Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
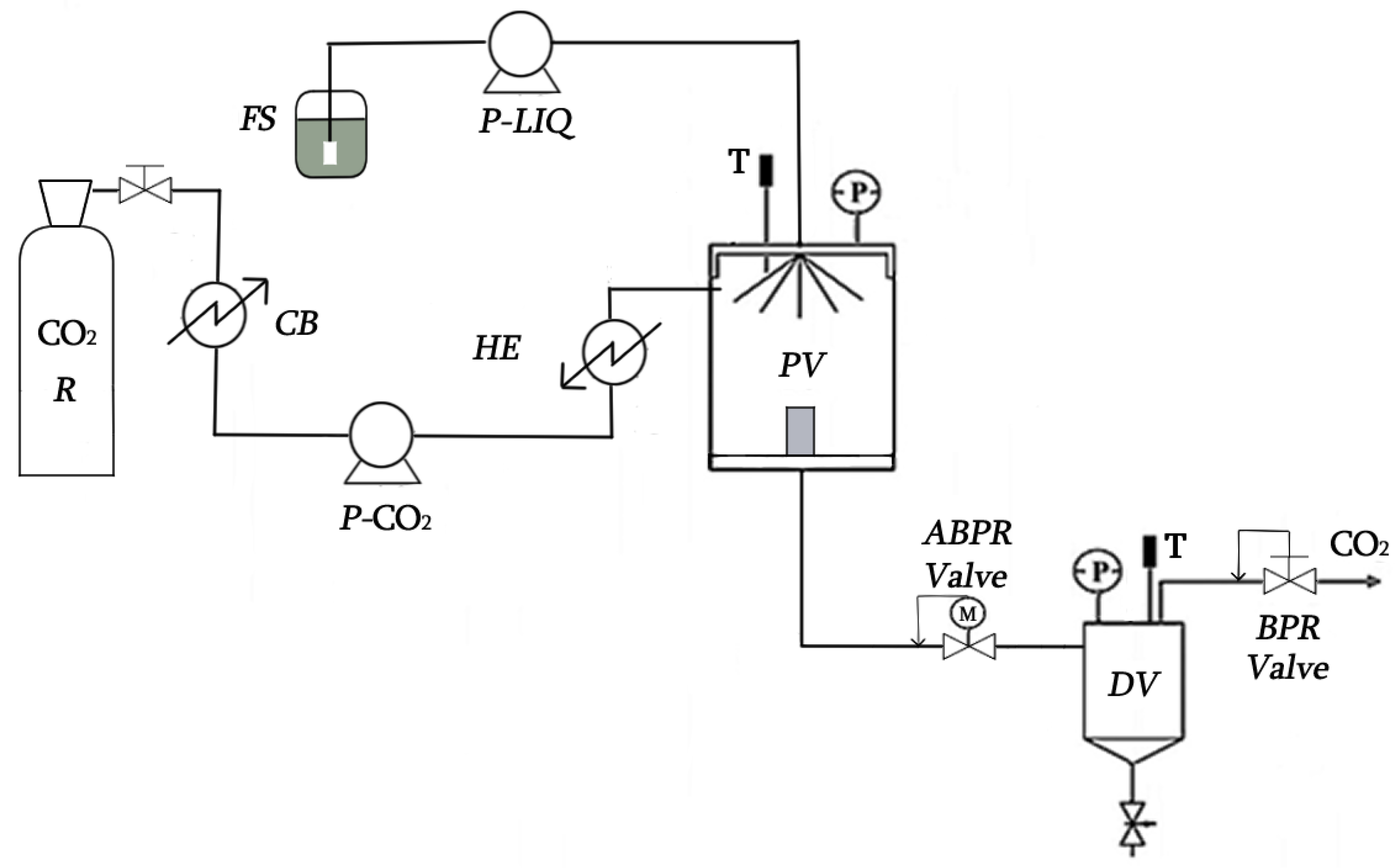
2.3. Supercritical CO2 Extraction (SCE)
2.4. Maceration and Supercritical Antisolvent Fractionation (SAF) Processes
2.5. HPLC Analysis
2.6. Experimental Design and Statistical Analysis
2.7. Application of the Skin Model
3. Results and Discussion
3.1. SCE and Feed Solution Preparation
3.2. SAF Yields Statistical Analysis
3.3. Enrichment Ratio Analysis
3.4. Results of the Skin Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, D.; Rani, A.; Sharma, A. A Review on Phytochemistry and Ethnopharmacological Aspects of Genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines, 3rd ed.; Pharmaceutical Press: London, UK; Grayslake, IL, USA, 2007; ISBN 978-0-85369-623-0. [Google Scholar]
- European Scientific Cooperative on Phytotherapy. ESCOP Monographs, The Scientific Foundation for Herbal Medicinal Products. Online Series. Calendulae Flos (Calendula Flower); ESCOP: Exeter, UK, 2019; ISBN 978-1-901964-61-5. [Google Scholar]
- European Medicines Agency Overview of Comments Recieved on Community Herbal Monograph on Calendula officinalis L., Flos. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-calendula-officinalis-l-flos-revision-1_en.pdf (accessed on 26 July 2021).
- World Health Organization Geneva. WHO Monographs on Selected Medicinal Plants; WHO Graphics: Malta, 2002; Volume 2, ISBN 92-4-154537-2. [Google Scholar]
- Mishra, A.; Mishra, A.; Chattopadhyay, P. Calendula officinalis: An Important Herb with Valuable Therapeutic Dimensions—An Overview. J. Global Pharma Technol. 2010, 10, 2. [Google Scholar] [CrossRef]
- European Comission CosIng—Cosmetics—Calendula officinalis Callus Extract. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=95567 (accessed on 21 July 2021).
- European Commission CosIng—Cosmetics—Calendula officinalis Flower Oil. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=74930 (accessed on 21 July 2021).
- European Commission CosIng—Cosmetics—Calendula officinalis Flower Water. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=83485 (accessed on 21 July 2021).
- Mishra, A.; Chattopadhyay, P. Assessment of In Vitro Sun Protection Factor of Calendula officinalis L. (Asteraceae) Essential Oil Formulation. J. Young Pharm. JYP 2012, 4, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial Activity of Calendula Officinalis Petal Extracts against Fungi, as Well as Gram-Negative and Gram-Positive Clinical Pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Klouchek-Popova, E.; Popov, A.; Pavlova, N.; Krŭsteva, S. Influence of the Physiological Regeneration and Epithelialization Using Fractions Isolated from Calendula Officinalis. Acta Physiol. Pharmacol. Bulg. 1982, 8, 63–67. [Google Scholar]
- Duran, V.; Matic, M.; Jovanovć, M.; Mimica, N.; Gajinov, Z.; Poljacki, M.; Boza, P. Results of the Clinical Examination of an Ointment with Marigold (Calendula officinalis) Extract in the Treatment of Venous Leg Ulcers. Int. J. Tissue React 2005, 27, 101–106. [Google Scholar] [PubMed]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Cardoso, J.C.; Cavalcanti De Albuquerque Junior, R.L.; Vieira Fonseca, M.J. Efficacy of Marigold Extract-Loaded Formulations Against UV-Induced Oxidative Stress. J. Pharm. Sci. 2011, 100, 2182–2193. [Google Scholar] [CrossRef]
- ZitterlEglseer, K.; Sosa, S.; Jurenitsch, J.; SchubertZsilavecz, M.; DellaLoggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-Oedematous Activities of the Main Triterpendiol Esters of Marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Loggia, R.D.; Tubaro, A.; Sosa, S.; Becker, H.; Saar, S.; Isaac, O. The Role of Triterpenoids in the Topical Anti-Inflammatory Activity of Calendula officinalis Flowers. Planta Med. 1994, 60, 516–520. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-Inflammatory, Anti-Tumor-Promoting, and Cytotoxic Activities of Constituents of Marigold (Calendula Officinalis) Flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef]
- Jimenez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A New Extract of the Plant Calendula officinalis Produces a Dual In Vitro Effect: Cytotoxic Anti-Tumor Activity and Lymphocyte Activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef]
- Shivasharan, B.D.; Nagakannan, P.; Thippeswamy, B.S.; Veerapur, V.P.; Bansal, P.; Unnikrishnan, M.K. Protective Effect of Calendula officinalis Linn. Flowers against 3-Nitropropionic Acid Induced Experimental Huntington’s Disease in Rats. Drug Chem. Toxicol. 2013, 36, 466–473. [Google Scholar] [CrossRef]
- Ray, D.; Mukherjee, S.; Falchi, M.; Bertelli, A.; Braga, P.C.; Das, D.K. Amelioration of Myocardial Ischemic Reperfusion Injury with Calendula officinalis. Curr. Pharm. Biotechnol. 2010, 11, 849–854. [Google Scholar] [CrossRef]
- Urbaniak, A.; Kujawski, J.; Czaja, K.; Szelag, M. Antioxidant Properties of Several Caffeic Acid Derivatives: A Theoretical Study. C. R. Chim. 2017, 20, 1072–1082. [Google Scholar] [CrossRef]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-Protective and Antioxidant Properties of Caffeic Acid and Chlorogenic Acid: Mechanistic Role of Angiotensin Converting Enzyme, Cholinesterase and Arginase Activities in Cyclosporine Induced Hypertensive Rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-Wrinkle and Anti-Inflammatory Effects of Active Garlic Components and the Inhibition of MMPs via NF-KB Signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef]
- Dias Alves, G.d.A.; de Souza, R.O.; Ghislain Rogez, H.L.; Masaki, H.; Vieira Fonseca, M.J. Cecropia Obtusa Extract and Chlorogenic Acid Exhibit Anti Aging Effect in Human Fibroblasts and Keratinocytes Cells Exposed to UV Radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; Gunawan, K.Y.; Widowati, W. Anti-Inflammatory and Antiaging Properties of Chlorogenic Acid on UV-Induced Fibroblast Cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Lewandowska, K.; Sionkowska, A. Modification of Collagen Properties with Ferulic Acid. Materials 2020, 13, 3419. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Lo Cascio, R.; Trombetta, D.; Proteggente, A.; De Pasquale, A.; Uccella, N.; Bonina, F. Ferulic and Caffeic Acids as Potential Protective Agents against Photooxidative Skin Damage. J. Sci. Food Agric. 1999, 79, 476–480. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Zhang, Z.; Wang, W.; Yao, S. Laser Flash Photolysis Study on Antioxidant Properties of Hydroxycinnamic Acid Derivatives. Radiat. Environ. Biophys. 2006, 45, 73–77. [Google Scholar] [CrossRef]
- Staniforth, V.; Chiu, L.-T.; Yang, N.-S. Caffeic Acid Suppresses UVB Radiation-Induced Expression of Interleukin-10 and Activation of Mitogen-Activated Protein Kinases in Mouse. Carcinogenesis 2006, 27, 1803–1811. [Google Scholar] [CrossRef]
- Taofiq, O.; Gonzalez-Paramas, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Vegetable Matrices: Applications, Trends and Future Perspectives of a Convincing Green Technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-by-Products, Algae and Microalgae. A Review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of Bioactive Compounds and Essential Oils from Mediterranean Herbs by Conventional and Green Innovative Techniques: A Review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, M.; Adler, S.; Baumann, D.; Forg, A.; Weinreich, B. Preparative Purification of the Major Anti-Inflammatory Triterpenoid Esters from Marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338. [Google Scholar] [CrossRef]
- Danielski, L.; Campos, L.M.A.S.; Bresciani, L.F.V.; Hense, H.; Yunes, R.A.; Ferreira, S.R.S. Marigold (Calendula officinalis L.) Oleoresin: Solubility in SC-CO2 and Composition Profile. Chem. Eng. Process. 2007, 46, 99–106. [Google Scholar] [CrossRef]
- Petrovic, L.; Lepojevic, Z.; Sovilj, V.; Adamovic, D.; Tesevic, V. An Investigation of CO2 Extraction of Marigold (Calendula officinalis L.). J. Serb. Chem. Soc. 2007, 72, 407–413. [Google Scholar] [CrossRef]
- Petrovic, L.; Lepojevic, Z.; Sovilj, V.; Adamovic, D.; Tesevic, V. Composition of Essential Oil Obtained from Tubular, Head and Ligulate Flowers of Calendula officinalis L. by Steam Distillation of Plant Material and CO2 Extracts. J. Essent. Oil Res. 2010, 22, 143–146. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Langa, E.; Urieta, J.S.; Hernaiz, M.J.; Mainar, A.M. Supercritical Antisolvent Fractionation of Antioxidant Compounds from Lavandula Luisieri (Rozeira) Riv.-Mart. J. Supercrit. Fluids 2020, 161, 104821. [Google Scholar] [CrossRef]
- Martin, L.; Gonzalez-Coloma, A.; Adami, R.; Scognamiglio, M.; Reverchon, E.; Della Porta, G.; Urieta, J.S.; Mainar, A.M. Supercritical Antisolvent Fractionation of Ryanodol from Persea Indica. J. Supercrit. Fluids 2011, 60, 16–20. [Google Scholar] [CrossRef]
- Sanchez-Camargo, A.P.; Mendiola, J.A.; Valdes, A.; Castro-Puyana, M.; Garcia-Canas, V.; Cifuentes, A.; Herrero, M.; Ibanez, E. Supercritical Antisolvent Fractionation of Rosemary Extracts Obtained by Pressurized Liquid Extraction to Enhance Their Antiproliferative Activity. J. Supercrit. Fluids 2016, 107, 581–589. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Loran, S.; Mainar, A.M.; Hernaiz, M.J.; Rota, C. Supercritical Carbon Dioxide Antisolvent Fractionation for the Sustainable Concentration of Lavandula luisieri (Rozeira) Riv.- Mart Antimicrobial and Antioxidant Compounds and Comparison with Its Conventional Extracts. Plants 2019, 8, 455. [Google Scholar] [CrossRef]
- Langa, E.; Pardo, J.I.; Gimenez-Rota, C.; Gonzalez-Coloma, A.; Hernaiz, M.J.; Mainar, A.M. Supercritical Anti-Solvent Fractionation of Artemisia Absinthium L. Conventional Extracts: Tracking Artemetin and Casticin. J. Supercrit. Fluids 2019, 151, 15–23. [Google Scholar] [CrossRef]
- Schwöbel, J.A.H.; Klamt, A. Mechanistic Skin Penetration Model by the COSMOperm Method: Routes of Permeation, Vehicle Effects and Skin Variations in the Healthy and Compromised Skin. Comput. Toxicol. 2019, 11, 50–64. [Google Scholar] [CrossRef]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.-N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative Structure-Skin Permeability Relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Ates, G.; Steinmetz, F.P.; Doktorova, T.Y.; Madden, J.C.; Rogiers, V. Linking Existing In Vitro Dermal Absorption Data to Physicochemical Properties: Contribution to the Design of a Weight-of-Evidence Approach for the Safety Evaluation of Cosmetic Ingredients with Low Dermal Bioavailability. Regul. Toxicol. Pharmacol. 2016, 76, 74–78. [Google Scholar] [CrossRef]
- Williams, F.M.; Rothe, H.; Barrett, G.; Chiodini, A.; Whyte, J.; Cronin, M.T.D.; Monteiro-Riviere, N.A.; Plautz, J.; Roper, C.; Westerhout, J.; et al. Assessing the Safety of Cosmetic Chemicals: Consideration of a Flux Decision Tree to Predict Dermally Delivered Systemic Dose for Comparison with Oral TTC (Threshold of Toxicological Concern). Regul. Toxicol. Pharmacol. 2016, 76, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A.; Deyoe, C.W.; Pfost, H.B. A Method for Determining and Expressing the Size of Feed Particles by Sieving. Poult. Sci. 1970, 49, 9–13. [Google Scholar] [CrossRef]
- Mur, R.; Pardo, J.I.; Pino-Otín, M.R.; Urieta, J.S.; Mainar, A.M. Supercritical Antisolvent Fractionation of Antioxidant Compounds from Salvia Officinalis. Int. J. Mol. Sci. 2021, 22, 9351. [Google Scholar] [CrossRef]
- Marqués, J.L.; Porta, G.D.; Reverchon, E.; Renuncio, J.A.R.; Mainar, A.M. Supercritical Antisolvent Extraction of Antioxidants from Grape Seeds after Vinification. J. Supercrit. Fluids 2013, 82, 238–243. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-Like Screening Model for Real Solvents—A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics. In Proceedings of the 28th European Symposium on Computer Aided Process Engineering, Graz, Sunday, 10–13 June 2018; Friedl, A., Klemes, J.J., Radl, S., Varbanov, P.S., Wallek, T., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2018; Volume 43, p. 9, ISBN 978-0-444-64235-6. [Google Scholar]
- Buggert, M.; Cadena, C.; Mokrushina, L.; Smirnova, I.; Maginn, E.J.; Arlt, W. COSMO-RS Calculations of Partition Coefficients: Different Tools for Conformational Search. Chem. Eng. Technol. 2009, 32, 977–986. [Google Scholar] [CrossRef]
- Klamt, A.; Huniar, U.; Spycher, S.; Keldenich, J. COSMOmic: A Mechanistic Approach to the Calculation of Membrane-Water Partition Coefficients and Internal Distributions within Membranes and Micelles. J. Phys. Chem. B 2008, 112, 12148–12157. [Google Scholar] [CrossRef] [PubMed]
- Jakobtorweihen, S.; Zuniga, A.C.; Ingram, T.; Gerlach, T.; Keil, F.J.; Smirnova, I. Predicting Solute Partitioning in Lipid Bilayers: Free Energies and Partition Coefficients from Molecular Dynamics Simulations and COSMOmic. J. Chem. Phys. 2014, 141, 045102. [Google Scholar] [CrossRef]
- Droge, S.T.J.; Hermens, J.L.M.; Gutsell, S.; Rabone, J.; Hodges, G. Predicting the Phospholipophilicity of Monoprotic Positively Charged Amines. Environ. Sci.-Process. Impacts 2017, 19, 307–323. [Google Scholar] [CrossRef]
- Yordanova, D.; Ritter, E.; Gerlach, T.; Jensen, J.H.; Smirnova, I.; Jakobtorweihen, S. Solute Partitioning in Micelles: Combining Molecular Dynamics Simulations, COSMOmic, and Experiments. J. Phys. Chem. B 2017, 121, 5794–5809. [Google Scholar] [CrossRef]
- Bittermann, K.; Linden, L.; Goss, K.-U. Screening Tools for the Bioconcentration Potential of Monovalent Organic Ions in Fish. Environ. Sci.-Process. Impacts 2018, 20, 845–853. [Google Scholar] [CrossRef]
- Klamt, A.; Schwoebel, J.; Huniar, U.; Koch, L.; Terzi, S.; Gaudin, T. COSMOplex: Self-Consistent Simulation of Self-Organizing Inhomogeneous Systems Based on COSMO-RS. Phys. Chem. Chem. Phys. 2019, 21, 9225–9238. [Google Scholar] [CrossRef]
- Naegel, A.; Heisig, M.; Wittum, G. Detailed Modeling of Skin Penetration-An Overview. Adv. Drug Deliv. Rev. 2013, 65, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.; Adler, S.; Gruner, S.; Otto, F.; Weinreich, B.; Hamburger, M. Supercritical Carbon Dioxide Extraction of Marigold at High Pressures: Comparison of Analytical and Pilot-Scale Extraction. Phytochem. Anal. 2004, 15, 226–230. [Google Scholar] [CrossRef]
- Lopez-Padilla, A.; Ruiz-Rodriguez, A.; Reglero, G.; Fornari, T. Supercritical Carbon Dioxide Extraction of Calendula Officinalis: Kinetic Modeling and Scaling up Study. J. Supercrit. Fluids 2017, 130, 292–300. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; El-Denshary, E.S.M.; Hassan, N.S.; Mannaa, F.A.; Abdel-Wahhab, M.A. Dietary Supplementation of Calendula Officinalis Counteracts the Oxidative Stress and Liver Damage Resulted from Aflatoxin. ISRN Nutr. 2013, 2013, e538427. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.E.; Villanueva-Bermejo, D.; Reglero, G.; Garcia-Risco, M.R.; Fornari, T. Supercritical Antisolvent Particle Precipitation and Fractionation of Rosemary (Rosmarinus officinalis L.) Extracts. J. CO2 Util. 2019, 34, 479–489. [Google Scholar] [CrossRef]
- Martin, A.; Gutierrez, L.; Mattea, F.; Cocero, M.J. Precipitation of Mandelic Acid with a Supercritical Antisolvent Process: Experimental and Theoretical Analysis, Optimization, and Scaleup. Ind. Eng. Chem. Res. 2007, 46, 1552–1562. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, Z.; Wang, Q.; Qin, B.; Dai, L.; Jiang, F.; Liu, W.; Qian, H. Prediction Model, Experimental Optimization, and Verification for Yield of High-Pressure Crystallization: A Case Study of Citric Acid. Sep. Sci. Technol. 2020, 55, 135–143. [Google Scholar] [CrossRef]
- Reverchon, E.; Caputo, G.; De Marco, I. Role of Phase Behavior and Atomization in the Supercritical Antisolvent Precipitation. Ind. Eng. Chem. Res. 2003, 42, 6406–6414. [Google Scholar] [CrossRef]
- Cardoso, M.A.T.; Cabral, J.M.S.; Palavra, A.M.F.; Geraldes, V. CFD Analysis of Supercritical Antisolvent (SAS) Micronization of Minocycline Hydrochloride. J. Supercrit. Fluids 2008, 47, 247–258. [Google Scholar] [CrossRef]
- Sikroria, T.; Kushari, A.; Syed, S.; Lovett, J.A. Experimental Investigation of Liquid Jet Breakup in a Cross Flow of a Swirling Air Stream. J. Eng. Gas Turbines Power-Trans. ASME 2014, 136, 061501. [Google Scholar] [CrossRef]
- Rantakyla, M.; Jantti, M.; Aaltonen, O.; Hurme, M. The Effect of Initial Drop Size on Particle Size in the Supercritical Antisolvent Precipitation (SAS) Technique. J. Supercrit. Fluids 2002, 24, 251–263. [Google Scholar] [CrossRef]
- Werling, J.O.; Debenedetti, P.G. Numerical Modeling of Mass Transfer in the Supercritical Antisolvent Process. J. Supercrit. Fluids 1999, 16, 167–181. [Google Scholar] [CrossRef]
- Werling, J.O.; Debenedetti, P.G. Numerical Modeling of Mass Transfer in the Supercritical Antisolvent Process: Miscible Conditions. J. Supercrit. Fluids 2000, 18, 11–24. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Dalvi, S.V. Mass and Heat Transfer Analysis of SAS: Effects of Thermodynamic States and Flow Rates on Droplet Size. J. Supercrit. Fluids 2004, 30, 333–348. [Google Scholar] [CrossRef]
- Baldyga, J. Mixing and Fluid Dynamics Effects in Particle Precipitation Processes. KONA Powder Part. J. 2016, 33, 127–149. [Google Scholar] [CrossRef]
- Cardoso, F.a.R.; Vogel, E.M.; Souza, M.F.; Cardozo-Filho, L. Mathematical Modeling to Predict the Size and Nucleation Rate of Micro and Nanoparticles Using the Scale-up Process with Supercritical CO2. J. Supercrit. Fluids 2019, 154, 104608. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Shen, Y.; Dave, R.; Pfeffer, R. Development in Modeling Submicron Particle Formation in Two Phases Flow of Solvent-Supercritical Antisolvent Emulsion. Adv. Colloid Interface Sci. 2007, 134–135, 72–88. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Banerjee, N.; Chaudhari, P. Investigation on Crystallization Phenomena with Supercritical Carbon Dioxide (CO2) as the Antisolvent. Int. J. Chem. React. Eng. 2021, 19, 861–871. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Influence of Pressure, Temperature and Concentration on the Mechanisms of Particle Precipitation in Supercritical Antisolvent Micronization. J. Supercrit. Fluids 2011, 58, 295–302. [Google Scholar] [CrossRef]
- Mihalovits, M.; Horvath, A.; Lorincz, L.; Szekely, E.; Kemeny, S. Model Building on Selectivity of Gas Antisolvent Fractionation Method Using the Solubility Parameter. Period. Polytech.-Chem. Eng. 2019, 63, 294–302. [Google Scholar] [CrossRef]
- Mihalovits, M.; Korosi, M.; Szekely, E. New Formula for the Hydrogen-Bonding Hansen Component of Methanol, Ethanol, and n-Propanol for Non-Ambient Conditions-Application in Gas Antisolvent Fractionation-Based Optical Resolution. ACS Omega 2021, 6, 18964–18974. [Google Scholar] [CrossRef] [PubMed]
- Pasca, M.B.; Pallag, A.; Gitea, D. The quantitative determination of active principles from Calendula officinalis L. Inflorescences 2013, XII, 317–320. [Google Scholar]
- Ferreira, C.; Pereyra, A.; Patriarca, A.; Mazzobre, M.; Polak, T.; Abram, V.; Buera, M.; PoklarUlrihd, N. Phenolic Compounds in Extracts from Eucalyptus Globulus Leaves and Calendula officinalis Flowers. J. Nat. Prod. Resour. 2016, 2, 53–57. [Google Scholar]
- Zhang, K.; Sun, W.; Fahr, A.; Zeng, X.; Ge, L.; Chen, M.; Yang, L.; Wu, S.; Fei, J.; Zhou, B. Skin-Permeating Components of Lonicera Japonica Flos: A Comprehensive Study from Observations and Model Computations. New J. Chem. 2019, 43, 12538–12547. [Google Scholar] [CrossRef]
- Farrell, T.L.; Poquet, L.; Dew, T.P.; Barber, S.; Williamson, G. Predicting Phenolic Acid Absorption in Caco-2 Cells: A Theoretical Permeability Model and Mechanistic Study. Drug Metab. Dispos. 2012, 40, 397–406. [Google Scholar] [CrossRef]
- Mortele, O.; Jorissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.N.; Hermans, N. Demonstrating the Involvement of an Active Efflux Mechanism in the Intestinal Absorption of Chlorogenic Acid and Quinic Acid Using a Caco-2 Bidirectional Permeability Assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Adv. Drug Deliv. Rev. 1996, 22, 67–84. [Google Scholar] [CrossRef]
- Sugino, M.; Todo, H.; Suzuki, T.; Nakada, K.; Tsuji, K.; Tokunaga, H.; Jinno, H.; Sugibayashi, K. Safety Prediction of Topically Exposed Biocides Using Permeability Coefficients and the Desquamation Rate at the Stratum Corneum. J. Toxicol. Sci. 2014, 39, 475–485. [Google Scholar] [CrossRef][Green Version]
- Kimura, E.; Kawano, Y.; Todo, H.; Ikarashi, Y.; Sugibayashi, K. Measurement of Skin Permeation/Penetration of Nanoparticles for Their Safety Evaluation. Biol. Pharm. Bull. 2012, 35, 1476–1486. [Google Scholar] [CrossRef]




| Variable | Symbol | Factor Levels | ||||
|---|---|---|---|---|---|---|
| {−1.44 | −1 | 0 | 1 | 1.44} | ||
| Pressure (bar) | XP | 80 | 92 | 120 | 148 | 160 |
| CO2 flow rate (g/min) | XQCO2 | 10 | 17 | 35 | 53 | 60 |
| Compartment | Abbreviation | Description |
|---|---|---|
| Stratum corneum | SC | Horny layer, the outermost layer and the main barrier to permeability within the skin |
| Stratum granulosum | SG | Granular layer |
| Stratum spinosum | SS | Spinous or prickle layer, release neutral barrier lipids |
| Stratum basale | SB | Basal layer, metabolically active |
| Appendageal compartment | Shunt | Responsible for transport of chemical through the hair follicles sweat glands, and sebaceous glands |
| Run | Exp. Run Order | XP (bar) | XQCO2 (g/min) | YPV (wt%) | YDV (wt%) | YSAF (wt%) | ECHA/PV | ECAF/PV | EFA/PV | EALL/PV |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 80 | 35 | 47.0 | 1.3 | 48.3 | 0.86 | 1.19 | 0.69 | 0.79 |
| 2 | 4 | 92 | 17 | 41.0 | 21.5 | 62.5 | 1.04 | 1.25 | 1.10 | 1.09 |
| 3 | 10 | 53 | 16.1 | 15.0 | 31.1 | 1.08 | 1.33 | 1.16 | 1.21 | |
| 4 | 13 | 120 | 10 | 26.0 | 32.2 | 58.2 | 1.51 | 1.48 | 1.67 | 1.64 |
| 5 | 2 | 35 | 26.1 | 20.3 | 46.4 | 1.53 | 1.36 | 1.55 | 1.52 | |
| 6 | 3 | 35 | 22.0 | 25.8 | 47.8 | 1.52 | 1.37 | 1.54 | 1.52 | |
| 7 | 6 | 35 | 18.5 | 30.0 | 48.5 | 1.40 | 1.44 | 1.56 | 1.66 | |
| 8 | 7 | 35 | 15.1 | 30.5 | 45.6 | 1.59 | 1.40 | 1.73 | 1.66 | |
| 9 | 11 | 35 | 13.6 | 29.6 | 43.2 | 1.72 | 1.39 | 1.70 | 1.67 | |
| 10 | 12 | 60 | 12.3 | 30.4 | 42.7 | 1.14 | 1.30 | 1.25 | 1.27 | |
| 11 | 8 | 148 | 17 | 23.4 | 31.3 | 54.7 | 1.18 | 1.40 | 1.32 | 1.32 |
| 12 | 5 | 53 | 30.9 | 42.8 | 73.7 | 1.33 | 1.41 | 1.52 | 1.46 | |
| 13 | 1 | 160 | 35 | 31.1 | 34.3 | 65.4 | 1.28 | 1.37 | 1.55 | 1.46 |
| YPV/wt% | YDV/wt% | YSAF/wt% | ECHA/PV | EFA/PV | EALL/PV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient Value | p | Coefficient Value | p | Coefficient Value | p | Coefficient Value | p | Coefficient Value | p | Coefficient Value | p | |
| β0 | 284.3 | 0.000 | −40.3 | 0.000 | 242.6 | 0.000 | −3.889 | 0.000 | −4.11 | 0.000 | −3.840 | 0.000 |
| β1 | −3.594 | 0.075 | 1.241 | 0.000 | −2.343 | 0.000 | 0.0796 | 0.026 | 0.0835 | 0.003 | 0.0802 | 0.004 |
| β2 | −2.204 | 0.019 | −1.691 | 0.833 | −3.835 | 0.003 | 0.0253 | 0.381 | 0.0160 | 0.454 | 0.0166 | 0.479 |
| β11 | 0.01215 | 0.000 | −0.00493 | 0.035 | 0.00717 | 0.001 | −0.000314 | 0.001 | −0.000315 | 0.002 | −0.000308 | 0.001 |
| β22 | - | - | 0.00898 | 0.105 | 0.00812 | 0.040 | −0.000395 | 0.034 | −0.000262 | 0.189 | −0.000264 | 0.125 |
| β12 | 0.01620 | 0.006 | 0.00900 | 0.058 | 0.02520 | 0.000 | - | - | - | - | - | - |
| R2 | 88.28 | - | 91.26 | - | 96.74 | - | 83.00 | - | 82.91 | - | 84.54 | - |
| s | 4.42 | - | 4.00 | - | 2.66 | - | 0.13 | - | 0.15 | - | 0.13 | - |
| Parameter | Caffeic Acid | Chlorogenic Acid | Ferulic Acid |
|---|---|---|---|
| Vehicle | water | water | water |
| Skin membrane | epidermis | epidermis | epidermis |
| Rate limiting step | SC via polar trans-corneocyte pathway | SS via interstitial space | SC via polar trans-corneocyte pathway |
| logKvehicle:water | 0.00 | 0.00 | 0.00 |
| RSC,inter (s/m) | 2.15 × 1012 | 8.20 × 1016 | 2.55 × 1011 |
| RSC,trans (s/m) | 4.07 × 107 | 1.76 × 108 | 3.23E × 107 |
| RSC (s/m) | 4.07 × 107 | 1.76 × 108 | 3.23E × 107 |
| LogRSC (cm/s) | −5.09 | −6.71 | −4.21 |
| RSG,inter (s/m) | 1.26 × 108 | 5.14 × 108 | 1.23 × 108 |
| RSG,trans (s/m) | 1.34 × 107 | 5.13 × 1011 | 1.63 × 106 |
| RSG (s/m) | 1.22 × 107 | 5.13E × 108 | 1.61 × 106 |
| LogRSG (cm/s) | −5.32 | −7.06 | −4.46 |
| RSS,inter (s/m) | 2.86 × 108 | 1.17 × 109 | 2.80 × 108 |
| RSS,trans (s/m) | 2.26 × 107 | 8.54 × 1011 | 2.91 × 106 |
| RSS (s/m) | 2.09 × 107 | 1.16 × 109 | 2.88 × 106 |
| LogRSS (cm/s) | −4.57 | −5.91 | −3.80 |
| RSB,inter (s/m) | 1.98 × 107 | 8.07 × 107 | 1.94 × 107 |
| RSB,trans (s/m) | 4.58E × 106 | 1.71 × 1011 | 6.50 × 105 |
| RSB (s/m) | 3.72 × 106 | 8.06 × 107 | 6.29 × 105 |
| LogRSB (cm/s) | −5.89 | −7.27 | −5.57 |
| Rcells (s/m) | 7.75 × 107 | 1.93 × 109 | 3.74E × 107 |
| Rshunt (s/m) | 5.00 × 1010 | 5.00 × 1010 | 5.00 × 1010 |
| Rskin (s/m) | 7.74 × 107 | 1.86 × 109 | 3.74E × 107 |
| logKp (pred.) (cm/s) | −5.89 | −7.27 | −5.57 |
| logKp (pred.) + cte offset (cm/s) | −7.01 | −8.39 | −6.69 |
| logKp (calc.) epidermis (cm/s) | −6.85 | - | −7.15 |
| Deviation | 0.16 | - | 0.46 |
| logKp (exp.) Caco−2 (cm/s) | −5.84 | −5.60 | −4.98 |
| Deviation | 1.17 | 2.79 | 1.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mur, R.; Langa, E.; Pino-Otín, M.R.; Urieta, J.S.; Mainar, A.M. Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use. Antioxidants 2022, 11, 96. https://doi.org/10.3390/antiox11010096
Mur R, Langa E, Pino-Otín MR, Urieta JS, Mainar AM. Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use. Antioxidants. 2022; 11(1):96. https://doi.org/10.3390/antiox11010096
Chicago/Turabian StyleMur, Raquel, Elisa Langa, M. Rosa Pino-Otín, José S. Urieta, and Ana M. Mainar. 2022. "Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use" Antioxidants 11, no. 1: 96. https://doi.org/10.3390/antiox11010096
APA StyleMur, R., Langa, E., Pino-Otín, M. R., Urieta, J. S., & Mainar, A. M. (2022). Concentration of Antioxidant Compounds from Calendula officinalis through Sustainable Supercritical Technologies, and Computational Study of Their Permeability in Skin for Cosmetic Use. Antioxidants, 11(1), 96. https://doi.org/10.3390/antiox11010096









