Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress
Abstract
1. Introduction: Male Reproductive Health and Oxidative Stress at Brief
2. Chromium: Biochemistry and Derivates
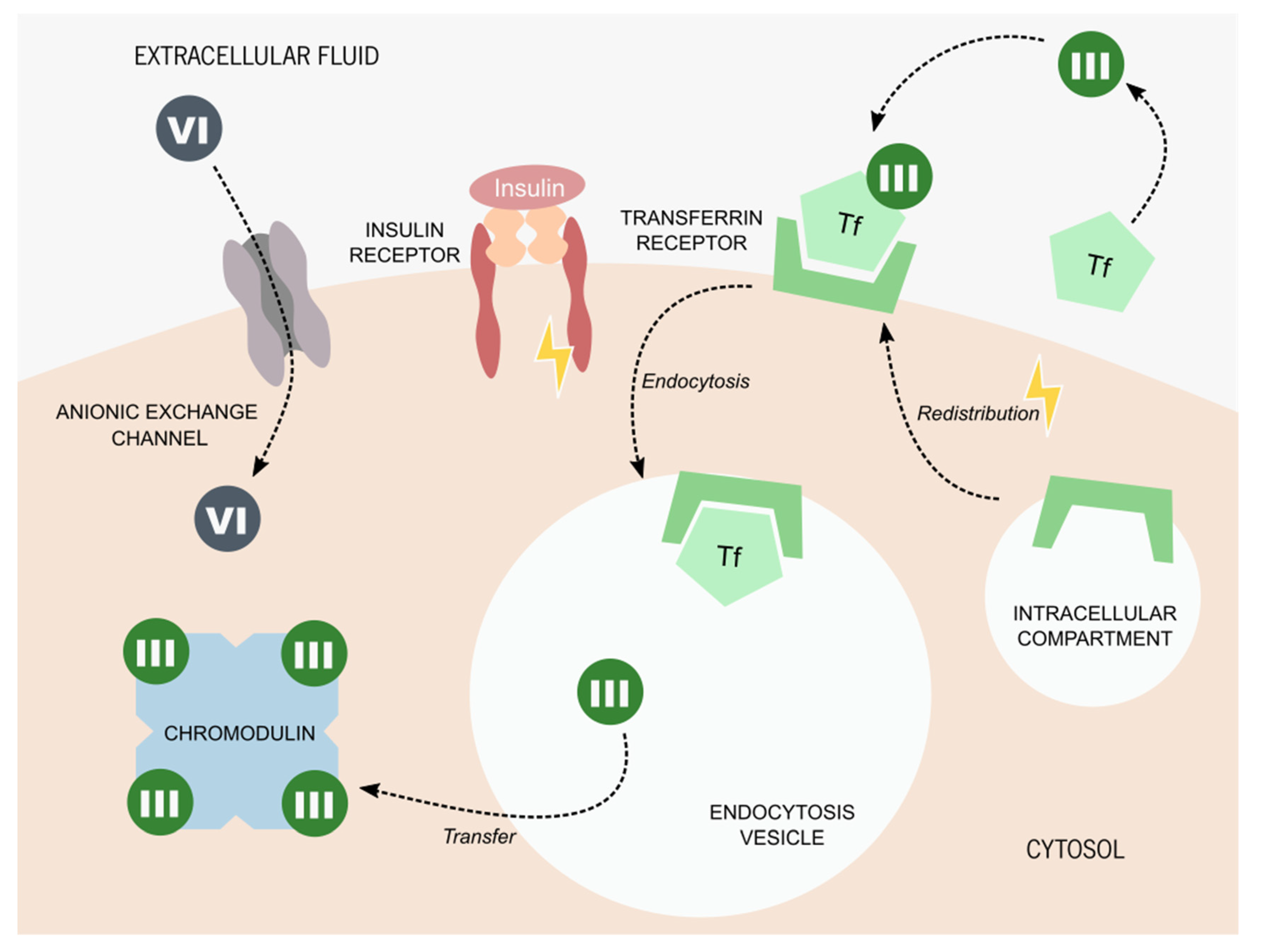
3. Chromium Picolinate Applications and Mechanisms of Action
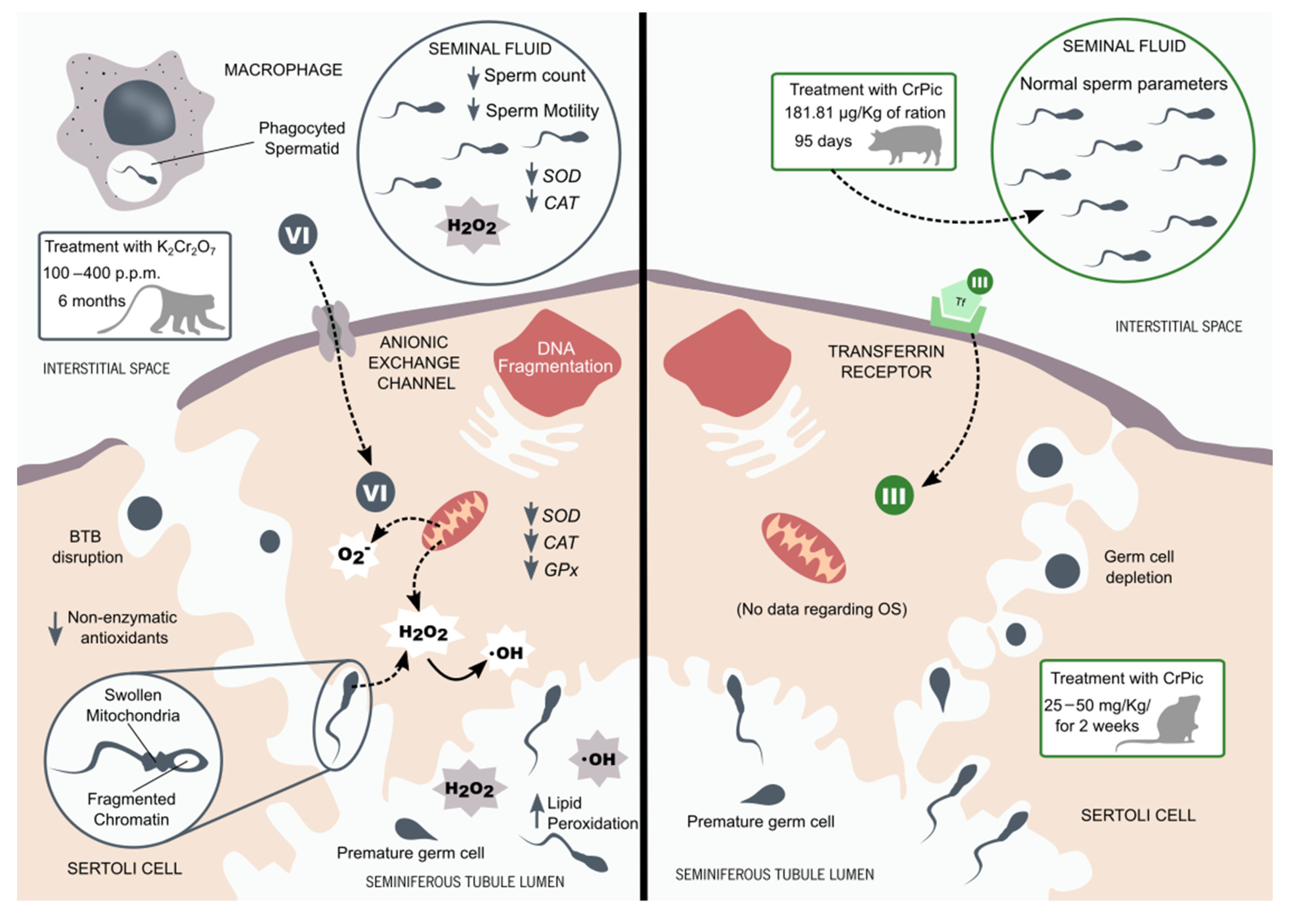
4. Chromium Compounds and Reproductive Health
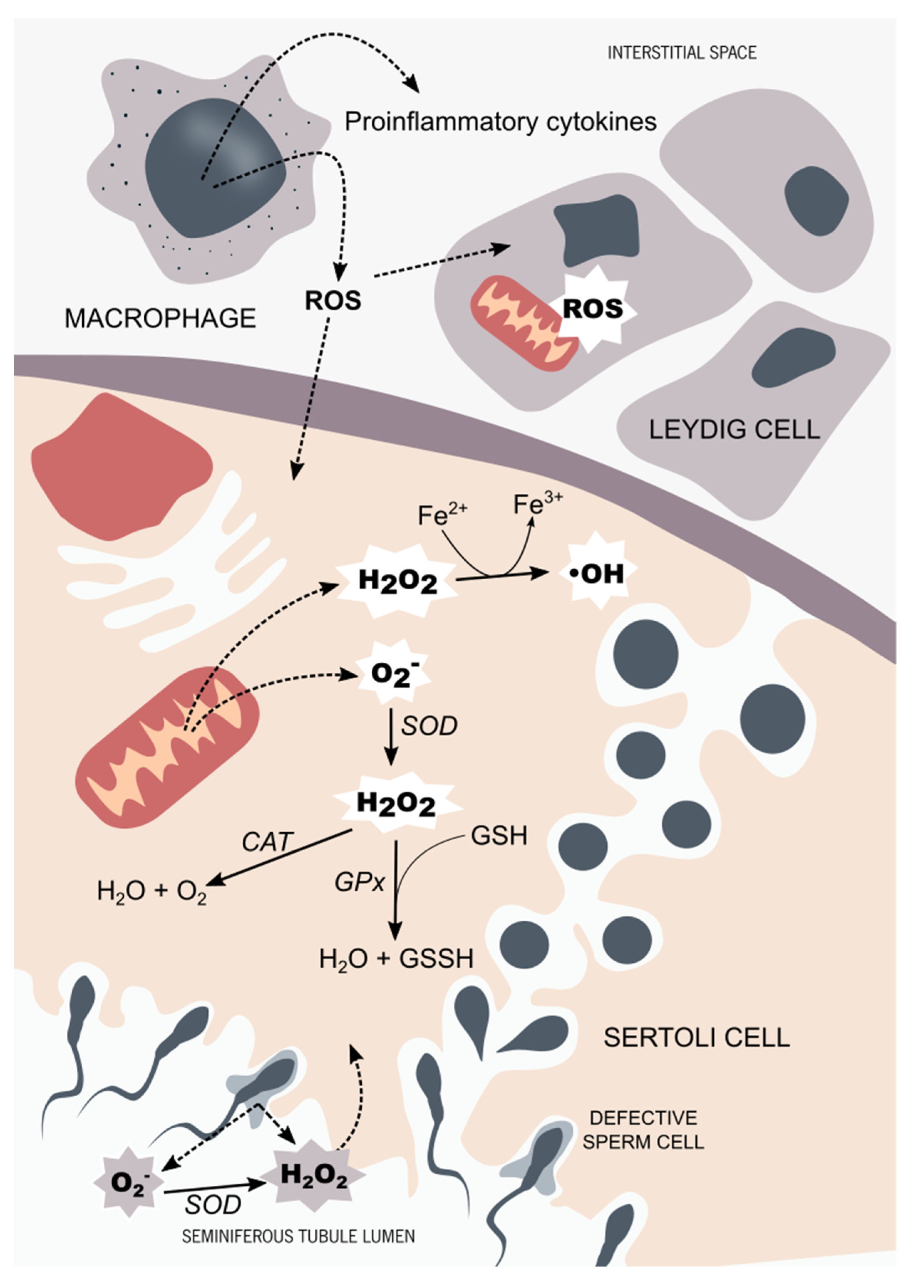
5. Redox Balance in Male Reproductive Tissues and Chromium Compounds
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Saleh, R.A.; Sharma, R.K.; Lewis-Jones, I.; Esfandiari, N.; Thomas, A.J., Jr.; Agarwal, A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004, 81, 349–354. [Google Scholar] [CrossRef]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. JCDR 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Johnson, L.; Zane, R.S.; Petty, C.S.; Neaves, W.B. Quantification of The Human Sertoli Cell Population: Its Distribution, Relation To Germ Cell Numbers, and Age-Related Decline. Biol. Reprod. 1984, 31, 785–795. [Google Scholar] [CrossRef]
- Johnson, L.; Thompson, D.L.; Varner, D.D. Role of Sertoli cell number and function on regulation of spermatogenesis. Anim. Reprod. Sci. 2008, 105, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Print, C.G.; Loveland, K.L. Germ cell suicide: New insights into apoptosis during spermatogenesis. BioEssays 2000, 22, 423–430. [Google Scholar] [CrossRef]
- Ranawat, P.; Bansal, M.P. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: Implications in spermatogenesis. Mol. Cell. Biochem. 2009, 330, 83. [Google Scholar] [CrossRef]
- Stocco, D. The Role of The Star Protein in Steroidogenesis: Challenges for The Future. Eur. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive Oxygen Disrupts Mitochondria in MA-10 Tumor Leydig Cells and Inhibits Steroidogenic Acute Regulatory (StAR) Protein and Steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef]
- Hanukoglu, I. Antioxidant Protective Mechanisms against Reactive Oxygen Species (ROS) Generated by Mitochondrial P450 Systems in Steroidogenic Cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef]
- Beattie, M.C.; Chen, H.; Fan, J.; Papadopoulos, V.; Miller, P.; Zirkin, B.R. Aging and Luteinizing Hormone Effects on Reactive Oxygen Species Production and DNA Damage in Rat Leydig Cells. Biol. Reprod. 2013, 88, 100. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Vincent, A.; Frederic, C. MANAGEMENT OF ENDOCRINE DISEASE: Management of Cushing’s syndrome during pregnancy: Solved and unsolved questions. Eur. J. Endocrinol. 2018, 178, R259–R266. [Google Scholar]
- Vernet, P.; Aitken, R.; Drevet, J. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of Hydrogen Peroxide in Sperm Capacitation and Acrosome Reaction1. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Zarghami, N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 117–121. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction 1987, 81, 459–469. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Holland, M.K.; Storey, B.T. Oxygen metabolism of mammalian spermatozoa. Generation of hydrogen peroxide by rabbit epididymal spermatozoa. Biochem. J. 1981, 198, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tosic, J.; Walton, A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem. J. 1950, 47, 199. [Google Scholar] [CrossRef] [PubMed]
- John Aitken, R.; Clarkson, J.S.; Fishel, S. Generation of Reactive Oxygen Species, Lipid Peroxidation, and Human Sperm Function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. The role of genetics and oxidative stress in the etiology of male infertility—A unifying hypothesis? Front. Endocrinol. 2020, 11, 750. [Google Scholar] [CrossRef]
- Moein, M.R.; Dehghani, V.; Tabibnezhad, N.; Vahidi, S. Reactive Oxygen Species (ROS) level in seminal plasma of infertile men and healthy donors. Iran J. Reprod. Med. 2007, 5, 51–55. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Alshahrani, S.; Durairajanayagam, D.; Sabanegh, E. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod. Biol. Endocrinol. 2014, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef]
- Vatannejad, A.; Tavilani, H.; Sadeghi, M.R.; Amanpour, S.; Shapourizadeh, S.; Doosti, M. Evaluation of ROS-TAC Score and DNA Damage in Fertile Normozoospermic and Infertile Asthenozoospermic Males. Urol. J. 2017, 14, 2973–2978. [Google Scholar] [PubMed]
- Pasqualotto, F.F.; Sharma, R.K.; Pasqualotto, E.B.; Agarwal, A. Poor semen quality and ROS-TAC scores in patients with idiopathic infertility. Urol. Int. 2008, 81, 263–270. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Ni, W.; Huang, Y.; Wang, X.; Zhang, J.; Wu, K. Associations of neonatal lead, cadmium, chromium and nickel co-exposure with DNA oxidative damage in an electronic waste recycling town. Sci. Total Environ. 2014, 472, 354–362. [Google Scholar] [CrossRef]
- Permenter, M.G.; Lewis, J.A.; Jackson, D.A. Exposure to nickel, chromium, or cadmium causes distinct changes in the gene expression patterns of a rat liver derived cell line. PLoS ONE 2011, 6, e27730. [Google Scholar] [CrossRef]
- Azeez, N.A.; Dash, S.S.; Gummadi, S.N.; Deepa, V.S. Nano-remediation of toxic heavy metal contamination: Hexavalent chromium [Cr(VI)]. Chemosphere 2021, 266, 129204. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Chromium: Celebrating 50 years as an essential element? Dalton Trans. 2010, 39, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Shampo, M.A. Nicolas-Louis Vauquelin—discoverer of chromium. Mayo Clin. Proc. 1989, 64, 643. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Nieboer, E. Chromium in the Natural and Human Environments; John Wiley &Sons: Hoboken, NJ, USA, 1988; Volume 20. [Google Scholar]
- Mertz, W.; Schwarz, K. Impaired intravenous glucose tolerance as an early sign of dietary necrotic liver degeneration. Arch. Biochem. Biophys. 1955, 58, 504–506. [Google Scholar] [CrossRef]
- Schwarz, K.; Mertz, W. A glucose tolerance factor and its differentiation from factor 3. Arch. Biochem. Biophys. 1957, 72, 515–518. [Google Scholar] [CrossRef]
- Hwang, D.L.; Lev-Ran, A.; Papoian, T.; Beech, W.K. Insulin-like activity of chromium-binding fractions from brewer’s yeast. J. Inorg. Biochem. 1987, 30, 219–225. [Google Scholar] [CrossRef]
- Simonoff, M.; Shapcott, D.; Alameddine, S.; Sutter-Dub, M.T.; Simonoff, G. The isolation of glucose tolerance factors from brewer’s yeast and their relation to chromium. Biol. Trace Elem. Res. 1992, 32, 25–38. [Google Scholar] [CrossRef]
- The National Academies Collection: Reports funded by National Institutes of Health. In Recommended Dietary Allowances, 10th ed.; National Academies Press: Washington, DC, USA, 1989.
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Alexander, J.; Aaseth, J. Uptake of chromate in human red blood cells and isolated rat liver cells: The role of the anion carrier. Analyst 1995, 120, 931–933. [Google Scholar] [CrossRef]
- Mertz, W.; Roginski, E.E. The Effect of Trivalent Chromium on Galactose Entry in Rat Epididymal Fat Tissue. J. Biol. Chem. 1963, 238, 868–872. [Google Scholar] [CrossRef]
- Mertz, W.; Roginski, E.E.; Schwarz, K. Effect of Trivalent Chromium Complexes on Glucose Uptake by Epididymal Fat Tissue of Rats. J. Biol. Chem. 1961, 236, 318–322. [Google Scholar] [CrossRef]
- Glinsmann, W.H.; Mertz, W. Effect of trivalent chromium on glucose tolerance. Metab. Clin. Exp. 1966, 15, 510–520. [Google Scholar] [CrossRef]
- Hopkins, L.L.J.; Ransome-Kuti, O.; Majaj, A.S. Improvement of Impaired Carbohydrate Metabolism by Chromium(III) in Malnourished Infants. Am. J. Clin. Nutr. 1968, 21, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Gürson, C.T.; Saner, G. Effect of chromium on glucose utilization in marasmic protein-calorie malnutrition. Am. J. Clin. Nutr. 1971, 24, 1313–1319. [Google Scholar] [CrossRef]
- Anderson, R.A.; Polansky, M.M.; Bryden, N.A.; Roginski, E.E.; Mertz, W.; Glinsmann, W. Chromium supplementation of human subjects: Effects on glucose, insulin, and lipid variables. Metabolism 1983, 32, 894–899. [Google Scholar] [CrossRef]
- Evans, G.W.; Pouchnik, D.J. Composition and biological activity of chromium-pyridine carboxylate complexes. J. Inorg. Biochem. 1993, 49, 177–187. [Google Scholar] [CrossRef]
- Evans, G.W.; Bowman, T.D. Chromium picolinate increases membrane fluidity and rate of insulin internalization. J. Inorg. Biochem. 1992, 46, 243–250. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bryden, N.A.; Polansky, M.M.; Gautschi, K. Dietary chromium effects on tissue chromium concentrations and chromium absorption in rats. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 1996, 9, 11–25. [Google Scholar] [CrossRef]
- Harris, D.C. Different metal-binding properties of the two sites of human transferrin. Biochemistry 1977, 16, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L.L.; Schwarz, K. Chromium (III) binding to serum proteins, specifically siderophilin. Biochim. Biophys. Acta (BBA) Gen. Subj. 1964, 90, 484–491. [Google Scholar] [CrossRef]
- Borguet, F.; Cornelis, R.; Lameire, N. Speciation of chromium in plasma and liver tissue of endstage renal failure patients on continuous ambulatory peritoneal dialysis. Biol. Trace Elem. Res. 1990, 26, 449–460. [Google Scholar] [CrossRef]
- Davis, R.J.; Corvera, S.; Czech, M.P. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J. Biol. Chem. 1986, 261, 8708–8711. [Google Scholar] [CrossRef]
- Tanner, L.I.; Lienhard, G.E. Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J. Biol. Chem. 1987, 262, 8975–8980. [Google Scholar] [CrossRef]
- Biswas, S.; Tapryal, N.; Mukherjee, R.; Kumar, R.; Mukhopadhyay, C.K. Insulin promotes iron uptake in human hepatic cell by regulating transferrin receptor-1 transcription mediated by hypoxia inducible factor-1. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 293–301. [Google Scholar] [CrossRef]
- Yokomori, N.; Iwasa, Y.; Aida, K.; Inoue, M.; Tawata, M.; Onaya, T. Transcriptional regulation of ferritin messenger ribonucleic acid levels by insulin in cultured rat glioma cells. Endocrinology 1991, 128, 1474–1480. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Emamaullee, J.; Hepburn, D.D.; Chakov, N.E.; Nettles, H.S.; Vincent, J.B. The trail of chromium(III) in vivo from the blood to the urine: The roles of transferrin and chromodulin. J. Biol. Inorg. Chem. 2001, 6, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Wada, O.; Ono, T. A low-molecular-weight, chromium-binding substance in mammals. Toxicol. Appl. Pharmacol. 1981, 59, 515–523. [Google Scholar] [CrossRef]
- Davis, C.M.; Vincent, J.B. Chromium in carbohydrate and lipid metabolism. JBIC J. Biol. Inorg. Chem. 1997, 2, 675–679. [Google Scholar] [CrossRef]
- Yamamoto, A.; Wada, O.; Ono, T. Distribution and chromium-binding capacity of a low-molecular-weight, chromium-binding substance in mice. J. Inorg. Biochem. 1984, 22, 91–102. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bryden, N.A.; Polansky, M.M.; Reiser, S. Urinary chromium excretion and insulinogenic properties of carbohydrates. Am. J. Clin. Nutr. 1990, 51, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Love, S.T.; Di Bona, K.R.; Sinha, S.H.; McAdory, D.; Skinner, B.R.; Rasco, J.F.; Vincent, J.B. Urinary Chromium Excretion in Response to an Insulin Challenge Is Not a Biomarker for Chromium Status. Biol. Trace Elem. Res. 2013, 152, 57–65. [Google Scholar] [CrossRef]
- Clodfelder, B.J.; Vincent, J.B. The time-dependent transport of chromium in adult rats from the bloodstream to the urine. JBIC J. Biol. Inorg. Chem. 2005, 10, 383–393. [Google Scholar] [CrossRef]
- Anderson, R.A.; Cheng, N.; Bryden, N.A.; Polansky, M.M.; Cheng, N.; Chi, J.; Feng, J. Elevated Intakes of Supplemental Chromium Improve Glucose and Insulin Variables in Individuals With Type 2 Diabetes. Diabetes 1997, 46, 1786. [Google Scholar] [CrossRef]
- Cheng, N.; Zhu, X.; Shi, H.; Wu, W.; Chi, J.; Cheng, J.; Anderson, R.A. Follow-up survey of people in China with type 2 diabetes mellitus consuming supplemental chromium. J. Trace Elem. Exp. Med. 1999, 12, 55–60. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharya, B.; Mukherjee, B.; Manna, B.; Sinha, M.; Chowdhury, J.; Chowdhury, S. Role of chromium supplementation in Indians with type 2 diabetes mellitus. J. Nutr. Biochem. 2002, 13, 690–697. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Domenico, P. Clinical studies on chromium picolinate supplementation in diabetes mellitus—A review. Diabetes Technol. Ther. 2006, 8, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Kaats, G.R.; Blum, K.; Pullin, D.; Keith, S.C.; Wood, R. A randomized, double-masked, placebo-controlled study of the effects of chromium picolinate supplementation on body composition: A replication and extension of a previous study. Curr. Ther. Res. 1998, 59, 379–388. [Google Scholar] [CrossRef]
- Kaats, G.R.; Blum, K.; Fisher, J.A.; Adelman, J.A. Effects of chromium picolinate supplementation on body composition: A randomized, double-masked, placebo-controlled study. Curr. Ther. Res. 1996, 57, 747–756. [Google Scholar] [CrossRef]
- Hasten, D.L.; Rome, E.P.; Franks, B.D.; Hegsted, M. Effects of chromium picolinate on beginning weight training students. Int. J. Sport Nutr. 1992, 2, 343–350. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Siders, W.A.; Penland, J.G. Chromium picolinate supplementation in women: Effects on body weight, composition, and iron status. Nutrition 2007, 23, 187–195. [Google Scholar] [CrossRef]
- Livolsi, J.M.; Adams, G.M.; Laguna, P.L. The effect of chromium picolinate on muscular strength and body composition in women athletes. J. Strength Cond. Res. 2001, 15, 161–166. [Google Scholar]
- Walker, L.S.; Bemben, M.G.; Bemben, D.A.; Knehans, A.W. Chromium picolinate effects on body composition and muscular performance in wrestlers. Med. Sci. Sports Exerc. 1998, 30, 1730–1737. [Google Scholar] [CrossRef]
- Clancy, S.P.; Clarkson, P.M.; DeCheke, M.E.; Nosaka, K.; Freedson, P.S.; Cunningham, J.J.; Valentine, B. Effects of chromium picolinate supplementation on body composition, strength, and urinary chromium loss in football players. Int. J. Sport Nutr. 1994, 4, 142–153. [Google Scholar] [CrossRef]
- Stearns, D.M.; Silveira, S.M.; Wolf, K.K.; Luke, A.M. Chromium (III) tris (picolinate) is mutagenic at the hypoxanthine (guanine) phosphoribosyltransferase locus in Chinese hamster ovary cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2002, 513, 135–142. [Google Scholar] [CrossRef]
- Stearns, D.M.; Wise, J.P., Sr.; Patierno, S.R.; Wetterhahn, K.E. Chromium (III) picolinate produces chromosome damage in Chinese hamster ovary cells 1. FASEB J. 1995, 9, 1643–1649. [Google Scholar] [CrossRef]
- Manygoats, K.R.; Yazzie, M.; Stearns, D.M. Ultrastructural damage in chromium picolinate-treated cells: A TEM study. JBIC J. Biol. Inorg. Chem. 2002, 7, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Stearns, D.M.; Belbruno, J.J.; Wetterhahn, K.E. A prediction of chromium (III) accumulation in humans from chromium dietary supplements. FASEB J. 1995, 9, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Bryden, N.; Polansky, M.M. Lack of toxicity of chromium chloride and chromium picolinate in rats. J. Am. Coll. Nutr. 1997, 16, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003, 33, 213–230. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Aruldhas, M.M.; Subramanian, S.; Sekar, P.; Vengatesh, G.; Chandrahasan, G.; Govindarajulu, P.; Akbarsha, M. Chronic chromium exposure-induced changes in testicular histoarchitecture are associated with oxidative stress: Study in a non-human primate (Macaca radiata Geoffroy). Hum. Reprod. 2005, 20, 2801–2813. [Google Scholar] [CrossRef]
- Aruldhas, M.M.; Subramanian, S.; Sekhar, P.; Vengatesh, G.; Govindarajulu, P.; Akbarsha, M.A. In vivo spermatotoxic effect of chromium as reflected in the epididymal epithelial principal cells, basal cells, and intraepithelial macrophages of a nonhuman primate (Macaca radiata Geoffroy). Fertil. Steril. 2006, 86, 1097–1105. [Google Scholar] [CrossRef]
- Aruldhas, M.M.; Subramanian, S.; Sekhar, P.; Hasan, G.C.; Govindarajulu, P.; Akbarsha, M. Microcanalization in the epididymis to overcome ductal obstruction caused by chronic exposure to chromium–a study in the mature bonnet monkey (Macaca radiata Geoffroy). Reproduction 2004, 128, 127–137. [Google Scholar] [CrossRef]
- Marouani, N.; Tebourbi, O.; Hallègue, D.; Mokni, M.; Yacoubi, M.T.; Sakly, M.; Benkhalifa, M.; Rhouma, K.B. Mechanisms of chromium hexavalent-induced apoptosis in rat testes. Toxicol. Ind. Health 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Zheng, W.; Ge, F.; Wu, K.; Chen, X.; Li, X.; Chen, Y.; Lv, Y.; Lian, Q.; Ge, R.-S. In utero exposure to hexavalent chromium disrupts rat fetal testis development. Toxicol. Lett. 2018, 299, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Q.; Li, S.; Yao, W.; Li, L.; Shi, X.; Wang, L.; Castranova, V.; Vallyathan, V.; Ernst, E. Effect of Cr (VI) exposure on sperm quality: Human and animal studies. Ann. Occup. Hyg. 2001, 45, 505–511. [Google Scholar] [CrossRef]
- Danadevi, K.; Rozati, R.; Reddy, P.; Grover, P. Semen quality of Indian welders occupationally exposed to nickel and chromium. Reprod. Toxicol. 2003, 17, 451–456. [Google Scholar] [CrossRef]
- Yan, L.; Qiaoyan, G.; Mingcai, L.; Mengyang, L.; Xueming, G. Cadmium, chromium, and copper concentration plus semen-quality in environmental pollution site, China. Iran. J. Public Health 2014, 43, 35. [Google Scholar]
- Yang, Y.; Liu, H.; Xiang, X.-H.; Liu, F.-Y. Outline of occupational chromium poisoning in China. Bull. Environ. Contam. Toxicol. 2013, 90, 742–749. [Google Scholar] [CrossRef]
- Hjollund, N.H.I.; Bonde, J.P.E.; Jensen, T.K.; Henriksen, T.B.; Andersson, A.-M.; Kolstad, H.A.; Ernst, E.; Giwercman, A.; Skakkebæk, N.E.; Olsen, J. Male-mediated spontaneous abortion among spouses of stainless steel welders. Scand. J. Work. Environ. Health 2000, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- McAdory, D.; Rhodes, N.R.; Briggins, F.; Bailey, M.M.; Di Bona, K.R.; Goodwin, C.; Vincent, J.B.; Rasco, J.F. Potential of chromium (III) picolinate for reproductive or developmental toxicity following exposure of male CD-1 mice prior to mating. Biol. Trace Elem. Res. 2011, 143, 1666–1672. [Google Scholar] [CrossRef]
- Ferreira, M.; Santos, T.; Pereira, M. Light microscopy studies on mice testis after the nutritional supplement chromium (III)-tris (picolinate). Microsc. Microanal. 2013, 19, 47–48. [Google Scholar] [CrossRef][Green Version]
- Dallago, B.S.L.; Braz, S.; Marçola, T.G.; McManus, C.; Caldeira, D.F.; Campeche, A.; Gomes, E.F.; Paim, T.P.; Borges, B.O.; Louvandini, H. Blood parameters and toxicity of chromium picolinate oral supplementation in lambs. Biol. Trace Elem. Res. 2015, 168, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Horký, P.; Jančíková, P.; Zeman, L. The effect of a supplement of chromium (picolinate) on the level of blood glucose, insulin activity and changes in laboratory evaluation of the ejaculate of breeding boars. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 60, 49–56. [Google Scholar] [CrossRef]
- Shanmugam, M.; Prakash, B.; Panda, A. Effect of dietary organic zinc and chromium supplementation on semen quality in layer breeders. Indian J. Poult. Sci. 2020, 55, 133–138. [Google Scholar] [CrossRef]
- Biswas, A.; Divya, S.; Mandal, A.; Majumdar, S.; Singh, R. Effects of dietary supplementation of organic chromium (picolinate) on physical and biochemical characteristics of semen and carcass traits of male turkeys. Anim. Reprod. Sci. 2014, 151, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Maysa, M.H. Influence of adding organic chromium in diet on productive traits, serum constituents and immune status of Bandarah laying hens and semen physical properties for cocks in winter season. Egypt. Poult. Sci. J. 2011, 31, 203–216. [Google Scholar]
- Subramanian, S.; Rajendiran, G.; Sekhar, P.; Gowri, C.; Govindarajulu, P.; Aruldhas, M.M. Reproductive toxicity of chromium in adult bonnet monkeys (Macaca radiata Geoffrey). Reversible oxidative stress in the semen. Toxicol. Appl. Pharmacol. 2006, 215, 237–249. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Jebur, A.B.; Nasr, H.M.; Hamid, H.M. Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ. Toxicol. 2019, 34, 330–339. [Google Scholar] [CrossRef]
- Chandra, A.K.; Chatterjee, A.; Ghosh, R.; Sarkar, M. Vitamin E-supplementation protect chromium (VI)-induced spermatogenic and steroidogenic disorders in testicular tissues of rats. Food Chem. Toxicol. 2010, 48, 972–979. [Google Scholar] [CrossRef]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Protective role of cactus cladodes extract on sodium dichromate-induced testicular injury and oxidative stress in rats. Biol. Trace Elem. Res. 2014, 159, 304–311. [Google Scholar] [CrossRef]
- Acharya, U.R.; Mishra, M.; Tripathy, R.R.; Mishra, I. Testicular dysfunction and antioxidative defense system of Swiss mice after chromic acid exposure. Reprod. Toxicol. 2006, 22, 87–91. [Google Scholar] [CrossRef]
- Acharya, U.R.; Mishra, M.; Mishra, I.; Tripathy, R.R. Potential role of vitamins in chromium induced spermatogenesis in Swiss mice. Environ. Toxicol. Pharmacol. 2004, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.C.; Oliveira, P.F.; Oliveira, S.R.; Pereira, M.d.L.; Alves, M.G. Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress. Antioxidants 2021, 10, 1365. https://doi.org/10.3390/antiox10091365
Pereira SC, Oliveira PF, Oliveira SR, Pereira MdL, Alves MG. Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress. Antioxidants. 2021; 10(9):1365. https://doi.org/10.3390/antiox10091365
Chicago/Turabian StylePereira, Sara C., Pedro F. Oliveira, Sónia Rodrigues Oliveira, Maria de Lourdes Pereira, and Marco G. Alves. 2021. "Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress" Antioxidants 10, no. 9: 1365. https://doi.org/10.3390/antiox10091365
APA StylePereira, S. C., Oliveira, P. F., Oliveira, S. R., Pereira, M. d. L., & Alves, M. G. (2021). Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress. Antioxidants, 10(9), 1365. https://doi.org/10.3390/antiox10091365









