Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice
Abstract
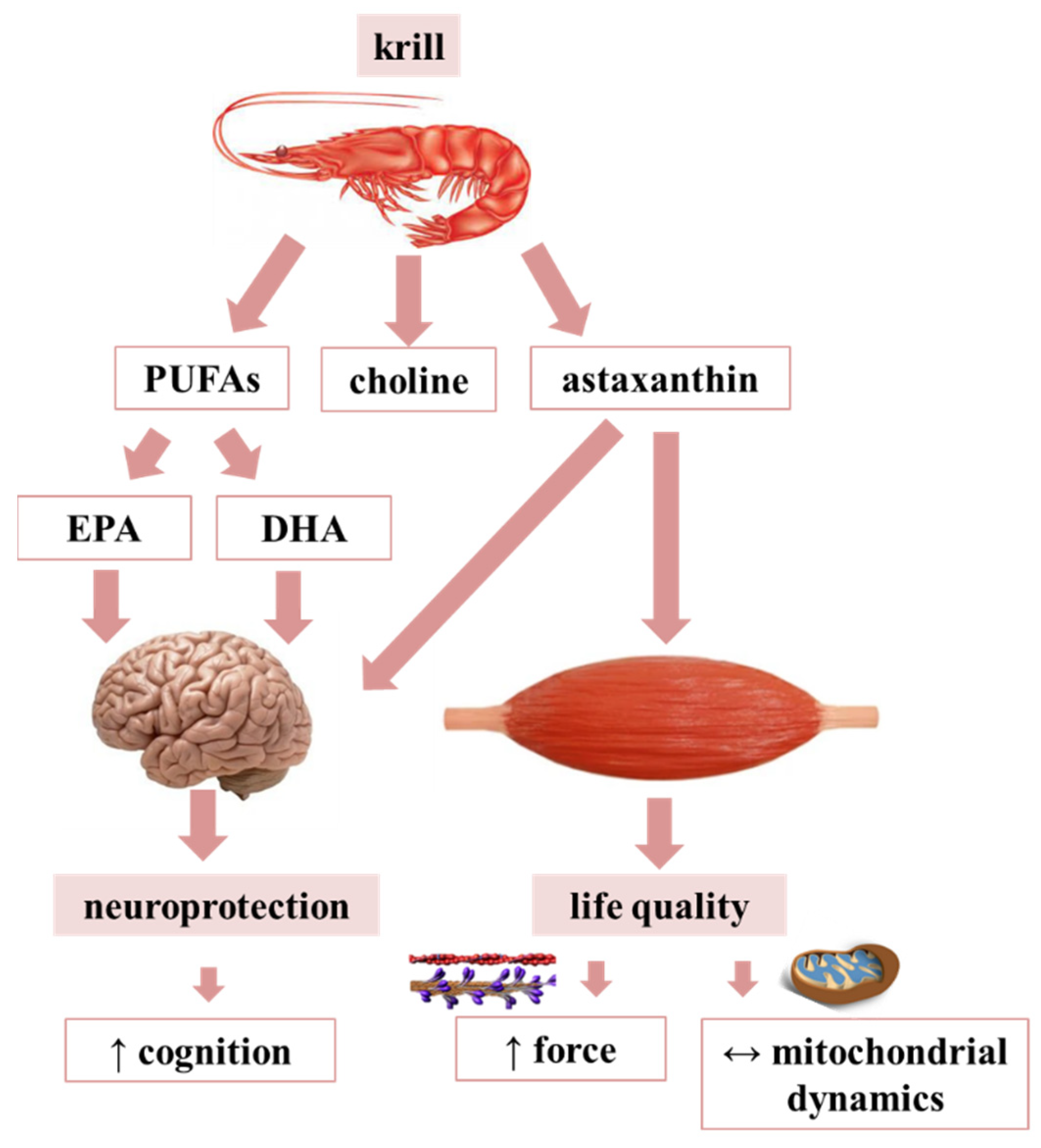
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nutraceutical Diet Regimen
2.3. In Vivo Experiments
2.3.1. Body Weight Measurement
2.3.2. Forepaw Grip Test
2.3.3. Barnes–Maze Protocol
2.3.4. Video Tracking Using Animal Tracker
2.4. In Vitro Experiments
2.4.1. Measurement of EDL Muscle Force
2.4.2. Isolating Single FDB Muscle Fibers
2.4.3. Voltage Clamp
2.4.4. Mitochondrial Calcium Uptake
2.4.5. Confocal Microscopy and Image Analysis
2.5. Molecular Biology
2.5.1. RNA Preparation, Reverse Transcription (RT) and Quantitative Polymerase Chain Reaction (qPCR)
2.5.2. Western Blot
2.6. Chemicals and Solutions
2.7. Statistical Analysis
3. Results
3.1. Increased Grip Force in Nutraceutical Supplemented Aging Mice
3.2. Increased Twitch and Tetanic Force in EDL Muscles from Nutraceutical Treated Mice
3.3. Minor Effects on Excitation-Contraction Coupling Mechanism Following Nutraceutical Administration
3.4. Unaltered SR Calcium Transients and Fluxes by Nutraceutical Diet Regimen
3.5. Preserved Activity Dependent Mitochondrial Calcium Uptake Following Nutraceutical Application
3.6. Variable Effects of Nutraceuticals on Mitochondrial Dynamics
3.7. Enhanced Spatial Learning and Memory in Aging Mice upon Krill Oil Supplementation
4. Discussion
4.1. Nutraceuticals and Skeletal Muscle Performance
4.2. Nutraceuticals and Skeletal Muscle Calcium Handling
4.3. Nutraceuticals and Mitochondrial Function and Antioxidant Features
4.4. Krill Oil and Cognition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and physical frailty: Two sides of the same coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing 2017 Report. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Report.pdf (accessed on 7 July 2021).
- Austad, S.N.; Allison, D.B.; Antoine, L.H.; Ballinger, S.W.; Bamman, M.M.; Biga, P.; Darley-Usmar, V.M.; Fisher, G.; Gohlke, J.M.; Halade, G.V.; et al. Aging and energetics’ “Top 40” future research opportunities 2010–2013. F1000Research 2014, 3, 219. [Google Scholar] [CrossRef] [Green Version]
- Park, D.C.; Yeo, S.G. Aging. Korean J. Audiol. 2013, 17, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Romanello, V.; Sandri, M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell. Mol. Life Sci. 2021, 78, 1305–1328. [Google Scholar] [CrossRef]
- De Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610. [Google Scholar] [CrossRef]
- Ainbinder, A.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 2015, 57, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filadi, R.; Theurey, P.; Pizzo, P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 2017, 62, 1–15. [Google Scholar] [CrossRef]
- Sebastián, D.; Sorianello, E.; Segalés, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Muñoz, J.P.; Sánchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. Calcium signaling around Mitochondria Associated Membranes (MAMs). Cell Commun. Signal. 2011, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perocchi, F.; Gohil, V.M.; Girgis, H.S.; Bao, X.R.; McCombs, J.E.; Palmer, A.E.; Mootha, V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 2010, 467, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Jhun, B.S.; Hurst, S.; Ouchi, J.; Csordás, G.; Sheu, S. The Mitochondrial Ca 2 + Uniporter: Structure, Function and Pharmacology. Handb. Exp. Pharmacol. 2017, 240, 129–156. [Google Scholar]
- Arrabal, S.; Lucena, M.A.; Canduela, M.J.; Ramos-Uriarte, A.; Rivera, P.; Serrano, A.; Pavón, F.J.; Decara, J.; Vargas, A.; Baixeras, E.; et al. Pharmacological blockade of cannabinoid CB1 receptors in diet-induced obesity regulates mitochondrial dihydrolipoamide dehydrogenase in muscle. PLoS ONE 2015, 10, e0145244. [Google Scholar] [CrossRef] [Green Version]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; De Fonseca, F.R.; et al. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- SANDOW, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- Margreth, A.; Damiani, E.; Bortoloso, E. Sarcoplasmic reticulum in aged skeletal muscle. Acta Physiol. Scand. 1999, 167, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; D’Amelio, L.; Fulle, S.; Fanò, G.; Protasi, F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 995–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delbono, O. Chapter 9 Calcium homeostasis and skeletal muscle alterations in aging. Adv. Cell Aging Gerontol. 2002, 10, 167–177. [Google Scholar] [CrossRef]
- Szalait, G.; Csordást, G.; Hantash, B.M.; Thomas, A.P.; Hajnóczky, G. Calcium signal transmission between ryanodine receptors and mitochondria. J. Biol. Chem. 2000, 275, 15305–15313. [Google Scholar] [CrossRef] [Green Version]
- Boncompagni, S.; Pecorai, C.; Michelucci, A.; Pietrangelo, L.; Protasi, F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca2+ Entry Units in Aged Muscle. Front. Physiol. 2021, 11, 1597. [Google Scholar] [CrossRef]
- Del Campo, A.; Jaimovich, E.; Tevy, M.F. Mitochondria in the Aging Muscles of Flies and Mice: New Perspectives for Old Characters. Oxid. Med. Cell. Longev. 2016, 2016, 9057593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrangelo, L.; D’Incecco, A.; Ainbinder, A.; Michelucci, A.; Kern, H.; Dirksen, R.T.; Boncompagni, S.; Protasi, F. Age-dependent uncoupling of mitochondria from Ca2+ release units in skeletal muscle. Oncotarget 2015, 6, 35358–35371. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a Putative Geroprotector: Molecular Basis and Focus on Brain Aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Janet Tomiyama, A.; Milush, J.M.; Lin, J.; Flynn, J.M.; Kapahi, P.; Verdin, E.; Sinclair, E.; Melov, S.; Epel, E.S. Long-term calorie restriction in humans is not associated with indices of delayed immunologic aging: A descriptive study. Nutr. Health Aging 2017, 4, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackman, M.R.; Mu, T.; Bellantoni, M.F.; Busby-whitehead, J.; Stevens, T.E.; Jayme, J.; Connor, K.G.O.; Christmas, C.; Tobin, J.D.; Stewart, K.J.; et al. Growth Hormone and Sex Steroid Administration. JAMA 2002, 288, 2282–2292. [Google Scholar] [CrossRef] [Green Version]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Tsai, Y.T.; Dimarco, N.M.; Long, M.A.; Sun, X.; Tang, L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci. Rep. 2011, 1, 67. [Google Scholar] [CrossRef] [Green Version]
- Fusco, D.; Colloca, G.; Lo Monaco, M.R.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [Green Version]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- Sztretye, M.; Singlár, Z.; Szabó, L.; Angyal, Á.; Balogh, N.; Vakilzadeh, F.; Szentesi, P.; Dienes, B.; Csernoch, L. Improved tetanic force and mitochondrial calcium homeostasis by astaxanthin treatment in mouse skeletal muscle. Antioxidants 2020, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Grimmig, B.; Hudson, C.; Moss, L.; Peters, M.; Subbarayan, M.; Weeber, E.J.; Bickford, P.C. Astaxanthin supplementation modulates cognitive function and synaptic plasticity in young and aged mice. GeroScience 2019, 41, 77–87. [Google Scholar] [CrossRef]
- Watanabe, M.; Takimoto, T.; Akiba, Y.; Takahashi, K. Uptake and distribution of astaxanthin in several tissues and plasma lipoproteins in male broiler chickens fed a yeast (Phaffia rhodozyma) with a high concentration of astaxanthin. Br. Poult. Sci. 2004, 45, 133–138. [Google Scholar] [CrossRef]
- Burri, L.; Johnsen, L. Krill products: An overview of animal studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [Green Version]
- Cheong, L.Z.; Sun, T.; Li, Y.; Zhou, J.; Lu, C.; Li, Y.; Huang, Z.; Su, X. Dietary krill oil enhances neurocognitive functions and modulates proteomic changes in brain tissues of d-galactose induced aging mice. Food Funct. 2017, 8, 2038–2045. [Google Scholar] [CrossRef]
- Li, Q.; Wu, F.; Wen, M.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. The Protective Effect of Antarctic Krill Oil on Cognitive Function by Inhibiting Oxidative Stress in the Brain of Senescence-Accelerated Prone Mouse Strain 8 (SAMP8) Mice. J. Food Sci. 2018, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr. Metab. 2011, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Bodnár, D.; Geyer, N.; Ruzsnavszky, O.; Oláh, T.; Hegyi, B.; Sztretye, M.; Fodor, J.; Dienes, B.; Balogh, Á.; Papp, Z.; et al. Hypermuscular mice with mutation in the myostatin gene display altered calcium signalling. J. Physiol. 2014, 592, 1353–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitts, M. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio-Protocol 2018, 8, 2018. [Google Scholar] [CrossRef] [Green Version]
- Gulyás, M.; Bencsik, N.; Pusztai, S.; Liliom, H.; Schlett, K. AnimalTracker: An ImageJ-Based Tracking API to Create a Customized Behaviour Analyser Program. Neuroinformatics 2016, 14, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Bodnár, D.; Ruzsnavszky, O.; Oláh, T.; Dienes, B.; Balatoni, I.; Ungvári, É.; Benkő, I.; Babka, B.; Prokisch, J.; Csernoch, L.; et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr. Metab. 2016, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Szentesi, P.; Jacquemond, V.; Kovács, L.; Csernoch, L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. J. Physiol. 1997, 505, 371–384. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Gönczi, M.; Sztretye, M.; Dienes, B.; Oláh, T.; Szabó, L.; Csoma, E.; Szentesi, P.; Szigeti, G.P.; Marty, I.; et al. Altered expression of triadin 95 causes parallel changes in localized Ca2+ release events and global Ca2+ signals in skeletal muscle cells in culture. J. Physiol. 2008, 586, 5803–5818. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Oláh, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royer, L.; Pouvreau, S.; Ríos, E. Evolution and modulation of intracellular calcium release during long-lasting, depleting depolarization in mouse muscle. J. Physiol. 2008, 586, 4609–4629. [Google Scholar] [CrossRef]
- Melzer, W.; Rios, E.; Schneider, M.F. Time course of calcium release and removal in skeletal muscle fibers. Biophys. J. 1984, 45, 637–641. [Google Scholar] [CrossRef] [Green Version]
- Melzer, W.; Rios, E.; Schneider, M.F. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys. J. 1987, 51, 849–863. [Google Scholar] [CrossRef] [Green Version]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxidants Redox Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef]
- Evans, W.J. Vitamin E, vitamin C, and exercise. Am. J. Clin. Nutr. 2000, 72, 647S–652S. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Ima-Nirwana, S.; Chin, K. Effects of astaxanthin on the protection of muscle health (Review). Exp. Ther. Med. 2020, 20, 2941–2952. [Google Scholar] [CrossRef]
- Colletti, A.; Cravotto, G.; Citi, V.; Martelli, A.; Testai, L.; Cicero, A.F.G. Advances in technologies for highly active omega-3 fatty acids from krill oil: Clinical applications. Mar. Drugs 2021, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Sharp, M.H.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; Hornberger, T.A.; Harding, F.; Johnson, J.H.; Peele, D.M.; Jäger, R. The Effects of Krill Oil on mTOR Signaling and Resistance Exercise: A Pilot Study. J. Nutr. Metab. 2018, 2018, 7625981. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Rodacki, C.L.N.; Rodacki, A.L.F.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, A.; Dror, E.; Handschin, C.; Furrer, R.; Perez-Schindler, J.; Bachmann, C.; Treves, S.; Zorzato, F. Over-expression of a retinol dehydrogenase (SRP35/DHRS7C) in skeletal muscle activates mTORC2, enhances glucose metabolism and muscle performance. Sci. Rep. 2018, 8, 636. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Li, R.; Heng, N.; Chen, Y.; Wang, L.; Li, Z.; Guo, Y.; Sheng, X.; Wang, X.; Xing, K.; et al. Effects of dietary supplementation of natural astaxanthin from Haematococcus pluvialis on antioxidant capacity, lipid metabolism, and accumulation in the egg yolk of laying hens. Poult. Sci. 2020, 99, 5874–5882. [Google Scholar] [CrossRef]
- Yoshihara, T.; Sugiura, T.; Shibaguchi, T.; Naito, H. Role of astaxanthin supplementation in prevention of disuse muscle atrophy: A review. J. Phys. Fit. Sport. Med. 2019, 8, 61–71. [Google Scholar] [CrossRef]
- Schiaffino, S. Tubular aggregates in skeletal muscle: Just a special type of protein aggregates? Neuromuscul. Disord. 2012, 22, 199–207. [Google Scholar] [CrossRef]
- Jiménez-Moreno, R.; Wang, Z.M.; Gerring, R.C.; Delbono, O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys. J. 2008, 94, 3178–3188. [Google Scholar] [CrossRef] [Green Version]
- Russ, D.W.; Grandy, J.S.; Toma, K.; Ward, C.W. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol. 2011, 201, 391–403. [Google Scholar] [CrossRef]
- González, E.; Messi, M.L.; Zheng, Z.; Delbono, O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J. Physiol. 2003, 552, 833–844. [Google Scholar] [CrossRef]
- Payne, A.M.; Zheng, Z.; González, E.; Wang, Z.M.; Messi, M.L.; Delbono, O. External Ca2+-dependent excitation-contraction coupling in a population of ageing mouse skeletal muscle fibres. J. Physiol. 2004, 560, 137–155. [Google Scholar] [CrossRef]
- Renganathan, M.; Messi, M.L.; Delbono, O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J. Membr. Biol. 1997, 157, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, S.; Pietrangelo, L.; Loefler, S.; Fruhmann, H.; Vogelauer, M.; Burggraf, S.; Pond, A.; Grim-Stieger, M.; Cvecka, J.; Sedliak, M.; et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, A.; Muller, F.L.; Liu, Y.; Ng, R.; Faulkner, J.; Hamilton, M.; Richardson, A.; Huang, T.T.; Epstein, C.J.; Van Remmen, H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech. Ageing Dev. 2006, 127, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Filadi, R.; Greotti, E.; Turacchio, G.; Luini, A.; Pozzan, T.; Pizzo, P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc. Natl. Acad. Sci. USA 2015, 112, E2174–E2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.patentdocs.org/2019/10/aker-biomarine-antarctic-as-v-rimfrost-as-fed-cir-2019.html (accessed on 16 August 2021).
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the neuroprotective role of astaxanthin: New perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, Y.; Komatsu, T.; Seki, S.; Sugawara, T. Dietary astaxanthin can accumulate in the brain of rats. Biosci. Biotechnol. Biochem. 2018, 82, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Müller, M.; Rainger, J.E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging 2016, 8, 1578–1582. [Google Scholar] [CrossRef] [Green Version]
- Spindler, S.R.; Mote, P.L.; Flegal, J.M. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age 2014, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Gamoh, S.; Michio Hashimoto, M.; Yanagimoto, K.; Katakura, M.; Md Abdul, H.; Shido, O. Krill-derived Phospholipids Rich in n-3 Fatty Acid Improve Spatial Memory in Adult Rats. J. Agric. Sci. 2011, 3, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Wibrand, K.; Berge, K.; Messaoudi, M.; Duffaud, A.; Panja, D.; Bramham, C.R.; Burri, L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V.; Griinari, M.; Carta, G.; Murru, E.; Ligresti, A.; Cordeddu, L.; Giordano, E.; Bisogno, T.; Collu, M.; Batetta, B.; et al. Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. Int. Dairy J. 2010, 20, 231–235. [Google Scholar] [CrossRef]

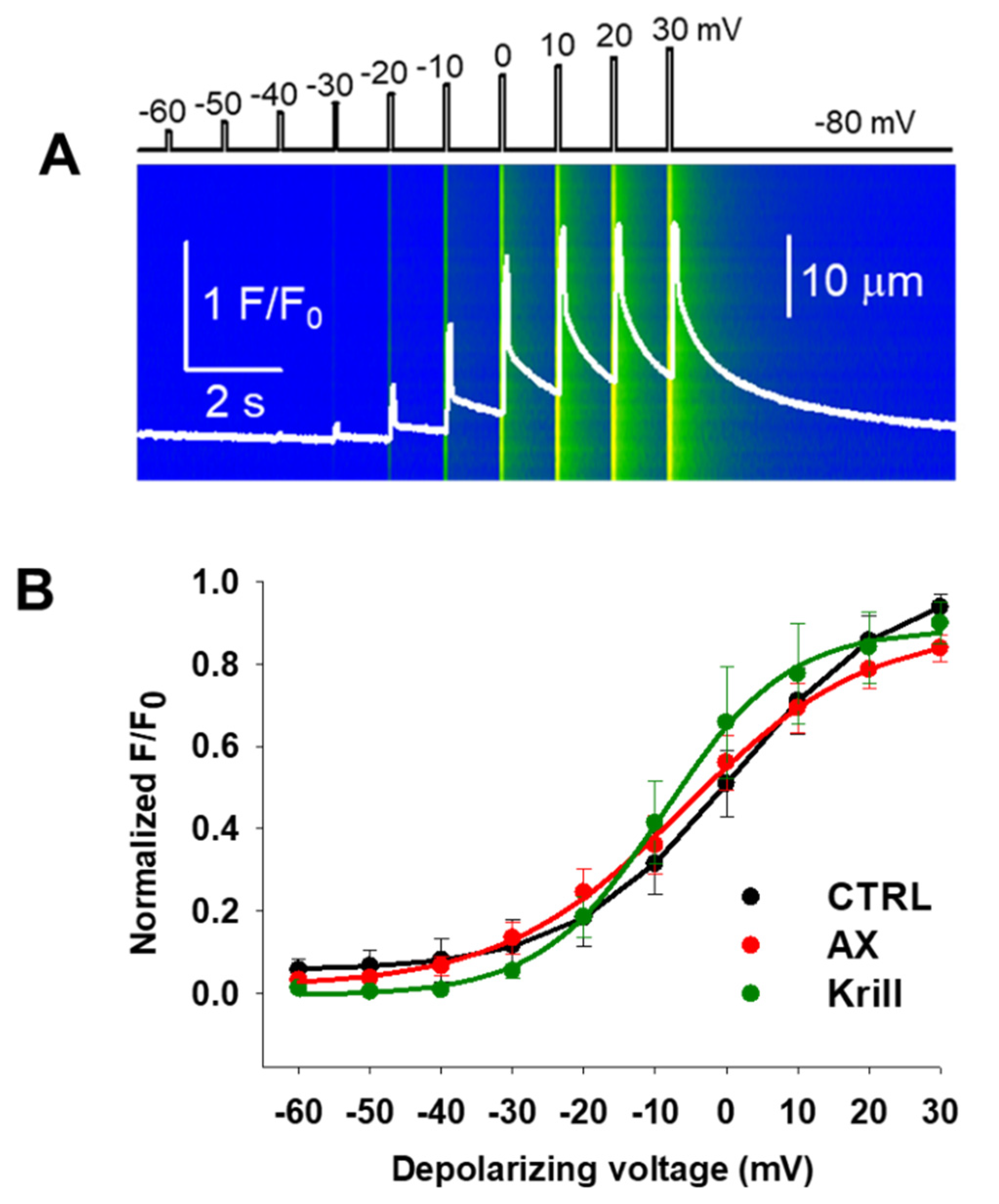
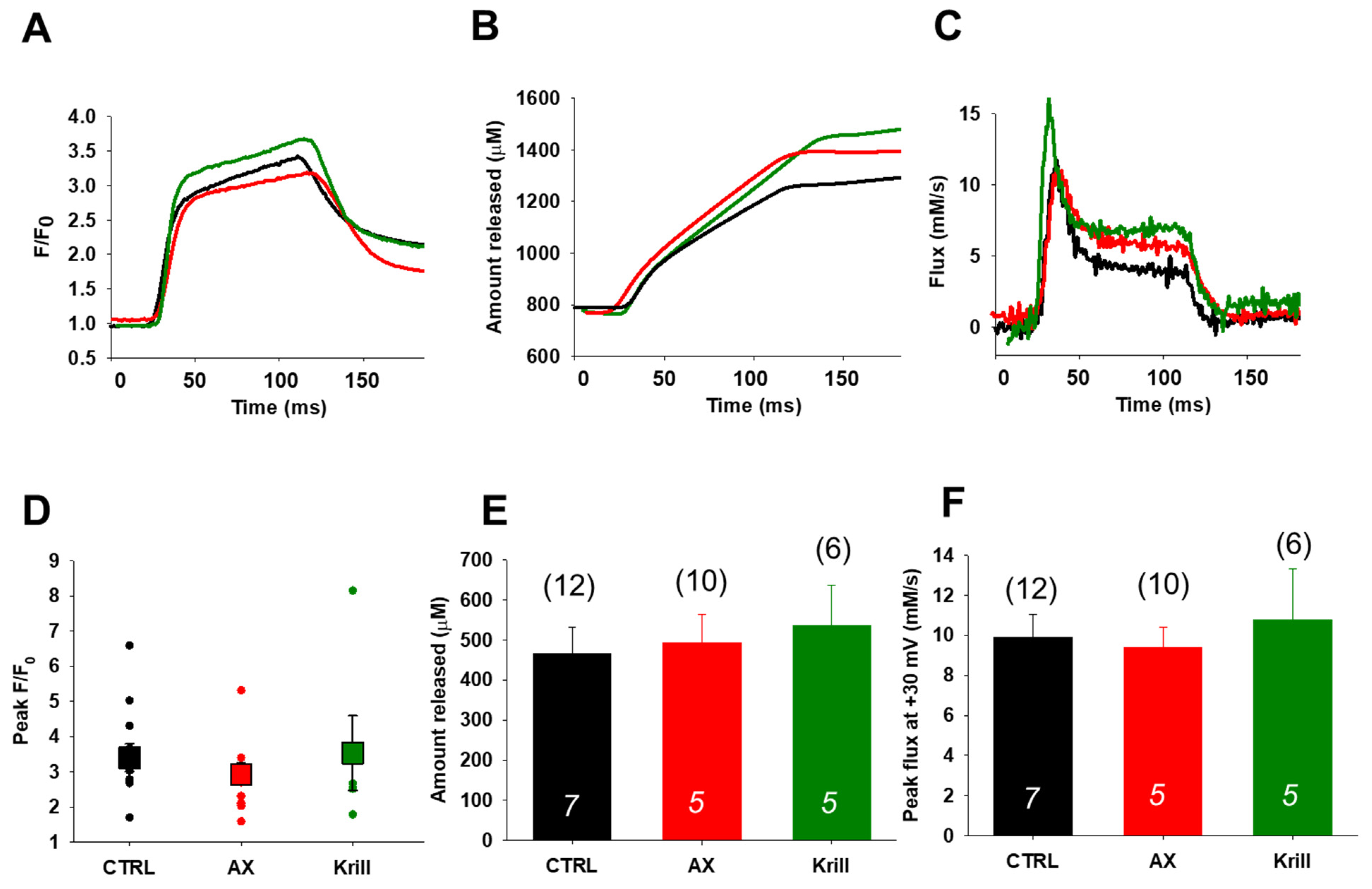
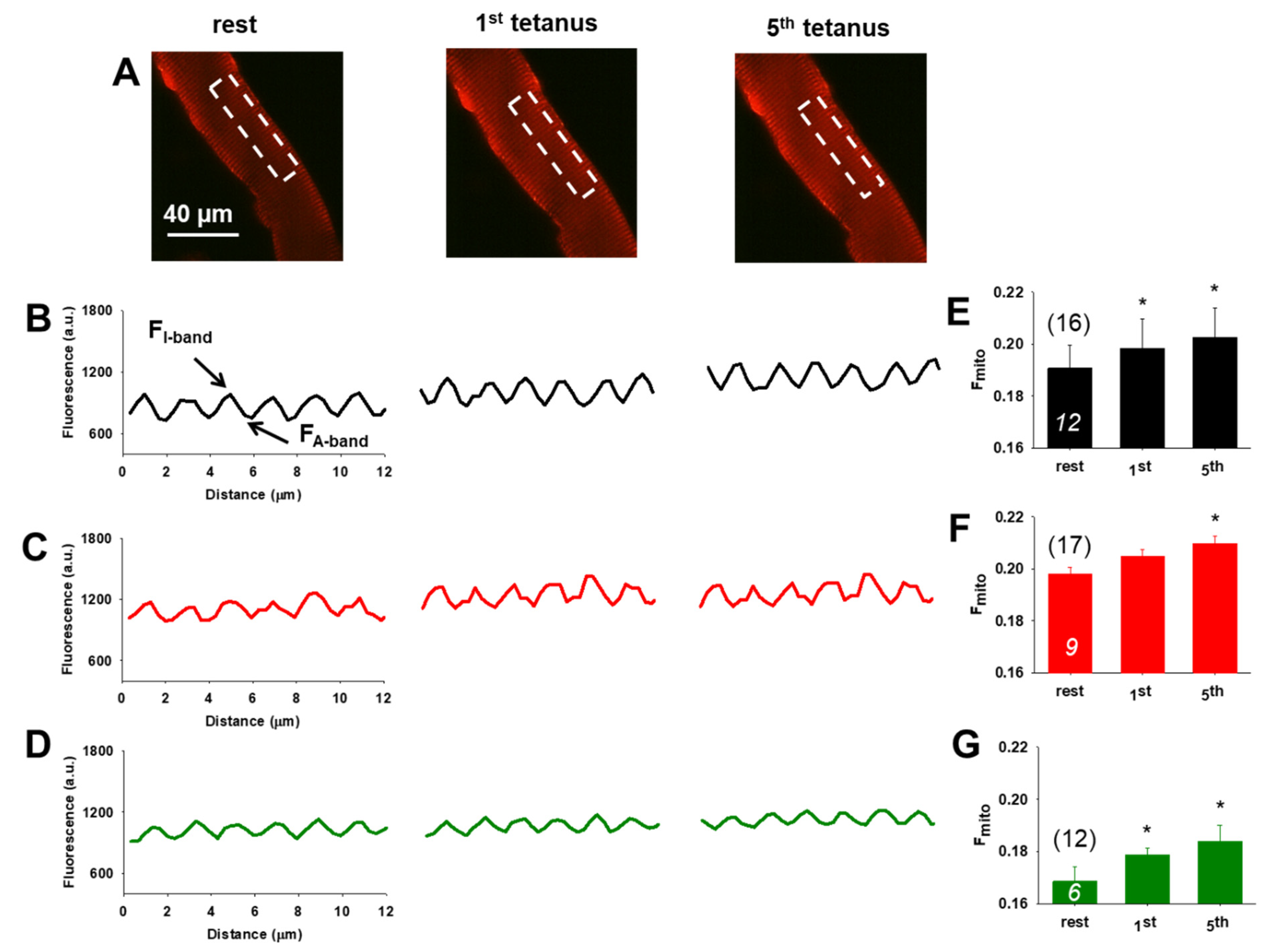

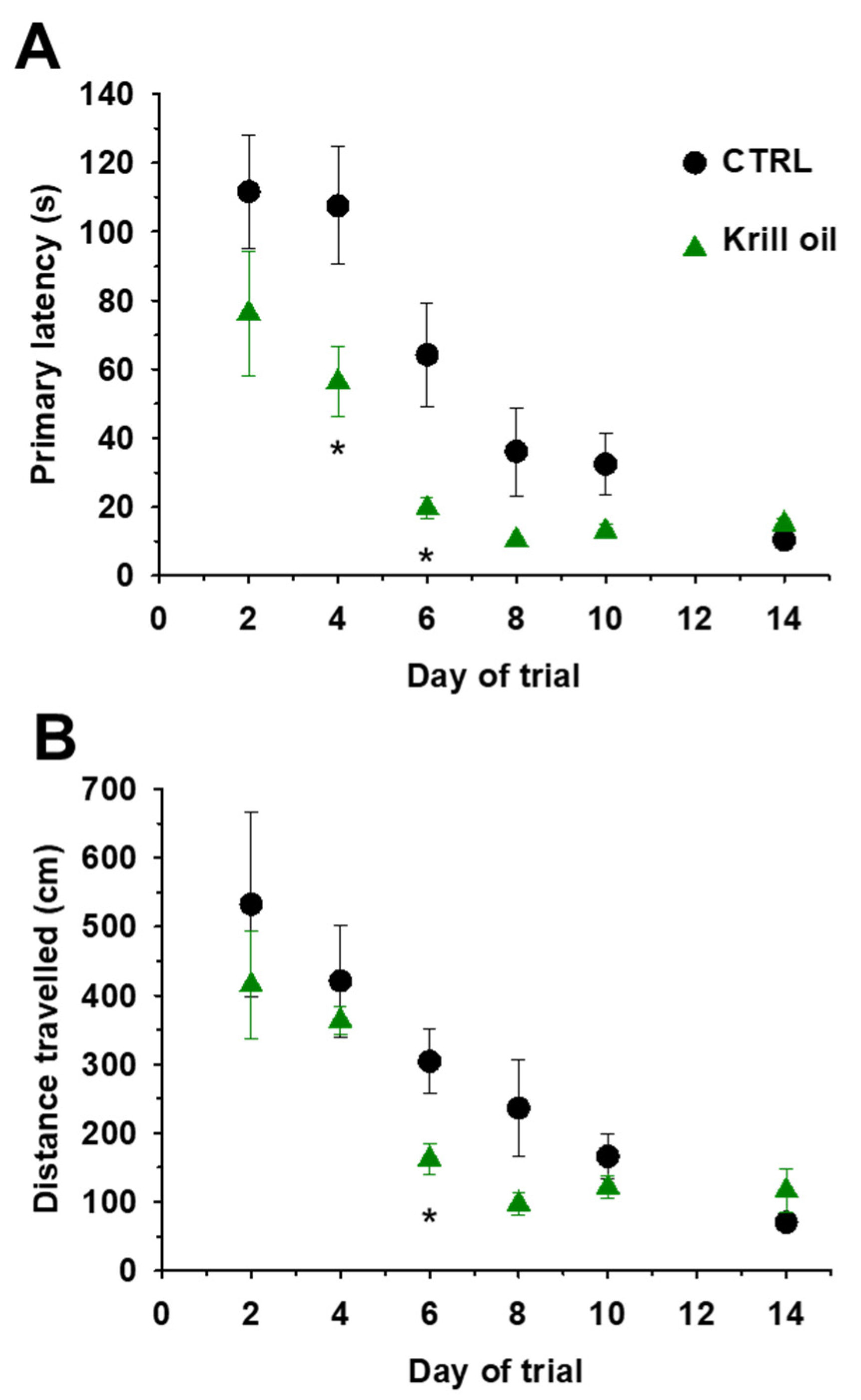
| Parameters | CTRL | AX | Krill Oil |
|---|---|---|---|
| Number of animals | 6 | 4 | 7 |
| Body weight change (g) | −1.04 ± 0.66 | −3.98 ± 0.31 *** | −0.73 ± 0.34 |
| Maximal force change (mN) | −6.45 ± 5.87 | −1.46 ± 6.08 | 11.09 ± 5.33 |
| Normalized force change (mN/g) | −0.11 ± 0.23 | 0.42 ± 0.23 * | 0.44 ± 0.16 * |
| Parameters | Twitch | Tetanus | ||||
|---|---|---|---|---|---|---|
| CTRL | AX | Krill Oil | CTRL | AX | Krill Oil | |
| Number of muscles | 30 | 12 | 9 | 30 | 12 | 9 |
| Force (mN/mm2) | 1.65 ±0.12 | 3.06 ± 0.63 ** | 2.33 ± 0.38 | 8.13 ± 0.40 | 14.52 ± 2.94 ** | 10.24 ± 1.11 * |
| TTP (ms) | 30.1 ± 0.3 | 31.0 ± 1.3 | 34.3 ± 1.7 *** | 173.7 ± 4.4 | 177.8 ± 7.8 | 190.1 ± 5.8 |
| HRT (ms) | 26.0 ± 0.6 | 26.0 ± 1.3 | 28.1 ± 1.4 | 80.2 ± 4.6 | 76.7 ± 8.3 | 65.7 ± 7.2 |
| Muscle weight (mg) | 17.6 ± 0.4 | 17.0 ± 0.9 | 18.6 ± 0.9 | |||
| Parameters | CTRL | AX | Krill Oil |
|---|---|---|---|
| Number of muscle fibers | 13 | 10 | 6 |
| k | 9.41 ± 0.74 | 12.54 ± 1.47 | 8.97 ± 1.56 |
| V50 (mV) | −0.95 ± 4.82 | −5.87 ± 3.89 | −2.62 ± 5.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singlár, Z.; Szentesi, P.; Fodor, J.; Angyal, Á.; Csernoch, L.; Sztretye, M. Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice. Antioxidants 2021, 10, 1415. https://doi.org/10.3390/antiox10091415
Singlár Z, Szentesi P, Fodor J, Angyal Á, Csernoch L, Sztretye M. Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice. Antioxidants. 2021; 10(9):1415. https://doi.org/10.3390/antiox10091415
Chicago/Turabian StyleSinglár, Zoltán, Péter Szentesi, János Fodor, Ágnes Angyal, László Csernoch, and Mónika Sztretye. 2021. "Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice" Antioxidants 10, no. 9: 1415. https://doi.org/10.3390/antiox10091415
APA StyleSinglár, Z., Szentesi, P., Fodor, J., Angyal, Á., Csernoch, L., & Sztretye, M. (2021). Assessing the Potential of Nutraceuticals as Geroprotectors on Muscle Performance and Cognition in Aging Mice. Antioxidants, 10(9), 1415. https://doi.org/10.3390/antiox10091415







