | N.I. | Filtered using Whatman #41 | TPC, DPPH |
| Alimoglu, Guzelmeric et al., 2021 | [24] | Turkey | multifloral | Stored at 20 °C | None | Agitation and Sonication | 70% Ethanol (Aqueous) | 5 g w/50 mL | 40 °C | 100 rpm, N.I. | 1 h and 15 min | N.I. | Filtered using filter paper, dried in vacuo | TPC, TFC, DPPH, CUPRAC, FRAP |
| Pascoal, Rodrigues et al., 2014 | [25] | Porugal and Spain | multifloral | Stored at 20 °C | None | Maceration | Methanol | 1:2 w/v | RT | None | 72 h | 2 | Filtered using Whatman #4, dried in vacuo, | TPC, TFC, TBARS, DPPH |
| Morais, Moreira et al., 2011 | [26] | Portugal | multifloral | Stored at −20 °C | None | Maceration | Methanol | 1:2 w/v | RT | None | 72 h | 2 | N.I. | TPC, DPPH, β-carotene bleaching |
| Leja, Mareczek et al., 2006 | [27] | Poland | multifloral | Stored at −18 °C | None | Maceration | 80% Methanol (Aqueous) | N.I. | N.I. | None | N.I. | N.I. | N.I. | TPC, TFC, anthocyanidins, phenylpropanoids, DPPH, TAA |
| Mărghitaş, Stanciu et al., 2009 | [28] | Romania | multifloral | N.I. | None | Maceration and Sonication | Methanol | 2 g w/15 mL | RT | None | 1 h and 15 min | 3 | Dried in vacuo | TPC, TFC, DPPH, FRAP, ABTS |
| Thakur and Nanda 2021 | [29] | India | Coconut, Coriander, Rapeseed, and multifloral | Stored at −18 °C | Ground (method not indicated) | Maceration and Sonication | 85% Methanol (Aqueous) | 0.15:1 | RT | None | 2 h and 30 min | N.I. | Centrifuged, and Dried in vacuo, | TPC, TFC, DPPH, FRAP, ABTS, MCA, |
| Machado De-Melo, Estevinho et al., 2018 | [30] | Brazil | Mimosa caesalpiniifolia, Eucalyptus spp., Rubiaceae, Astrocaryum aculeatissimum, Fabaceae, Cocos nucifera, M. verrucosa, Myrcia spp., Alternanthera spp., Asteraceae, Brassica spp., and multifloral | Stored at −4 °C | None | Maceration | Methanol | 1:2 w/v | RT | None | 72 h | 2 | N.I. | TPC, TFC, DPPH, ORAC |
| Machado De-Melo, Estevinho et al., 2018 | [30] | Brazil | Mimosa caesalpiniifolia, Eucalyptus spp., Rubiaceae, Astrocaryum aculeatissimum, Fabaceae, Cocos nucifera, M. verrucosa, Myrcia spp., Alternanthera spp., Asteraceae, Brassica spp. and multifloral | Stored at −4 °C | None | Agitation | 70% Ethanol (Aqueous) | 2 g/25 mL | 70 °C | 105 rpm | 30 min | N.I. | N.I. | TPC, TFC, DPPH, ORAC |
| Kaškonienė, Adaškevičiūtė et al., 2020 | [31] | Latvia and Lithuania | multifloral | Pasteurisation at 95 °C for 20 min and stored at 6–8 °C | None | Agitation | 80% Methanol (Aqueous) | 2 g/20 mL | RT | 180 rpm | N.I. | N.I. | 7–10 μm paper filter (Labbox), followed by a 0.22 μm polyvinylidene fluoride (PVDF) membrane filter, stored at 4 C. | TPC, TFC, DPPH |
| Wan Omar, Azhar et al., 2016 | [32] | Malaysia | L. terminate | N.I. | None | Sonication | Methanol | 10 g/25 mL | 41 °C | N.I. | 1 h | N.I. | Centrifuged, filtered using 0.2 mm filter | DPPH |
| Khongkarat, Ramadhan et al., 2020 | [33] | Thailand | Sunflower (Helianthus annuus L.) | Dried at 40 °C and stored at 25 °C | None | Agitation | Methanol | 140 g/800 mL | 15 °C | 100 rpm | 18 h | N.I. | Centrifuged and dried in vacuo | DPPH |
| Zhang, Wang et al., 2015 | [34] | China | Rape (Brassica campestris L.) | Vacuum dried at 50 °C and stored at −18 °C | Ground (method not indicated) | Maceration | Water | 1:20, w/v | RT | None | 24 h | N.I. | Centrifuged, reconstituted to 100 mL | TPC, TFC, ABTS, DPPH, Reducing power |
| Zhang, Wang et al., 2015 | [34] | China | Rape (Brassica campestris L.) | Rape (Brassica campestris L.) | Ground (method not indicated) | Maceration | 25% Ethanol (Aqueous) | 1:20, w/v | RT | None | 24 h | N.I. | Centrifuged, reconstituted to 100 mL | TPC, TFC, ABTS, DPPH, Reducing power |
| Zhang, Wang et al., 2015 | [34] | China | Rape (Brassica campestris L.) | Vacuum dried at 50 °C and stored at −18 °C | Ground (method not indicated) | Maceration | 50% Ethanol (Aqueous) | 1:20, w/v | RT | None | 24 h | N.I. | Centrifuged, reconstituted to 100 mL | TPC, TFC, ABTS, DPPH, Reducing power |
| Zhang, Wang et al., 2015 | [34] | China | Rape (Brassica campestris L.) | Vacuum dried at 50 °C and stored at −18 °C | Ground (method not indicated) | Maceration | 75% Ethanol (Aqueous) | 1:20, w/v | RT | None | 24 h | N.I. | Centrifuged, reconstituted to 100 mL | TPC, TFC, ABTS, DPPH, Reducing power |
| Zhang, Wang et al., 2015 | [34] | China | Rape (Brassica campestris L.) | Vacuum dried at 50 °C and stored at −18 °C | Ground (method not indicated) | Maceration | Ethanol | 1:20, w/v | RT | None | 24 h | N.I. | Centrifuged, reconstituted to 100 mL | TPC, TFC, ABTS, DPPH, Reducing power |
| Hemmami, Ben Seghir et al., 2020 | [35] | Algeria | multifloral | N.I. | Crushed in a commercial blender and homogenised. | Sonication | Methanol | 0.2 g w/2 mL | RT | N.I. | 30 min | N.I. | Centrifuged, filtered using Whatman # 1, dried in vacuo, stored in 4 °C in brown bottle | TPC, TFC, TAC |
| Izuta, Narahara et al., 2009 | [36] | Japan and Spain | Jara pringosa (Cistus ladanifer) and Jara blanca (Cistus albidus) [Spain] | N.I. | None | N.I. | 95% Ethanol (Aqueous) | N.I. | RT | N.I. | N.I. | N.I. | N.I. | DPPH |
| Zuluaga-Domínguez, Serrato-Bermudez et al., 2018 | [37] | Colombia | Hypochaeris radicata and Brassica sp. | Stored at 2 °C | None | Maceration | 96% Ethanol (Aqueous) | 1 g w/30 mL | N.I. | N.I. | 24 h | N.I. | Filtered using 3 hw filter paper and reconstituted to 100 mL | TPC, TFC, ABTS, FRAP |
| Keskin and Özkök 2020 | [38] | Turkey | multifloral | Dried at different conditions | None | Agitation | Ethanol | 3 g/20 mL | N.I. | N.I. | 12 h | N.I. | Filtered and the final volume was completed to 30 mL | TPC |
| Vasconcelos, Duarte et al., 2017 | [39] | Brazil | multifloral | N.I. | None | Agitation | 70% Ethanol (Aqueous) | 1 g/10 mL | 70 °C | 150 rpm | 30 min | 2 | Centrifuged | TPC, TFC, DPPH, FRAP |
| Suriyatem, Auras et al., 2017 | [40] | Thailand | Longan | Stored at 20 °C | None | Maceration | Methanol | 1:2 (w/v). | RT | Shaken by hand twice a day | 72 h | 2 | Filtered using Whatman #4 and dried in vacuo | TPC, DPPH, ABTS |
| Cosmulescu, Trandafir et al., 2015 | [41] | Romania | Walnut (Juglans regia L.) | N.I. | None | Sonication and maceration | Methanol | 1 g/10 mL | RT | N.I. | 60 min and 24 h | 1 | Filtered using 0.45 µm membrane filter | TPC, TFC, DPPH |
| Bridi, Atala et al., 2019 | [42] | Chile | Brassica rapa and Eschscholzia californica | Stored at −20 °C | None | Sonication | Ethanol | 1 g/10 mL | −20 °C | N.I. | 10 min | 3 | Centrifuged and filtered using Whatman No.1 and reconstituted to 50 mL | TPC, TFC, FRAP, ORAC |
| Lopes, Vasconcelos et al., 2019 | [43] | Brazil | multifloral | Stored at 4 °C | None | Maceration | 70% Ethanol (Aqueous) | 1:5 (m/v) | RT | None | 72 h (solvent renewal every 24 h) | 3 | Dried in vacuo | TPC, TFC, FRAP, DPPH, ABTS |
| Lopes, Vasconcelos et al., 2020 | [44] | Brazil | multifloral | Stored at 4 °C | None | Maceration | 70% Ethanol (Aqueous) | 1: 5 (m/v) | RT | None | 72 h (solvent renewal every 24 h) | 3 | Dried in vacuo, lyophilised | TPC, TFC, FRAP, DPPH, ABTS |
| Futui and Thongwai 2020 | [45] | Thailand | multifloral | Stored at −20 °C | Powdered (method not indicated) | Maceration | Water | 1:10 | 45 °C | None | 3 h | 3 | Filtered, dried in vacuo, lyophilised | TPC, TFC, DPPH |
| Futui and Thongwai 2020 | [45] | Thailand | multifloral | Stored at −20 °C | Powdered (method not indicated) | Maceration | 95% Ethanol (Aqueous) | 1:10 | RT | None | 72 h (solvent renewal every 24 h) | 3 | Filtered, dried in vacuo, lyophilised | TPC, TFC, DPPH |
| Futui and Thongwai 2020 | [45] | Thailand | multifloral | Stored at −20 °C | Powdered (method not indicated) | Sonication | Water | 1:10 | RT | N.I. | 30 min | 2 | Filtered, dried in vacuo, lyophilised | TPC, TFC, DPPH |
| Nurdianah, Ahmad Firdaus et al., 2016 | [46] | Malaysia | multifloral | Stored at 4 °C | None | Maceration | Ethanol | 10 g/100 mL | RT | N.I. | 24 h | 1 | Filtered, dried in vacuo, lyophilised, 4 | DPPH |
| Fatrcova-Sramkova, Nozkova et al., 2013 | [47] | Slovak | Poppy (Papaver somniferum L.), Rape (Brassica napus subsp. napus L.), Sunflower (Helianthus annuus L.). | Stored at −18 °C | Homogenised | Maceration | 90% Ethanol (Aqueous) | 5 g/50 mL | 70 °C | N.I. | 30 min | N.I. | DPPH, Reducing Power | |
| Sun, Guo et al., 2017 | [48] | China | Rape | Defatted with hexane | None | Sonication | 70% Methanol (Aqueous) | N.I | RT | N.I | 60 min | N.I. | TPC, TFC, DPPH, FRAP, ABTS | |
| Borycka, Grabek-Lejko et al., 2016 | [10] | Poland | multifloral | N.I. | Ground using mortar and pestle | Agitation | Water | 2 g/15 mL | 70 °C | N.I. | 30 min | Filtered using Whatman #1 and dried in vacuo | TPC, TFC, DPPH, FRAP, ABTS, | |
| Borycka, Grabek-Lejko et al., 2016 | [10] | Poland | multifloral | N.I. | Ground using mortar and pestle | Agitation | 70% Ethanol (Aqueous) | 2 g/15 mL | 70 °C | N.I. | 30 min | Filtered usingWhatman #1 and dried in vacuo | TPC, TFC, DPPH, FRAP, ABTS | |
| Borycka, Grabek-Lejko et al., 2016 | [10] | Poland | multifloral | N.I. | Ground using mortar and pestle | Agitation | 70% Methanol (Aqueous) | 2 g/15 mL | 70 °C | N.I. | 30 min | 1 | Filtered, using Whatman #1 and dried in vacuo | TPC, TFC, DPPH, FRAP, ABTS |
| Su, Yang et al., 2020 | [49] | China | Camellia, Rape, Rose, and Lotus | N.I. | Mechanical pulverisation | Sonication | Methanol | 1:10 | 30 °C | 100 W | 1 h | 1 | Filtered, dried in vacuo | TPC, DPPH, RP, ABTS |
| Uçar, Barlak et al., 2017 | [50] | Turkey | multifloral | N.I. | Ground (method not indicated) | Agitation | DMSO | 5 g w/20 mL | 60 °C | 150 rpm | 24 h | 1 | Centrifuged | TPC, TFC, FRAP, ABTS |
| Uçar, Barlak et al., 2017 | [50] | Turkey | multifloral | N.I. | Ground (method not indicated) | Agitation | Water | 5 g w/20 mL | 60 °C | 150 rpm | 24 h | 1 | Centrifuged | TPC, TFC, FRAP, ABTS |
| Oroian, Ursachi et al., 2020 | [51] | Romania | multifloral | Stored at −20 °C | None | Sonication | 80% Methanol (Aqueous) | 1:10–30 | 35 °C, 50 °C, 65 °C | 100 W. | 10–30 min | 1 | N.I. | TPC, TFC |
| Barbieri, Gabriele et al., 2020 | [52] | Italy | multifloral | Stored at −20 °C | Powdered with mortar and pestle | Agitation | 95% Ethanol (Aqueous) | 50 mg/mL | RT | N.I. | 1 h | N.I. | N.I. | TPC, TFC, FRAP |
| Shen, Geng et al., 2019 | [53] | China | Schisandra chinensis | Dried at 37 °C | Pulverised (method not indicated) | Refluxed | 70% Ethanol (Aqueous) | 1:15 | Boling Point | None | 2 h | N.I. | Centrifuged, dried in vacuo, freeze dried | ABTS, FRAP |
| Saral, Yildiz et al., 2016 | [54] | Turkey | Castanea sativa L. | N.I. | None | Maceration and sonication | Methanol | 5 g/100 mL | RT | N.I. | 24 h, 3 h | N.I. | Filtered, fried in vacuo | TPC, FRAP, DPPH |
| Pérez-Pérez, Vit et al., 2012 | [55] | Venezuela | multifloral | N.I. | Ground in a mortar, frozen | homogenised | Water | 0.1 g/5 mL | 4 °C | N.I. | N.I. | N.I. | Centrifuged | TPC, ABTS |
| Pérez-Pérez, Vit et al., 2012 | [55] | Venezuela | multifloral | N.I. | Ground in a mortar, frozen | homogenised | Methanol | 0.1 g/5 mL | 4 °C | N.I. | N.I. | N.I. | Centrifuged | TPC, ABTS |
| Pérez-Pérez, Vit et al., 2012 | [55] | Venezuela | multifloral | N.I. | Ground in a mortar, frozen | homogenised | 95% Ethanol (Aqueous) | 0.1 g/5 mL | 4 °C | N.I. | N.I. | N.I. | Centrifuged | TPC, ABTS |
| Daudu 2019 | [56] | Nigeria | multifloral | Oven dried at 40 °C | Ground (method not indicated) | Maceration | 50% Ethanol (Aqueous) | 0.5 g/5 mL | N.I. | N.I. | N.I. | N.I. | Filtered | TPC, TFC, NO2, DPPH, TAC, RP, Metal Chelating |
| Daudu 2019 | [56] | Nigeria | multifloral | Oven dried at 40 °C | Ground (method not indicated) | Maceration | Methanol | 0.5 g/5 mL | N.I. | N.I. | N.I. | N.I. | Filtered | TPC, TFC, NO2, DPPH, TAC, RP, Metal Chelating |
| Daudu 2019 | [56] | Nigeria | multifloral | Oven dried at 40 °C | Ground (method not indicated) | Maceration | Water | 0.5 g/5 mL | N.I. | N.I. | N.I. | N.I. | Filtered | TPC, TFC, NO2, DPPH, TAC, RP, Metal Chelating |
| Daudu 2019 | [56] | Nigeria | multifloral | Oven dried at 40 °C | Ground (method not indicated) | Maceration | Ethanol | 0.5 g/5 mL | N.I. | N.I. | N.I. | N.I. | Filtered | TPC, TFC, NO2, DPPH, TAC, RP, Metal Chelating |
| Stanciu, Dezmirean et al., 2016 | [57] | Romania | multifloral | Stored at −18 °C | Ground (method not indicated) | N.I. | Hexane:dichloromethane 1:1 | N.I. | N.I. | N.I. | 1 h | N.I. | N.I. | TPC, Total carotenoid, ORAC |
| Stanciu, Dezmirean et al., 2016 | [57] | Romania | multifloral | Stored at −18 °C | Ground (method not indicated) | N.I. | acetone:water:acetic acid 70:29.5:0.5 | N.I. | N.I. | N.I. | 1 h | N.I. | N.I. | TPC, Total carotenoid, ORAC |
| Mayda, Özkök et al., 2020 | [58] | Turkey | multifloral | Stored at −18 °C | None | Agitation and sonication | 95% Ethanol (Aqueous) | 1.5 g/10 mL | 40 °C | Vortex Mixer | 60 min | N.I. | Filtered through 0.45 μm filter | TPC, TFC, DPPH, ABTS |
| Anjos, Fernandes et al., 2019 | [59] | Portugal | multifloral | Stored at −18 °C | None | Agitation | 80% Ethanol (Aqueous) | 11 g/200 mL | RT | 4× g | 24 h | N.I. | Centrifuged, dried in vacuo, freeze dried | TPC, TFC, DPPH, RP |
| Almeida, Reis et al., 2017 | [60] | Brazil | multifloral | N.I. | None | Agitation | 80% Ethanol (Aqueous) | 10 g/100 mL | 40 °C | 150 rpm | 60 min | N.I. | Filtered, dried in vacuo, freeze dried | TPC, TFC, ABTS, DPPH, FRAP, Coupled oxidation of b-carotene and linoleic acid assay |
| Fatrcova-Sramkova, Nozkova et al., 2016 | [61] | Slovakia | Helianthus annuus | Stored at 35 °C | Homogenised (method not indicated) | Maceration | 90% Ethanol (Aqueous) | 5 g/50 mL | 70 °C | N.I. | 30 min | N.I. | Stored at 5 °C | TPC, Caroteniods, RP, Flavonoids |
| Mosic, Trifkovic et al., 2019 | [62] | Serbia | multifloral | N.I. | None | Sonication | 70% Methanol (Aqueous) | 1 g/10 mL | N.I. | N.I. | 1 h | N.I. | N.I. | TPC |
| El Ghouizi, Menyiy et al., 2020 | [63] | Morocco | multifloral | N.I. | None | Agitation | 70% Ethanol (Aqueous) | 2 g/15 mL | 70 °C | N.I. | 30 min | N.I | Filtered using Whatman #5 | TPC, TFC, TAC, RP |
| Rebiai and Lane 2012 | [64] | Algeria | Carrot, Rosemary, Eucalyptus, and multifloral | Stored at 4 °C | Homogenised in blender | Soxhlet | Methanol | 5 g w/175 mL | 70 C | None | 2 h | 1 | Dried in vacuo | TPC, TFC, TAC |
| Araujo, Chambo et al., 2017 | [65] | Brazil | Cocos nucifera, Miconia spp., Spondias spp., Myrcia spp., Eucalyptus spp. | N.I. | None | Agitation | Methanol | 1:1 | N.I. | N.I. | 24 h | 3 | Dried in vacuo | TPC, TFC, DPPH, A BTS |
| Barbara, Machado et al., 2015 | [66] | Brazil | multifloral | N.I. | None | Agitation | Methanol | 1:1 | N.I. | N.I. | 24 h | 3 | Dried in vacuo | TPC, TFC |
| Carpes, Mourao et al., 2009 | [67] | Brazil | multifloral | Stored at −12 to −15 °C | Crushed using commercial blender | Maceration | 70% Ethanol (Aqueous) | 2 g/15 mL | 70 °C | None | 30 min | N.I. | Filtered and stored | TPC, TFC, DPPH |
| Sartini, Djide et al., 2019 | [68] | Indonesia | multifloral | N.I. | Coarse powder (method not indicated) | Maceration | 80% Ethanol (Aqueous) | 100 g/1 L | RT | None | 120 h | N.I. | Dried in vacuo, freeze dried | TPC, TFC, DPPH |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | Water | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | Methanol | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | Ethanol | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | Propanol | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | 2-propanol | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | Acetone | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | DMF | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Le Blanc, Davis et al., 2009 | [1] | USA | Mesquite, Yucca, Palm, Terpentine Bush, Mimosa and Chenopod | N.I. | None | Sonication | ACN | 50 mg/mL | 41 °C | None | 90 min | N.I. | N.I. | TPC, Total flavones and flavonol, total flavonones, DPPH, FRAP |
| Ceksteryte, Kurtinaitiene et al., 2016 | [12] | Lithuania | multifloral | Stored at 5–8 °C | None | Agitation | 80% Methanol (Aqueous) | 6 g/10 mL | N.I. | None | 5 min | N.I. | Centrifuged, dried in vacuo, freeze dried | TPC, DPPH, ABTS, ORAC |
| Özcan, Aljuhaimi et al., 2019 | [69] | Brazil | multifloral | Stored at −18 °C | None | Sonication | Methanol | 0.5 g/12 mL | N.I. | None | 10 min | N.I. | Centrifuged, dried in vacuo | TPC, DPPH, Carotenoid, Minerals |
| Zuluaga-Dominguez and Quicazan 2019 | [70] | Colombia | Hypochaeris spp., and Brassica spp. | N.I. | None | Agitation | 96% Ethanol (Aqueous) | 1 g/30 mL | N.I. | N.I. | 24 h | N.I. | Filtered, volume completed quantitatively to 100 mL | TPC, ABTS, FRAP |
| Duran A 2019 | [71] | Colombia | multifloral | N.I. | None. | Agitation | 96% Ethanol (Aqueous) | 1 g/30 mL | N.I. | N.I. | 24 h | N.I. | Filtered, volume completed quantitatively to 100 mL | TPC, TEAC, FRAP |
| Aleksieva, Mladenova et al., 2021 | [72] | Bulgaria | multifloral | Lyophilised | None | Agitation | Ethanol | 0.5 g/7.5 mL | RT | N.I. | 2 h | N.I. | filtered | TPC, TFC, DPPH, TEAC |
| Aleksieva, Mladenova et al., 2021 | [72] | Bulgaria | multifloral | Lyophilised | None | Agitation | Water | 0.5 g/7.5 mL | RT | N.I. | 2 h | N.I. | filtered | TPC, TFC, DPPH, TEAC |
| Atsalakis, Chinou et al., 2017 | [73], | Greece | Cistus creticus | Stored at −20 °C | None | Maceration | Cyclohexane, Dichloromethane, Butanol and Water | 27.5 g/150 mL | N.I. | None | N.I. | N.I. | N.I. | TPC, TFC, DPPH, ABTS |
| Saral, KiliÇArslan et al., 2019 | [74] | Turkey | multifloral | Stored at 4 °C | Blended | Maceration | Methanol | N.I. | RT | None | 24 h | N.I. | Filtered using Whatman filter paper #4 and then stored at 4 °C | TPC, TFC, CUPRAC, FRAP, DPPH |
| Al-Salem, Al-Yousef et al., 2020 | [75] | Saudi Arabia | multifloral | N.I. | None | Maceration | 95% Ethanol (Aqueous) | N.I. | RT | None | 48 h | N.I. | Filtered, dried in vacuo | Catalase (CAT) assay, Vitamin C (ascorbic acid) assay, Glutathione (GSH) assay, Glutathione S-Transferase (GST) activity |
| Gabriele, Parri et al., 2015 | [76] | Italy | multifloral | Stored at −20 °C | None | Agitation | 95% Ethanol (Aqueous) | N.I | RT | N.I. | 1 h | N.I | Filtered, stored at 4°C | TPC, TFC, DPPH, ORAC |
| Yildiz, Can et al., 2013 | [77] | Turkey | multifloral | Dried in 40 °C | Powder (method not indicated) | Sonication | Methanol | 1 g/10 mL | N.I. | None | 3 h | N.I. | Filtered | TPC, TFC, Total Anthocyanins, Total Carotenoids, FRAP, DPPH |
| Rocchetti, Castiglioni et al., 2019 | [78] | Italy | multifloral | Stored in the dark at room temperature | Ground (method not indicated) | Agitation | 70% Methanol (Aqueous) | 0.5 g/5 mL | N.I. | Shaking | 5 min | N.I | Stored at −20 °C | TPC, DPPH, ABTS, ORAC |
| Yan, Li et al., 2019 | [79] | China | Brassica campestris L. | N.I. | Superfine jet pulverisation Combined low-pressure jet-boiling device | Sonication | 80% Acetone (Aqueous) | 2 g/15 mL | N.I. | None | 30 min | N.I. | Freeze dried and stored at −40 °C | TPC |
| Rebiai and Lanez 2013 | [80] | Algeria | multifloral | N.I. | None | Maceration | Methanol | 5 g/50 mL | RT | None | 24 h | 3 | Filtered, refrigerated | TPC, TFC, cyclic voltammetry techniques |
| Cheng, Chen et al., 2019 | [81] | China | multifloral | N.I. | None | Reflux | 75% Ethanol (Aqueous) | 1:10 | 75 °C | None | 2 h | 2 | Centrifuged, dried in vacuo, | DPPH, FRAP |
| Muñoz, Velásquez et al., 2020 | [82] | Chile | Brassica campestris and Galega officinalis | Stored at −18 °C | None | Sonication | Methanol | 1 g/7.5 mL | N.I. | 50 Hz. | 30 min | 1 | Centrifuged, filtered at 0.45 um, refrigerated | TPC, TFC, FRAP |
| Yesiltas, Capanoglu et al., 2015 | [83] | Turkey and Spain | multifloral | Stored at −18 °C | Ground (method not indicated) | Maceration and sonication | Methanol | 2 g/15 mL | RT | N.I. | 3 d, 15 min | 1 | Centrifuged | TPC, TFC, ABTS, FRAP, DPPH, CUPRAC |
| Mejias and Montenegro 2012 | [84] | Chile | multifloral | N.I. | None | Suspension | Water | N.I. | N.I. | None | N.I. | N.I. | N.I. | TPC, DPPH, FRAP |
| Negri, Teixeira et al., 2011 | [85] | Brazil | multifloral | Stored at −18 °C | None | Maceration | 70% Methanol (Aqueous) | 1.0 g/75 mL | RT | N.I. | 45 min | N.I. | Filtered, volume completed quantitatively to 100 mL | TPC, DPPH |
| Campos, Webby et al., 2003 | [86] | New Zealand, Portugal | Salix atrocinera Brot., Ranunculus sardous Crantz, and Ulex europeus L. (Portugal and New Zealand); Eucalyptus globulus Labill., Cistus ladanifer L., Echium plantagineum L., and Erica australis L. (Portugal); and Metrosideros umbellata, Ixerba brexioides, and Knightia excelsa (New Zealand) | N.I. | None | Sonication | 50% Ethanol (Aqueous) | N.I. | N.I. | None | N.I. | N.I. | Centrifuged | DPPH |
| Ulusoy and Kolayli 2014 | [87] | Turkey | multifloral | Stored at 4 °C | None | Sonication | Methanol | N.I. | RT | None | 1 h | 3 | Filtered, dried in vacuo | TPC, FRAP, CUPRAC, DPPH |
| Mohdaly, Mahmoud et al., 2015 | [88] | Egypt | Maize (Zea mays) | Stored at 4 °C | Powdered (method not indicated) | Maceration | Methanol | 10.0 g/100 mL | RT | N.I. | 12 h | 1 | Filtered (Whatman #1), dried in vacuo | DPPH, ABTS |
| De-Melo, Estevinho et al., 2018 | [89] | Brazil | Alternanthera, Anadenanthera, Cocos nucifera, Mimosa caesalpiniaefolia, Myrcia, and Mimosa scabrella | Stored at 4 °C | Crushed using commercial blender | Maceration | 70% Ethanol (Aqueous) | 2 g/15 mL | 70 °C | None | 30 min | 1 | Filtered | TPC, TFC, DPPH |
| De-Melo, Estevinho et al., 2018 | [89] | Brazil | Alternanthera, Anadenanthera, Cocos nucifera, Mimosa caesalpiniaefolia, Myrcia, and Mimosa scabrella | Stored at 4 °C | Crushed using commercial blender | Maceration | Methanol | 2 g/15 mL | 70°C | None | 30 min | 1 | Filtered | TPC, TFC, DPPH |
| Sahin and Karkar 2019 | [90] | Turkey | Chestnut | N.I. | None | Sonication | Ethanol | 3 g/30 mL | 65 °C | None | 30 h | 1 | Filtered | TPC, FRAP, ABTS, CHROMAC |
| Belina-Aldemita, Schreiner et al., 2020 | [91] | Philippines | multifloral | Under argon at −24 °C, defatted with hexane 3 times | Ground (method not indicated) | Macerated | Methanol | 1 g/5 mL | RT | N.I. | 1 h | 3 | Centrifuged, stored at −20 °C | TPC, TFC, and TMAC |
| Belina-Aldemita, Schreiner et al., 2020 | [91] | Philippines | multifloral | Under argon at −24 °C, defatted with hexane 3 times | Ground (method not indicated) | Sonication | Methanol | 1 g/5 mL | RT | N.I. | 1 h | 3 | Centrifuged, stored at −20 °C | TPC, TFC, and TMAC |
| Belina-Aldemita, Schreiner et al., 2020 | [91] | Philippines | multifloral | Under argon at −24 °C, defatted with hexane 3 times | Ground (method not indicated) | Maceration | acidified Methanol solution (methanol and 1 N hydrochloric acid 85:15 v/v). | 1 g/5 mL | 50 °C | N.I. | 30 min | 3 | Centrifuged, stored at −20°C | TPC, TFC, and TMAC |
| Zuluaga-Domínguez, Castro- Mercado et al., 2019 | [92] | Colombia | Hypochaeris spp., and Brassica spp. | Stored at 4 °C | None | Agitation | 96% Ethanol (Aqueous) | 1 g/30 mL | N.I. | Low speed | 24 h | 1 | Filtered using 3 hw filter paper and volume completed quantitatively to 100 mL | TPC, TFC, Total Carotenoids, FRAP, ABTS |
| Bujang, Zakaria et al., 2021 | [93] | Malaysia | Maize (Zea mays L) | Stored at −20 °C | Crushed | Sonication | 70% Ethanol (Aqueous) | 2 g/15 mL | RT | None | 30 min | 1 | Centrifuged, filtered using Whatman #2 | TPC, TFC, DPPH |
| Yang, Zhang et al., 2019 | [16] | China | Rose | N.I. | Ball milled | Sonication | 70% Ethanol (Aqueous) | 50 mg/1 mL | 25 °C | 600 W and a frequency of 20 kHz | 4 h | 1 | Filtered | TFC, DPPH, ORAC, ABTS, In Vivo antioxidant |
| Kaskoniene, Kaskonas et al., 2015 | [94] | Lithuania | multifloral | N.I. | None | Maceration | 85% Methanol (Aqueous) | N.I. | RT | N.I. | 24 h | 3 | Filtered | TPC, TFC, DPPH |
| Kaškonienė, Katilevičiūtė et al., 2018 | [95] | Lithuania | multifloral | N.I. | None | Maceration | 85% Methanol (Aqueous) | N.I. | RT | N.I. | 24 h | 3 | Filtered | TPC, TFC, DPPH |
| Khider, Elbanna et al., 2013 | [96] | Egypt | Maize (Zea mays), clover (Trifolium alexandrinum), and Date palm (Phoenix dactylifera) | Stored at 4 °C | Powdered (method not indicated) | Maceration | Methanol | 50 g/500 mL | RT | None | 12 h | 1 | Filtered using Whatman paper # 5 | DPPH |
| Khider, Elbanna et al., 2013 | [96] | Egypt | Maize (Zea mays), clover (Trifolium alexandrinum), and date palm (Phoenix dactylifera) | Stored at 4 °C | Powdered (method not indicated) | Maceration | Hexane | 50 g/500 mL | RT | None | 12 h | 1 | Filtered using Whatman paper # 5 | DPPH |
| Canale, Benelli et al., 2016 | [97] | Italy | multifloral | Stored at −20 °C | None | Sonication and agitation | 80% Methanol (Aqueous) | 0.5 g/15 mL | N.I. and 4°C | None and N.I. | 30 min and 30 min | 2 | Filtered through 0.45 µm filter | TPC, TFC, Rutin |
| Freire, Lins et al., 2012 | [98] | Brazil. | multifloral | N.I. | None | Sonication | Ethanol | N.I. | N.I. | N.I. | N.I. | N.I. | Filtered, dried in vacuo | TPC, DPPH, ABTS, Fe Chelating |
| Kim, Jo et al., 2015 | [99] | Korea | multifloral | Dried at 40 °C and then stored in a freezer until use | None | Maceration | 80% Methanol (Aqueous) | N.I. | N.I. | N.I. | N.I. | 2 | N.I. | DPPH, TPC |
| Zhang, Yang et al., 2016 | [100] | China | Rapeseed (Brassica campestris L.) | N.I. | Powdered (method not indicated) | Sonication | 80% Ethanol (Aqueous) | 50 mL/g | 80 °C | 40 kHz | 30 min | 1 | Filtered through 0.45 µm | TPC, FRAP |
| Zhang, Liu et al., 2020 | [101] | China | Rapeseed (Brassica campestris L.) | Extracted with petroleum ether to remove the lipids | Powdered (method not indicated) | Sonication | 80% Ethanol (Aqueous) | 50 mL/g | 80 °C | 40 kHz | 30 min | 1 | Dried in vacuo, lyophilised | DPPH, ABTS, FRAP |
| Castagna, Benelli et al., 2020 | [102] | Italy | Chestnut | N.I. | None | Sonication | 80% Methanol (Aqueous) | N.I. | 4 °C | N.I. | 30 min | 1 | Centrifuged and filtered through 0.45 µm filter | TPC, TFC |
| Oyarzun, Andia et al., 2020 | [103] | Chile | multifloral | N.I. | None | Sonication | Ethanol | 1.0 g in 10 mL | RT | 37 kHz frequency and 240 W | 10 min | 3 | Centrifuged and filtered through Whatman # 1 | FRAP, ORAC, TPC, TFC |
| Asmae, Nawal et al., 2021 | [104] | Morocco | multifloral | Stored at −20 °C | None | Maceration | 70% Ethanol (Aqueous) | 1.0 g in 10 mL | RT | N.I. | 1 week | 1 | Filtered through Whatman # 1 | TPC, Flavones and Flavonols Content, TAC, DPPH, RP, ABTS |
| Feas, Vazquez-Tato et al., 2012 | [6] | Portugal | multifloral | N.I. | Ground (method not indicated) | Sonication and maceration | Methanol | 1:2 | RT | N.I. | 30 min and 2 days | 1 | Centrifuged, dried in vacuo | TPC, TFC, DPPH, β-Carotene Bleaching |
| Rodriguez-Gonzalez, Ortega-Toro et al., 2018 | [105] | Colombia | multifloral | N.I. | None | Microwave Assisted Extraction | Ethanol | 1 g/10 or 50 mL | Varies | 1350 W of power and 60 Hz | 6, 12 and 24 s | 1 | Filtered and stored in −20°C | TPC, ABTS, FRAP |
| Rodriguez-Gonzalez, Ortega-Toro et al., 2018 | [105] | Colombia | multifloral | N.I. | None | Sonication | Ethanol | 1 g/10 mL | N.I. | 5 kHz frequency and 250 W | 15 min | 1 | Filtered and stored in −20°C | TPC, ABTS, FRAP |
| Velasquez, Rodriguez et al., 2017 | [106] | Chile | multifloral | N.I. | None | Sonication | Water | 1:1 | N.I. | N.I. | 1 h | 5 | Filtered using Whatman #2 dried in vacuo and the dry extract was reconstituted to 10 mL, filtered (EDLAB CA syringe filter 0.45 µm) and stored at −20 °C. | Total Carotenoid, TPC, FRAP |
| Carpes, de Alencar et al., 2013 | [107] | Brazil | multifloral | N.I. | None | Maceration | 70% Ethanol (Aqueous) | 1:1 | 70 °C | N.I. | 30 min | N.I. | N.I. | TPC, TFC, DPPH, Antioxidant activity by the coupled oxidation of b-carotene and linoleic acid |
| Santa Bárbara, Moreira et al., 2020 | [108] | Brazil | multifloral | Oven dried, freeze dried, fresh | None | Agitation | 70% Ethanol (Aqueous) | 10 g/50 mL | RT | N.I. | 45 min | 1 | Filtered, Dried in vacuo | b-Carotene bleaching assay, FRAP, DPPH, TPC, TFC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 40% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 50% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 60% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 70% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 80% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Carpes, Begnini et al., 2007 | [109] | Brazil | multifloral | N.I. | Milled | Agitation | 90% Ethanol (Aqueous) | 2.0 g/15 mL | 70 °C | N.I. | 30 min | 2 | Stored at 5 ºC | oxidation of -carotene and linoleic acid, TPC |
| Zou, Hu et al., 2020 | [110] | South Korea, China | multifloral | N.I. | None | Maceration | 70% Ethanol (Aqueous) | N.I. | RT | N.I. | 48 h | 3 | Filtered, dried in vacuo | TPC, TFC, DPPH |
| Paradowska, Zielińska et al., 2017 | [111] | Poland | Buckwheat, Oilseeed Rape | N.I. | None | Sonication | 80% Methanol (Aqueous) | 0.2/10 mL | 25 °C | N.I. | N.I. | 1 | Filtered through a sintered glass filter funnel | TPC, TFC, DPPH, FRAP, ORAC |
| Yildiz, Karahalil et al., 2014 | [112] | Turkey | multifloral | N.I. | None | Suspension | Water | N.I. | N.I. | N.I. | N.I. | N.I. | Filtered | TPC, FRAP |
| Silva, Camara et al., 2009 | [113] | Brazil | multifloral | N.I. | None | Sonication | Ethanol | N.I. | N.I. | N.I. | N.I. | N.I. | Filtered, dried in vacuo | DPPH |
| Dai, Ding et al., 2013 | [114] | China | Rape, Rose, Camellia, Herba leonuri and Schizandra | N.I. | None | Sonication | Ethanol | 50 mg/10 mL | N.I. | N.I. | N.I. | N.I. | Filtered, completed to 10 mL | TPC, DPPH |
| Amalia, Diantini et al., 2020 | [115] | Indonesia | multifloral | N.I. | None | Suspension | Water | 1:10 | N.I. | N.I. | N.I. | N.I. | Filtered using Whatman® # 41 paper, freeze-dried | DPPH |
| Factors | Tested Conditions | Abbreviation |
|---|---|---|
| Pulverisation | Crude, non-pulverised | − |
| Pulverised | + | |
| Solvent | 70% Ethanol | E70:30 |
| Ethanol | EtOH | |
| Methanol | MtOH | |
| Water | H2O | |
| Extraction Process | Agitation | A |
| Maceration | M | |
| Reflux | R | |
| Sonication | S |
| Run | Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|---|
| Pulverisation | Solvent | Extraction | TPC | DPPH | FRAP | DPPH/FRAP Ratio | |
| 1 | − | E70:30 | A | 20.86 ± 4.07 | 320.11 ± 27.00 | 342.28 ± 55.57 | 1.07 |
| 2 | + | E70:30 | A | 20.85 ± 2.67 | 331.86 ± 43.20 | 357.36 ± 35.65 | 1.08 |
| 3 | − | E70:30 | M | 19.99 ± 1.08 | 267.92 ± 39.77 | 396.39 ± 14.23 | 1.48 |
| 4 | + | E70:30 | M | 19.42 ± 2.13 | 291.42 ± 56.06 | 349.19 ± 90.21 | 1.20 |
| 5 | − | E70:30 | R | 21.37 ± 1.70 | 309.28 ± 32.62 | 326.54 ± 34.19 | 1.17 |
| 6 | + | E70:30 | R | 20.46 ± 1.33 | 300.81 ± 48.42 | 328.85 ± 40.44 | 1.16 |
| 7 | − | E70:30 | S | 18.80 ± 2.20 | 266.37 ± 56.46 | 326.61 ± 62.16 | 1.23 |
| 8 | + | E70:30 | S | 19.68 ± 2.87 | 296.61 ± 55.53 | 338.49 ± 59.04 | 1.14 |
| 9 | − | EtOH | A | 16.61 ± 2.15 | 298.92 ± 14.55 | 286.19 ± 29.22 | 0.88 |
| 10 | + | EtOH | A | 16.92 ± 3.23 | 297.37 ± 15.99 | 289.85 ± 45.37 | 0.97 |
| 11 | − | EtOH | M | 17.03 ± 2.24 | 244.52 ± 55.49 | 302.95 ± 33.92 | 1.24 |
| 12 | + | EtOH | M | 16.84 ± 1.72 | 236.25 ± 52.65 | 297.27 ± 33.84 | 1.28 |
| 13 | − | EtOH | R | 19.35 ± 1.08 | 285.28 ± 32.63 | 323.98 ± 29.19 | 1.36 |
| 14 | + | EtOH | R | 18.84 ± 2.00 | 262.40 ± 37.24 | 310.51 ± 32.89 | 1.25 |
| 15 | − | EtOH | S | 15.67 ± 2.24 | 231.12 ± 52.88 | 269.77 ± 24.22 | 1.17 |
| 16 | + | EtOH | S | 16.57 ± 2.16 | 251.47 ± 37.37 | 286.61 ± 31.22 | 1.14 |
| 17 | − | H2O | A | 3.50 ± 0.52 | 34.67 ± 10.93 | 45.06 ± 12.36 | 1.30 |
| 18 | + | H2O | A | 3.68 ± 0.18 | 34.22 ± 6.14 | 44.05 ± 12.66 | 1.29 |
| 19 | − | H2O | M | 3.58 ± 0.55 | 20.91 ± 3.80 | 44.42 ± 6.33 | 2.13 |
| 20 | + | H2O | M | 3.34 ± 0.30 | 26.04 ± 10.26 | 43.01 ± 8.44 | 1.65 |
| 21 | − | H2O | R | 5.07 ± 1.45 | 49.21 ± 13.94 | 83.66 ± 23.00 | 2.83 |
| 22 | + | H2O | R | 4.98 ± 1.69 | 49.9 ± 20.24 | 80.89 ± 27.60 | 2.14 |
| 23 | − | H2O | S | 4.26 ± 0.29 | 31.72 ± 10.94 | 55.64 ± 9.42 | 1.75 |
| 24 | + | H2O | S | 4.35 ± 0.49 | 34.55 ± 4.53 | 56.99 ± 6.81 | 1.65 |
| 25 | − | MtOH | A | 19.55 ± 2.22 | 311.92 ± 33.70 | 196.83 ± 43.71 | 0.63 |
| 26 | + | MtOH | A | 19.25 ± 3.26 | 298.15 ± 47.53 | 237.14 ± 18.04 | 0.80 |
| 27 | − | MtOH | M | 19.17 ± 3.29 | 267.20 ± 62.25 | 221.76 ± 66.73 | 0.83 |
| 28 | + | MtOH | M | 18.58 ± 2.90 | 236.85 ± 46.00 | 268.84 ± 38.98 | 1.14 |
| 29 | − | MtOH | R | 21.15 ± 3.20 | 253.10 ± 25.66 | 254.02 ± 30.52 | 1.06 |
| 30 | + | MtOH | R | 20.00 ± 3.41 | 261.09 ± 41.84 | 270.53 ± 14.49 | 1.11 |
| 31 | − | MtOH | S | 17.85 ± 2.21 | 254.80 ± 65.14 | 216.45 ± 26.87 | 0.85 |
| 32 | + | MtOH | S | 17.95 ± 2.21 | 261.78 ± 70.17 | 241.74 ± 19.54 | 0.92 |
| Coefficient Estimate | |||
|---|---|---|---|
| Term | TPC | DPPH | FRAP |
| Intercept | 3.76 | 13.71 | 14.64 |
| A | - | - | 0.1503 |
| B[1] | 0.7323 * | 3.36 * | 3.93 * |
| B[2] | 0.3897 * | 2.26 * | 2.47 * |
| B[3] | −1.74 * | −8.15 * | −7.18 * |
| C[1] | −0.0195 * | 0.9483 * | −0.4800 * |
| C[2] | −0.0691 * | −0.4900 * | 0.0891 * |
| C[3] | 0.1706 * | −0.2780 * | 0.6496 * |
| AB[1] | - | - | −0.2061 |
| AB[2] | - | - | −0.0267 |
| AB[3] | - | - | −0.1826 |
| B[1]C[1] | 0.0937 * | - | 0.6036 * |
| B[2]C[1] | −0.0348 * | - | −0.0579 * |
| B[3]C[1] | −0.1043 * | - | −0.3087 * |
| B[1]C[2] | 0.0177 * | - | 0.6213 * |
| B[2]C[2] | 0.0365 * | - | 0.1917 * |
| B[3]C[2] | −0.0886 * | - | −0.9382 * |
| B[1]C[3] | −0.0886 * | - | −1.14 * |
| B[2]C[3] | 0.0498 * | - | 0.0415 * |
| B[3]C[3] | 0.0529 * | - | 0.9468 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants 2021, 10, 1113. https://doi.org/10.3390/antiox10071113
Lawag IL, Yoo O, Lim LY, Hammer K, Locher C. Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants. 2021; 10(7):1113. https://doi.org/10.3390/antiox10071113
Chicago/Turabian StyleLawag, Ivan Lozada, Okhee Yoo, Lee Yong Lim, Katherine Hammer, and Cornelia Locher. 2021. "Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents" Antioxidants 10, no. 7: 1113. https://doi.org/10.3390/antiox10071113
APA StyleLawag, I. L., Yoo, O., Lim, L. Y., Hammer, K., & Locher, C. (2021). Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants, 10(7), 1113. https://doi.org/10.3390/antiox10071113








