Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Mineral Components Content
2.3. Preparation of Extracts
2.4. Determinatio of Total Phenolic Content (TPC)
2.5. Evaluation of Total Antioxidant Status (TAS)
- ∆A blank—difference between the initial absorbance and the one measured after 3 min for the blank,
- ∆A standard—difference between initial absorbance and measured absorbance after 3 min for standard
2.6. Cell Culture
2.7. Exposure to UVB Radiation
- without UV radiation, as “control w/o UV”
- “UV 24 h”, radiation used after 24 h incubation with extracts in order to assess the protective effect of extracts,
- “UV 0 h”, radiation used before 24 h incubation with extracts where the repair properties of the test substances were assessed.
2.8. Cytotoxicity Assay
2.9. DNA Biosynthesis
2.10. Cell Cycle Assay
2.11. Expression of Metalloproteinases
2.12. Wound Healing Assay (Scratch Test)
2.13. GC-MS Analysis of Young Barley
2.14. Statistical Analysis
3. Results
3.1. Content of Mineral Components
3.2. Total Phenolic Content (TPC)
3.3. Evaluation of Total Antioxidant Status (TAS)
3.4. Viability of Fibroblasts
3.5. The Influence of EBG and WBJ on DNA Synthesis
3.6. Cell Cycle Assay
3.7. The Influence of EBG and WBJ on MMP Expresion
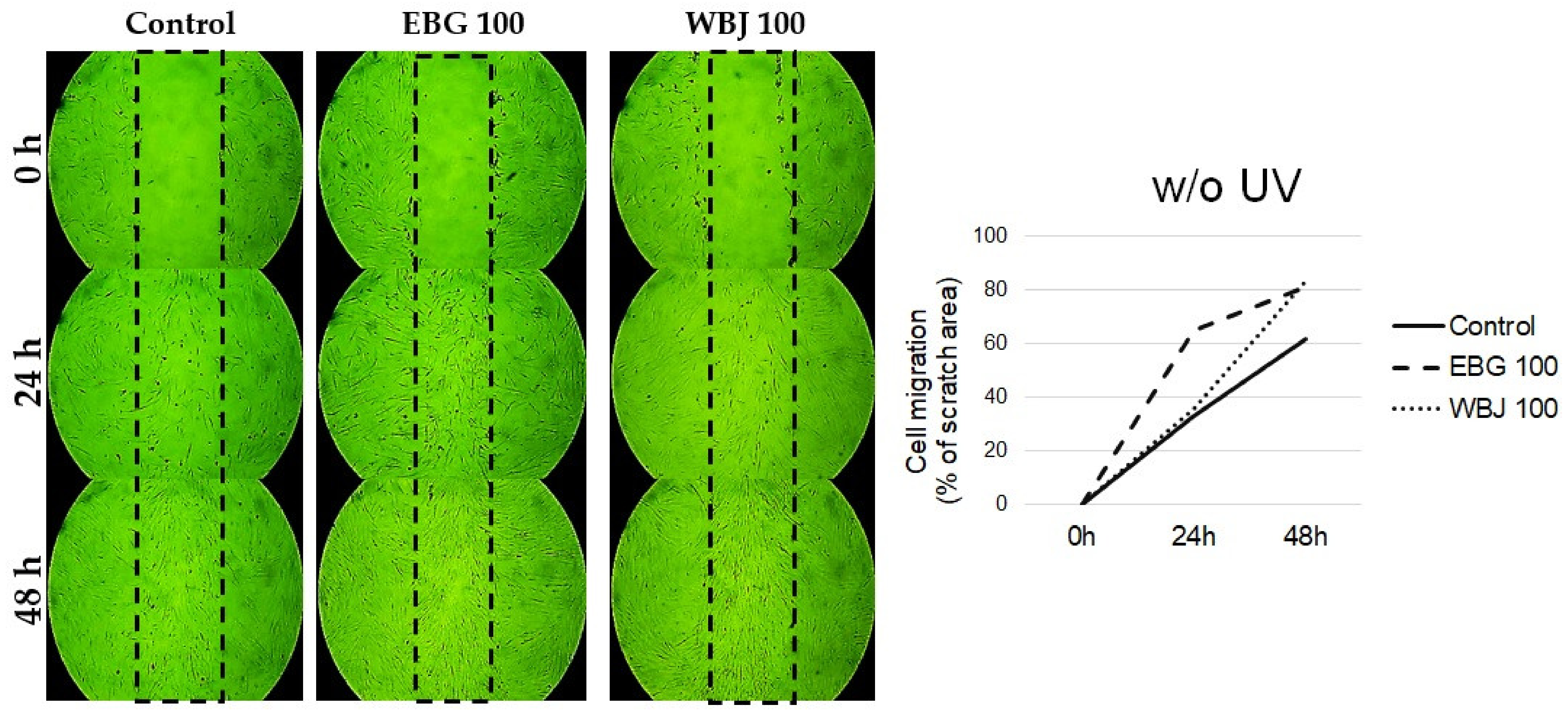
3.8. The Influence of EBG and WBJ on Cell Migration
3.9. Chemical Composition of EBG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Islam, M.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms. Antioxidants 2020, 9, 916. [Google Scholar] [CrossRef]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- De Oliveira, D.N.; Delafiori, J.; Ferreira, M.S.; Catharino, R.R. In vitro evaluation of Sun Protection Factor and stability of commercial sunscreens using mass spectrometry. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 988, 13–19. [Google Scholar] [CrossRef]
- Olsen, C.M.; Wilson, L.F.; Green, A.C.; Biswas, N.; Loyalka, J.; Whiteman, D.C. Prevention of DNA damage in human skin by topical sunscreens. Photodermatol. Photoimmunol. Photomed. 2017, 33, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [Green Version]
- Barone, A.N.; Hayes, C.E.; Kerr, J.J.; Lee, R.C.; Flaherty, D.B. Acute toxicity testing of TiO(2)-based vs. oxybenzone-based sunscreens on clownfish (Amphiprion ocellaris). Environ. Sci. Pollut. Res. Int. 2019, 26, 14513–14520. [Google Scholar] [CrossRef]
- PaulíčkoVá, I.; EhrENbErgEroVá, J.; FIEdlEroVá, V.; Gabrovska, D.; Havlova, P.; Holasova, M.; Kopáček, J.; Ouhrabková, J.; Pinkrová, J.; Rysová, J. Evaluation of barley grass as a potential source of some nutritional substances. Czech. J. Food Sci. 2007, 25, 65. [Google Scholar] [CrossRef] [Green Version]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant activity of flavonoids isolated from young green barley leaves toward biological lipid samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Brezinová Belcredi, N.; Ehrenbergerová, J.; Fiedlerová, V.; Bĕláková, S.; Vaculová, K. Antioxidant vitamins in barley green biomass. J. Agric. Food Chem. 2010, 58, 11755–11761. [Google Scholar] [CrossRef]
- Ehrenbergerová, J.; Brezinová Belcredi, N.; Kopácek, J.; Melisová, L.; Hrstková, P.; Macuchová, S.; Vaculová, K.; Paulícková, I. Antioxidant enzymes in barley green biomass. Plant. Foods Hum. Nutr. 2009, 64, 122–128. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørbaek, R.; Aaboer, D.B.; Bleeg, I.S.; Christensen, B.T.; Kondo, T.; Brandt, K. Flavone C-glycoside, phenolic acid, and nitrogen contents in leaves of barley subject to organic fertilization treatments. J. Agric. Food Chem. 2003, 51, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Norbaek, R.; Brandt, K.; Kondo, T. Identification of flavone C-glycosides including a new flavonoid chromophore from barley leaves (Hordeum vulgare L.) by improved NMR techniques. J. Agric. Food Chem. 2000, 48, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further knowledge on barley (Hordeum vulgare L.) leaves O-glycosyl-C-glycosyl flavones by liquid chromatography-UV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Markham, K.R.; Mitchell, K.A. The mis-identification of the major antioxidant flavonoids in young barley (Hordeum vulgare) leaves. Zeitschrift für Naturforschung C 2003, 58, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.E.; Suk, S.; Kwon, O.W.; Park, G.; Lim, T.G.; Seo, S.G.; Kim, J.R.; Kim, D.E.; Lee, M.; et al. A fermented barley and soybean formula enhances skin hydration. J. Clin. Biochem. Nutr. 2015, 57, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.W.; Venkatesan, R.; Do, M.H.; Ji, E.; Cho, D.W.; Lee, K.W.; Kim, S.Y. Dietary supplementation with a fermented barley and soybean mixture attenuates UVB-induced skin aging and dehydration in hairless mouse skin. Food Sci. Biotechnol. 2015, 24, 705–715. [Google Scholar] [CrossRef]
- Meng, T.X.; Irino, N.; Kondo, R. Melanin biosynthesis inhibitory activity of a compound isolated from young green barley (Hordeum vulgare L.) in B16 melanoma cells. J. Nat. Med. 2015, 69, 427–431. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, J.H.; Kim, M.H.; Lee, J.A.; Kim, Y.B.; Jung, E.; Kim, Y.S.; Lee, J.; Park, D. Hordenine, a single compound produced during barley germination, inhibits melanogenesis in human melanocytes. Food Chem. 2013, 141, 174–181. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004; Text with EEA Relevance; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- European Commission. Commission Regulation (EU) No 488/2014 of 12 May 2014 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Foodstuffs; (Text with EEA Relevance); European Commission: Brussels, Belgium, 2014. [Google Scholar]
- European Commission. Commission Regulation (EU) 2015/1005 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs; (Text with EEA Relevance); European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. Commission Regulation (EU) 2018/73 of 16 January 2018 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Mercury Compounds in or on Certain Products; (Text with EEA Relevance); European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Cho, T.H.; Lee, J.W.; Lee, M.H. Evaluating the cytotoxic doses of narrowband and broadband UVB in human keratinocytes, melanocytes, and fibroblasts. Photodermatol. Photoimmunol. Photomed. 2008, 24, 110–114. [Google Scholar] [CrossRef]
- Cho, S.; Won, C.H.; Kim, S.M.; Lee, S.; Lee, M.J.; Chung, J.H. Drink containing chlorophyll extracts improves signs of photoaging and increases type I procollagen in human skin in vivo. Korean J. Investig. Dermatol. 2006, 13, 111–119. [Google Scholar]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Moskwa, J.; Bielecka, J.; Grabia, M.; Borawska, M.; Socha, K. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother. 2021, 133, 111090. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, H.; Gu, Y.H.; Heo, J.H.; Milner, R.; Del Zoppo, G.J. Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods Mol. Biol. 2012, 814, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.J.; Nowakowski, P.; Strawa, J.W.; Borawska, M.H.; Tomczyk, M.; Socha, K. Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci. Rep. 2020, 10, 21127. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 1–5. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Park, K. Role of micronutrients in skin health and function. Biomol. Ther. 2015, 23, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and Skin Disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell Longev. 2018, 2018, 5895439. [Google Scholar] [CrossRef]
- Van der Pols, J.C.; Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C. Serum antioxidants and skin cancer risk: An 8-year community-based follow-up study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment. Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef]
- Panizo-Casado, M.; Déniz-Expósito, P.; Rodríguez-Galdón, B.; Afonso-Morales, D.; Ríos-Mesa, D.; Díaz-Romero, C.; Rodríguez-Rodríguez, E.M. The chemical composition of barley grain (Hordeum vulgare L.) landraces from the Canary Islands. J. Food Sci. 2020, 85, 1725–1734. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar] [PubMed]
- Matthews, N.H.; Fitch, K.; Li, W.Q.; Morris, J.S.; Christiani, D.C.; Qureshi, A.A.; Cho, E. Exposure to Trace Elements and Risk of Skin Cancer: A Systematic Review of Epidemiologic Studies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, A.; Schwerdtle, T. Interactions by carcinogenic metal compounds with DNA repair processes: Toxicological implications. Toxicol. Lett. 2002, 127, 47–54. [Google Scholar] [CrossRef]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med. Res. 2007, 126, 518–527. [Google Scholar]
- Kasperczyk, A.; Machnik, G.; Dobrakowski, M.; Sypniewski, D.; Birkner, E.; Kasperczyk, S. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 2012, 301, 79–84. [Google Scholar] [CrossRef]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, K. Ocena zawartości kadmu w suplementach diety. Bromatol. Chem. Toksyk. 2011, 44, 351–354. [Google Scholar]
- Socha, K. Ocena zawartości ołowiu w suplementach diety. Bromatol. Chem. Toksyk. 2010, 43, 529–532. [Google Scholar]
- Filipiak-Szok, A.; Kurzawa, M.; Szłyk, E. Determination of toxic metals by ICP-MS in Asiatic and European medicinal plants and dietary supplements. J. Trace Elem. Med. Biol. 2015, 30, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Korfali, S.I.; Hawi, T.; Mroueh, M. Evaluation of heavy metals content in dietary supplements in Lebanon. Chem. Cent. J. 2013, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, J.B.; Maruthi, R.; Wang, S.Q.; Lim, H.W. Sunscreens: An Update. Am. J. Clin. Dermatol. 2017, 18, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Arellano-Mendoza, M.I.; Stengel, F. Current challenges in photoprotection. J. Am. Acad. Dermatol. 2017, 76, S91–S99. [Google Scholar] [CrossRef] [Green Version]
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural Substances for Prevention of Skin Photoaging: Screening Systems in the Development of Sunscreen and Rejuvenation Cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Porównanie składu i właściwości antyoksydacyjnych ekstraktów siewek jęczmienia i pszenicy. Zeszyty Problemowe Postępów Nauk Rolniczych 2018, 593, 63–72. [Google Scholar] [CrossRef]
- Panthi, M.; Subba, R.K.; Raut, B.; Khanal, D.P.; Koirala, N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complement. Med. Ther. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olędzki, R. Znakowanie żywności pod względem wartości antyoksydacyjnej. Nauki Inżynierskie i Technologie 2013, 3, 80–91. [Google Scholar]
- Elif Korcan, S.; Aksoy, O.; Erdoğmuş, S.F.; Ciğerci, İ.; Konuk, M. Evaluation of antibacterial, antioxidant and DNA protective capacity of Chenopodium album’s ethanolic leaf extract. Chemosphere 2013, 90, 374–379. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Rzeski, W. Enhancement of chemopreventive properties of young green barley and chlorella extracts used together against colon cancer cells. Ann. Agric. Environ. Med. 2020, 27, 591–598. [Google Scholar] [CrossRef]
- Czerwonka, A.; Kawka, K.; Cykier, K.; Lemieszek, M.K.; Rzeski, W. Evaluation of anticancer activity of water and juice extracts of young Hordeum vulgare in human cancer cell lines HT-29 and A549. Ann. Agric. Environ. Med. 2017, 24, 345–349. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Lerma, D.; Nyakeriga, A.M.; Ross, J.A.; Kirken, R.A.; Aguilera, R.J.; Varela-Ramirez, A. Searching in mother nature for anti-cancer activity: Anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS ONE 2013, 8, e73508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biszczuk-Jakubowska, J.; Curyło, A. Słoneczne promieniowanie UV a zdrowie człowieka. Prace Instytutu Elektrotechniki 2010, 244, 93–106. [Google Scholar]
- Herman, K.N.; Toffton, S.; McCulloch, S.D. Detrimental effects of UV-B radiation in a xeroderma pigmentosum-variant cell line. Environ. Mol. Mutagen. 2014, 55, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Straface, E.; Vona, R.; Ascione, B.; Matarrese, P.; Strudthoff, T.; Franconi, F.; Malorni, W. Single exposure of human fibroblasts (WI-38) to a sub-cytotoxic dose of UVB induces premature senescence. FEBS Lett. 2007, 581, 4342–4348. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.P.; Bi, B.; Chen, L.; Yang, P.; Guo, Y.; Zhou, Y.Q.; Liu, T.Y. Repeated exposure of mouse dermal fibroblasts at a sub-cytotoxic dose of UVB leads to premature senescence: A robust model of cellular photoaging. J. Dermatol. Sci. 2014, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Greussing, R.; Hackl, M.; Charoentong, P.; Pauck, A.; Monteforte, R.; Cavinato, M.; Hofer, E.; Scheideler, M.; Neuhaus, M.; Micutkova, L.; et al. Identification of microRNA-mRNA functional interactions in UVB-induced senescence of human diploid fibroblasts. BMC Genom. 2013, 14, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of Skin Aging-Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef] [Green Version]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Kitamoto, D.; Habe, H. In vitro evaluation of glyceric acid and its glucosyl derivative, α-glucosylglyceric acid, as cell proliferation inducers and protective solutes. Biosci. Biotechnol. Biochem. 2014, 78, 1183–1186. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Hong, S.; Kim, E.Y.; Sohn, Y.J.; Jung, H.S. dl-Malic acid as a component of α-hydroxy acids: Effect on 2,4-dinitrochlorobenzene-induced inflammation in atopic dermatitis-like skin lesions in vitro and in vivo. Immunopharmacol. Immunotoxicol. 2019, 41, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Yang, J.H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Composite poly-L-lactic acid/poly-(α,β)-DL-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Merinville, E.; Cattley, K.; Al-Bader, T.; Hagforsen, E.; Nilsson, M.; Mavon, A. In vivo topical application of acetyl aspartic acid increases fibrillin-1 and collagen IV deposition leading to a significant improvement of skin firmness. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S1), 41–46. [Google Scholar] [CrossRef] [Green Version]
- Mavon, A. Acetyl aspartic acid, a novel active ingredient, demonstrates potential to improve signs of skin ageing: From consumer need to clinical proof. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S1), 1–2. [Google Scholar] [CrossRef] [Green Version]
- Lévêque, N.; Mac-Mary, S.; Muret, P.; Makki, S.; Aubin, F.; Kantelip, J.P.; Heusèle, C.; Humbert, P. Evaluation of a sunscreen photoprotective effect by ascorbic acid assessment in human dermis using microdialysis and gas chromatography mass spectrometry. Exp. Dermatol. 2005, 14, 176–181. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Kong, C.S.; Seo, Y. Antiphotoaging Effect of 3,5-Dicaffeoyl-epi-quinic Acid against UVA-Induced Skin Damage by Protecting Human Dermal Fibroblasts In Vitro. Int. J. Mol. Sci. 2020, 21, 7756. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Protective effect of 3,5-dicaffeoyl-epi-quinic acid against UVB-induced photoaging in human HaCaT keratinocytes. Mol. Med. Rep. 2019, 20, 763–770. [Google Scholar] [CrossRef]
- Pezza, M.; Carlomagno, V.; Casucci, G. Inositol and acne. Ital. Dermatol. Venereol. 2015, 150, 649–653. [Google Scholar]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Anti-inflammatory effect of 2-methoxy-4-vinylphenol via the suppression of NF-κB and MAPK activation, and acetylation of histone H3. Arch. Pharm. Res. 2011, 34, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Mathews-Roth, M.M. Protective role of butylated hydroxytoluene and certain carotenoids in photocarcinogenesis. Photochem. Photobiol. 1991, 53, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Koone, M.D.; Black, H.S. A mode of action for butylated hydroxytoluene-mediated photoprotection. J. Investig. Dermatol. 1986, 87, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Y.C.; Korivi, M.; Lin, F.Y.; Li, M.L.; Lin, R.W.; Wu, J.J.; Yang, H.L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Bobiński, R.; Wyszomirski, M.; Machnickam, A.; Pielesz, A.; Kawecki, M.; Waksmańska, W.; Staniszewski, L. The Effect of Lauric Acid on Pathogens Colonizing the Burn Wound: A Pilot Study. Altern. Ther. Health Med. 2020, 26, 23–27. [Google Scholar]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial property of lauric acid against Propionibacterium acnes: Its therapeutic potential for inflammatory acne vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, R.K.; Lascu, Z.; Puccetti, G.; Deshpande, A.A.; Paknikar, S.K. Design of a photostabilizer having built-in antioxidant functionality and its utility in obtaining broad-spectrum sunscreen formulations. Photochem. Photobiol. 2006, 82, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Mieremet, A.; Helder, R.; Nadaban, A.; Gooris, G.; Boiten, W.; El Ghalbzouri, A.; Bouwstra, J.A. Contribution of Palmitic Acid to Epidermal Morphogenesis and Lipid Barrier Formation in Human Skin Equivalents. Int. J. Mol. Sci. 2019, 20, 6069. [Google Scholar] [CrossRef] [Green Version]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, B.R.; Kim, J.E.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Lee, H.S.; Lee, C.Y.; Kim, B.H.; et al. Therapeutic Effects of Cold-Pressed Perilla Oil Mainly Consisting of Linolenic acid, Oleic Acid and Linoleic Acid on UV-Induced Photoaging in NHDF Cells and SKH-1 Hairless Mice. Molecules 2020, 25, 989. [Google Scholar] [CrossRef] [Green Version]
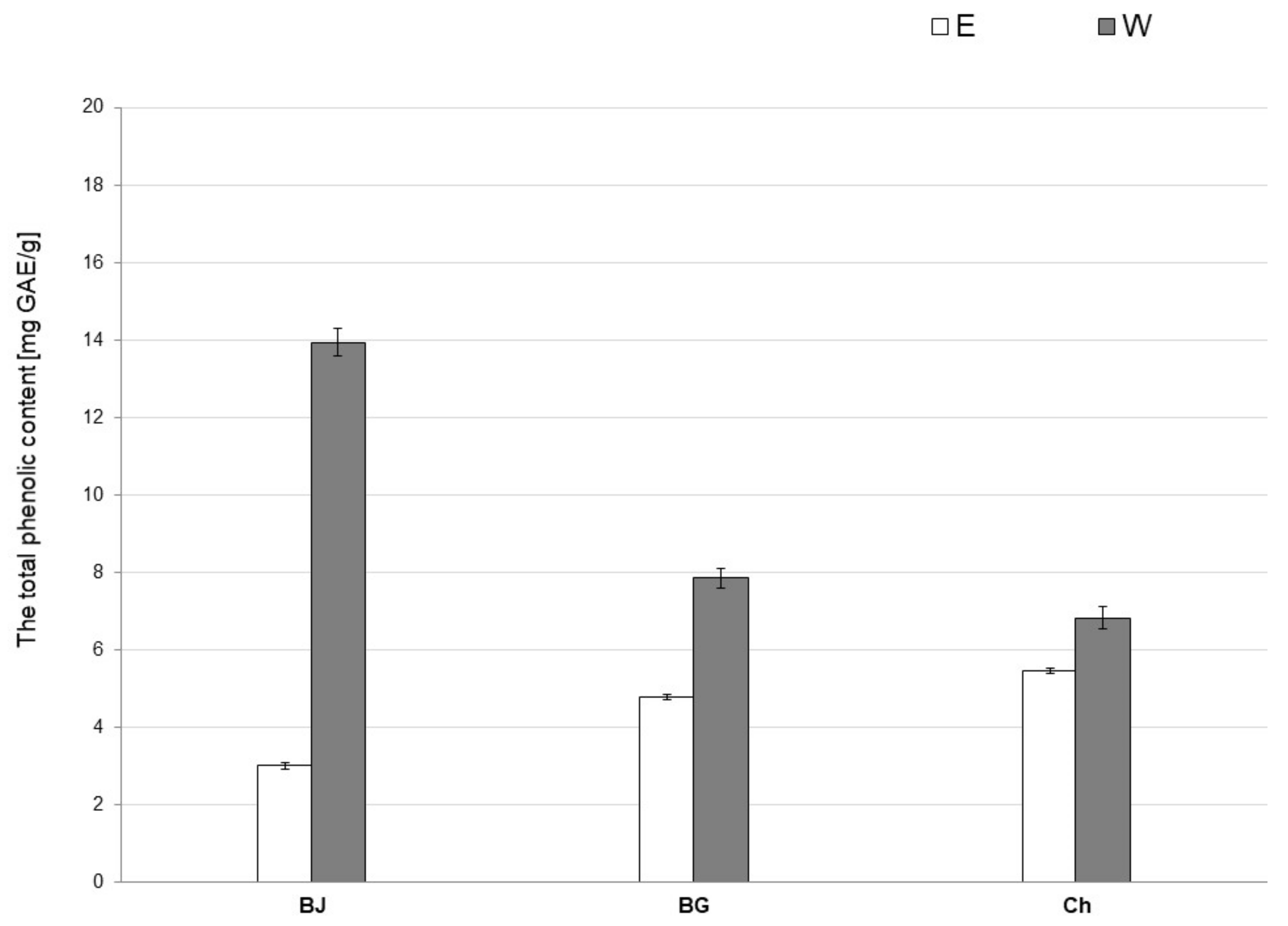













| Extract | Sample Volume (mL) | Sample Weight (g) | Water Weight (g) | Water Content (%) | Crude Extract Weight (g) | Crude Extract Content (%) |
|---|---|---|---|---|---|---|
| WBJ | 1 | 1.00 | 0.92 | 92.0 | 0.08 | 8.0 |
| WBG | 1 | 1.03 | 0.99 | 96.1 | 0.04 | 3.9 |
| WCh | 1 | 1.00 | 0.97 | 97.0 | 0.03 | 3.0 |
| Element (Unit) | Certified Value ±SD | Certified Value Range | Determined Element Content ± SD (n = 6) | Accuracy (%) | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| Zn (mg/kg) | 34.7 ± 2.7 | 32.0–37.4 | 34.8 ± 0.7 | 0.41 | 2.1 |
| Cu (mg/kg) | 20.4 ± 1.5 | 18.9–21.9 | 20.3 ± 0.7 | 0.54 | 3.5 |
| Se (µg/kg) | 76 * | - | 73.1 ± 0.4 | 3.96 | 5.5 |
| Cd (µg/kg) | 30.2 ± 4.0 | 26.2–34.2 | 30.4 ± 0.6 | 0.49 | 1.9 |
| Pb (µg/kg) | 1780 ± 240 | 1540–2020 | 1761 ± 42 | 1.06 | 2.4 |
| Hg (µg/kg) | 4.9 ± 0.7 | 4.2–5.6 | 5.0 ± 0.1 | 0.81 | 2.6 |
| Zn | Cu | Se | ||||
|---|---|---|---|---|---|---|
| Product | Content x ± SD (mg/kg) | % RVI (10 mg) * | Content x ± SD (mg/kg) | % RVI (1 mg) * | Content x ± SD (µg/kg) | % RVI (55 µg) * |
| BJ | 289.24 ± 12.87 | 15 | 14.22 ± 0.34 | 7.1 | 52.95 ± 6.71 | 0.48 |
| BG | 265.45 ± 17.10 | 2.6 | 6.76 ± 0.09 | 0.7 | 28.46 ± 3.72 | 0.05 |
| Ch | 232.59 ± 7.07 | 1.2 | 7722.6 ± 6.36 | 386.1 | 496.45 ± 12.87 | 0.5 |
| Cd | Pb | Hg | ||||
|---|---|---|---|---|---|---|
| Product | Content x ± SD (µg/kg) | Norm (µg/kg) * | Content x ± SD (µg/kg) | Norm (µg/kg) ** | Content x ± SD (µg/kg) | Norm (µg/kg) *** |
| BJ | 26.84 ± 2.84 | 1000 | 39.58 ± 5.41 | 3000 | 9.75 ± 2.19 | 100 |
| BG | 29.49 ± 4.65 | 0.62 ± 0.08 | 7.95 ± 1.05 | |||
| Ch | 52.28 ± 5.33 | 257.24 ± 18.30 | 37.67 ± 4.65 | |||
| No. | Components, TMS Derivative | Rt, min | LTPRI Exp | LTPRI Lit | Relative Composition (%) |
|---|---|---|---|---|---|
| 1 | Phosphoric acid | 8.42 | 1284 | 1285 | 1.62 |
| 2 | Butanedioic acid | 8.89 | 1318 | 1314 | 0.40 |
| 3 | Propanoic acid | 9.20 | 1341 | 1350 | 0.17 |
| 4 | 2-Piperidinecarboxylic acid | 9.62 | 1372 | 1378 | 0.54 |
| 5 | L-threonine | 9.97 | 1399 | 1367 | 0.14 |
| 6 | DL-Malic acid | 11.44 | 1501 | 1390 | 7.73 |
| 7 | L-Aspartic acid | 12.02 | 1530 | 1512 | 0.33 |
| 8 | Rythronic (Tetronic) acid | 12.63 | 1560 | 1595 | 0.13 |
| 9 | Threonic acid | 13.01 | 1580 | 1518 | 0.43 |
| 10 | Glutamine | 14.28 | 1630 | 1612 | 0.10 |
| 11 | β-D-Galactofuranose | 17.89 | 1731 | 1852 | 0.12 |
| 12 | D-glucofuranose | 18.05 | 1756 | 1873 | 0.16 |
| 13 | 2-Keto-l-gluconic acid | 19.44 | 1800 | 2073 | 0.38 |
| 14 | D-Fructose | 20.73 | 1840 | 1867 | 1.93 |
| 15 | d-(-)-Fructose | 21.01 | 1848 | 1805 | 8.26 |
| 16 | L-idofuranuronic acid | 22.15 | 1883 | 2082 | 0.29 |
| 17 | Quinic acid | 22.56 | 1896 | 1904 | 0.64 |
| 18 | Talose | 23.59 | 1929 | 1970 | 3.06 |
| 19 | Mannose | 24.87 | 1968 | 2052 | 0.12 |
| 20 | Myo-Inositol | 26.43 | 2009 | 2153 | 0.20 |
| 21 | Galactopyranose | 26.76 | 2015 | 2037 | 3.46 |
| 22 | Inositol | 29.75 | 2064 | 2194 | 0.36 |
| No. | Components, TMS Derivative | Rt, min | LTPRI Exp | LTPRI Lit | Relative Composition (%) |
|---|---|---|---|---|---|
| 1 | Levulinic acid | 14.69 | 1175 | 1130 | 0.10 |
| 2 | 2,3-dihydrobenzofuran | 21.31 | 1220 | 1224 | 0.23 |
| 3 | 1H-Pyrrole-2,5-dione, 3-ethyl-4-methyl (Maleimide) | 21.85 | 1232 | 1239 | 0.10 |
| 4 | Benzoic acid | 22.48 | 1246 | 1232 | 0.08 |
| 5 | Octanoic (Caprylic) acid | 23;43 | 1267 | 1260 | 0.12 |
| 6 | Benzeneacetic (Phenylacetic) acid | 24.83 | 1298 | 1273 | 0.16 |
| 7 | 2-Methoxy-4-vinylphenol | 25.42 | 1312 | 1317 | 0.52 |
| 8 | Nonanoic (Pelargonic) acid | 27.63 | 1364 | 1358 | 0.05 |
| 9 | 2,5-di-tert-Butyl-1,4-benzoquinone | 32.06 | 1471 | 1466 | 0.43 |
| 10 | Butylated hydroxytoluene | 33.71 | 1512 | 1503 | 2.58 |
| 11 | Dihydroactinidiolide | 34.26 | 1527 | 1532 | 0.89 |
| 12 | Cinnamic acid | 34.97 | 1545 | 1542 | 0.31 |
| 13 | 2′,4′-Dimethoxyacetophenone | 35.78 | 1566 | 1593 | 0.33 |
| 14 | Fumaric acid, 2,4-dimethylpent-3-yl ethyl ester | 36.03 | 1572 | 1528 | 0.34 |
| 15 | 3-Hydroxy-α-ionene | 38.45 | 1638 | 1646 | 0.20 |
| 16 | Dodecanoic acid (Lauric acid) | 39.17 | 1657 | 1651 | 0.10 |
| 17 | 3-Hydroxy-5,6-epoxy-beta-ionone | 40.32 | 1689 | 1642 | 0.36 |
| 18 | 9-hydroxy-beta-ionone | 40.46 | 1693 | 1646 | 0.22 |
| 19 | Carbamic acid | 40.95 | 1705 | 1781 | 0.12 |
| 20 | Isololiolide | 43.15 | 1766 | 1784 | 4.35 |
| 21 | Neophytadiene | 45.56 | 1840 | 1827 | 1.16 |
| 22 | Myristic acid | 46.05 | 1855 | 1850 | 0.33 |
| 23 | N, N (82, 81, 95, 43) | 46.39 | 1864 | - | 0.41 |
| 24 | N, N (82, 68, 95, 124) | 46.98 | 1883 | - | 0.32 |
| 25 | n-Hexadecanoic acid | 49.81 | 1971 | 1968 | 3.49 |
| 26 | Hexadecanal cyclic ethylene acetal | 52.21 | 2048 | 2030 | 0.64 |
| 27 | Palmitic acid | 52.48 | 2057 | 2039 | 5.25 |
| 28 | trans-Phytol | 54.42 | 2122 | 2104 | 1.82 |
| 29 | 9,12,15-Octadecatrienoic acid | 55.84 | 2171 | 2191 | 28.60 |
| 30 | 9,12,15-Octadecatrienoic acid, ethyl ester, | 56.17 | 2183 | 2166 | 0.91 |
| 31 | Phytol | 56.62 | 2198 | 2181 | 1.90 |
| 32 | α-Linolenic acid | 57.82 | 2241 | 2218 | 3.50 |
| 33 | Stearic acid | 58.62 | 2270 | 2236 | 0.35 |
| 34 | Furan-2-carboxamide, 5-benzoyl-N-(2-dimethylaminoethyl) | 65.45 | 2429 | 2388 | 0.02 |
| 35 | 2-Palmitoylglycerol | 66.02 | 2554 | 2519 | 0.15 |
| 36 | Glycerol 1-palmitate | 67.44 | 2511 | 2482 | 4.26 |
| 37 | N, N (44, 43, 55, 73) | 69.96 | 2717 | - | 0.82 |
| 38 | N, N (79, 67, 108, 55) | 75.42 | 2962 | - | 8.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromkowska-Kępka, K.J.; Markiewicz-Żukowska, R.; Nowakowski, P.; Naliwajko, S.K.; Moskwa, J.; Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Mielcarek, K.; Soroczyńska, J.; et al. Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies. Antioxidants 2021, 10, 1402. https://doi.org/10.3390/antiox10091402
Gromkowska-Kępka KJ, Markiewicz-Żukowska R, Nowakowski P, Naliwajko SK, Moskwa J, Puścion-Jakubik A, Bielecka J, Grabia M, Mielcarek K, Soroczyńska J, et al. Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies. Antioxidants. 2021; 10(9):1402. https://doi.org/10.3390/antiox10091402
Chicago/Turabian StyleGromkowska-Kępka, Krystyna Joanna, Renata Markiewicz-Żukowska, Patryk Nowakowski, Sylwia Katarzyna Naliwajko, Justyna Moskwa, Anna Puścion-Jakubik, Joanna Bielecka, Monika Grabia, Konrad Mielcarek, Jolanta Soroczyńska, and et al. 2021. "Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies" Antioxidants 10, no. 9: 1402. https://doi.org/10.3390/antiox10091402
APA StyleGromkowska-Kępka, K. J., Markiewicz-Żukowska, R., Nowakowski, P., Naliwajko, S. K., Moskwa, J., Puścion-Jakubik, A., Bielecka, J., Grabia, M., Mielcarek, K., Soroczyńska, J., & Socha, K. (2021). Chemical Composition and Protective Effect of Young Barley (Hordeum vulgare L.) Dietary Supplements Extracts on UV-Treated Human Skin Fibroblasts in In Vitro Studies. Antioxidants, 10(9), 1402. https://doi.org/10.3390/antiox10091402











