The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Samples
2.3. Cyclic Voltammetry
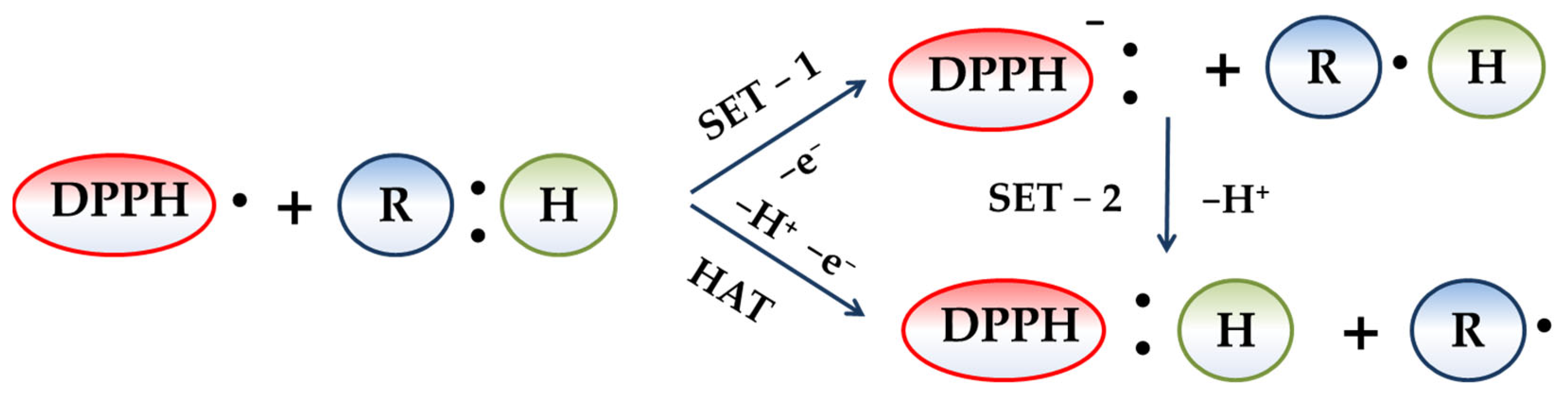
2.4. Spectrophotometric DPPH• Assay
3. Results and Discussion
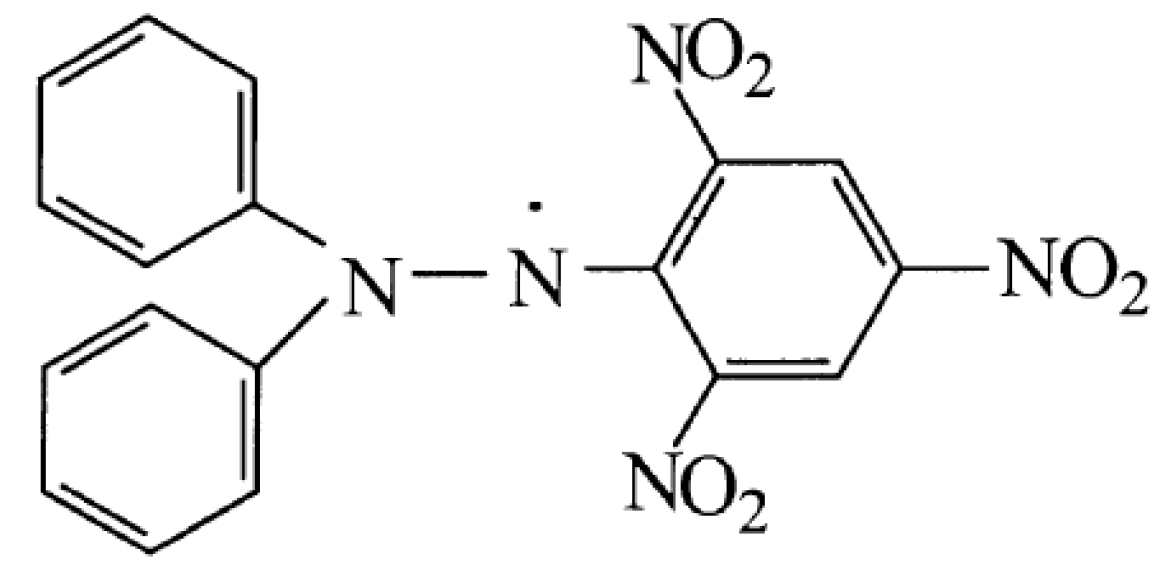
3.1. Redox Behavior of the 2,2-Diphenyl-1-Picrylhydrazyl Radical
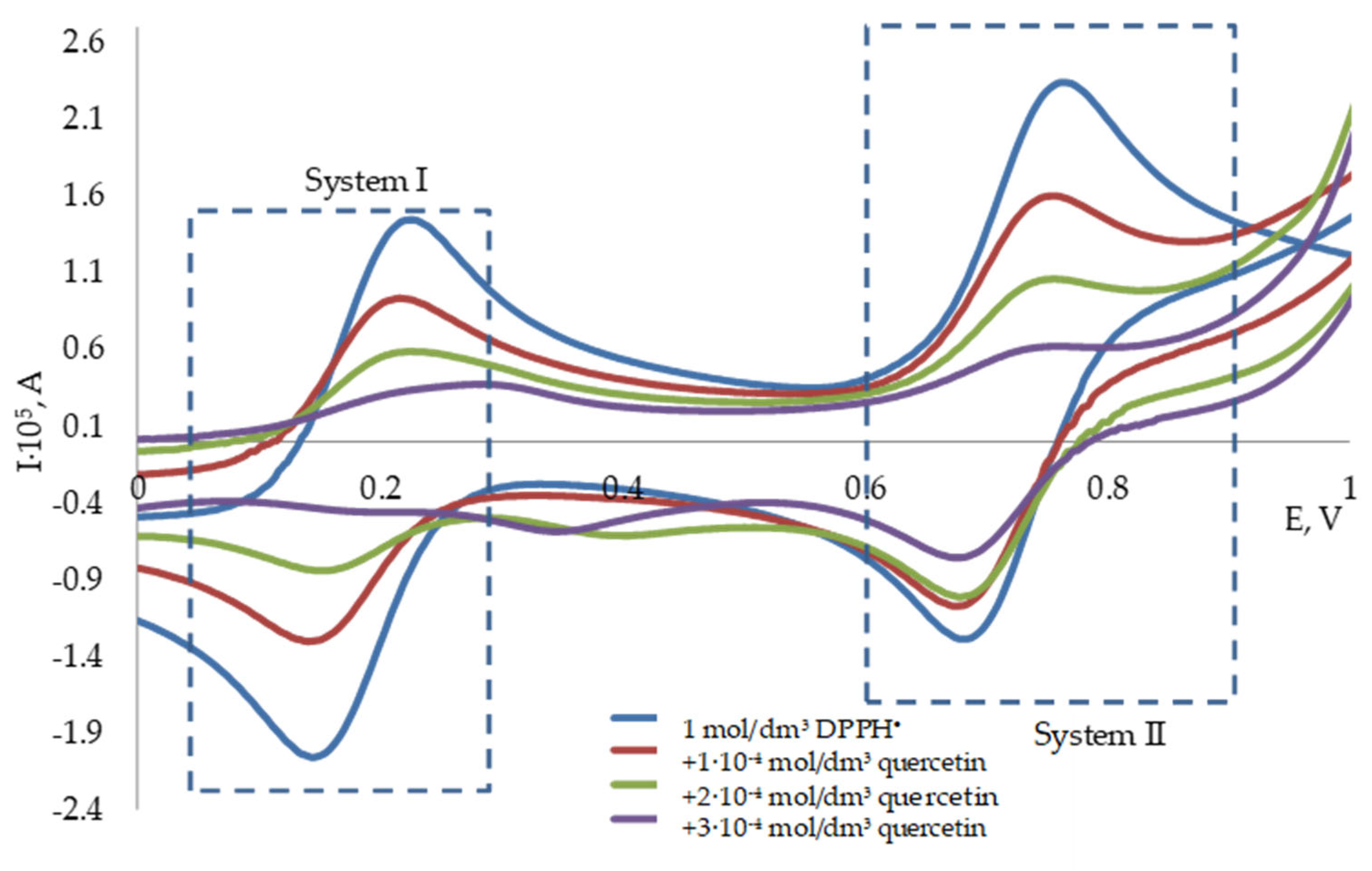
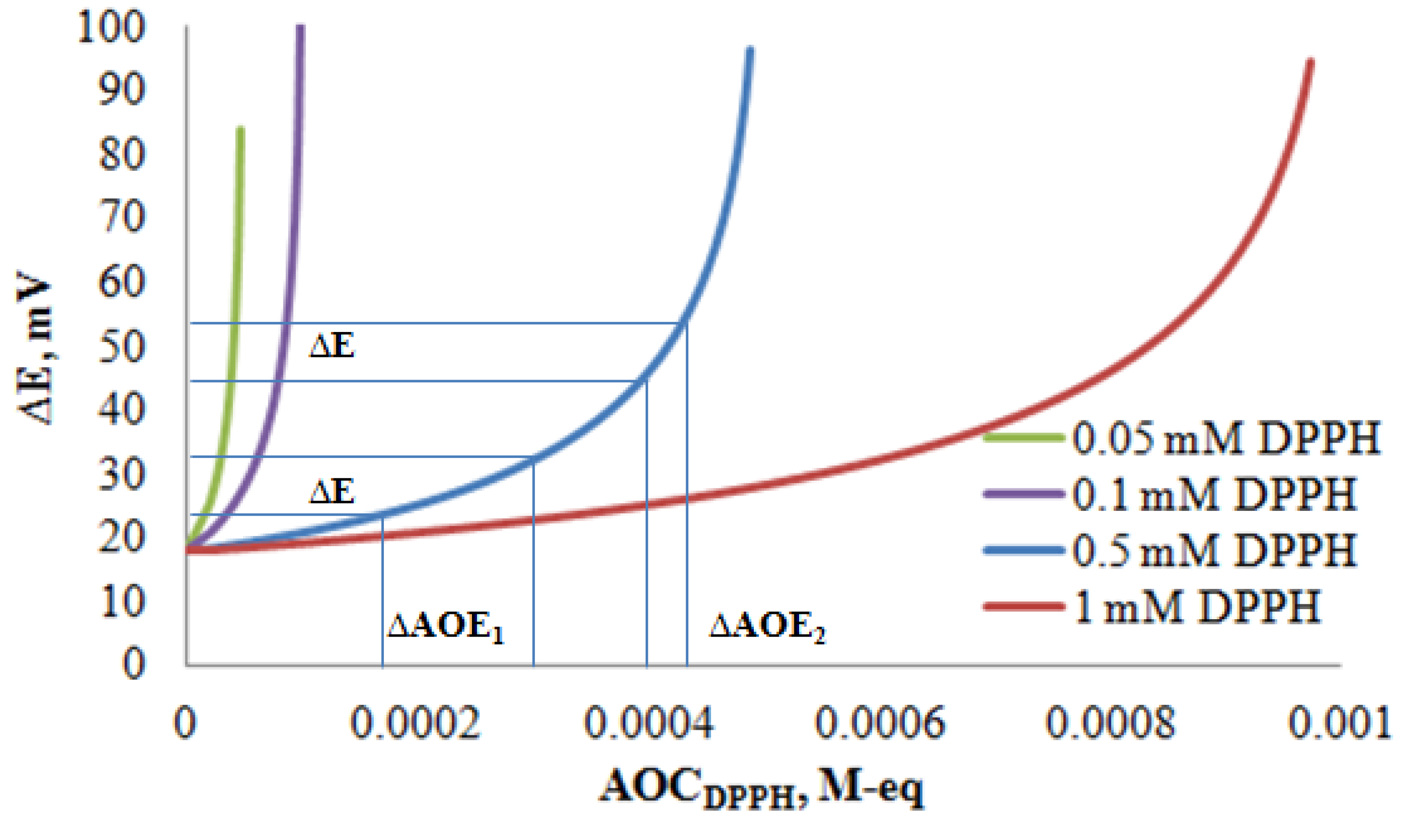
3.2. Potentiometric Investigation of the 2,2-Diphenyl-1-Picrylhydrazyl Radical
3.3. The Determination of the Antioxidant Capacity of Individual Antioxidants by Potentiometry and Spectrophotometry
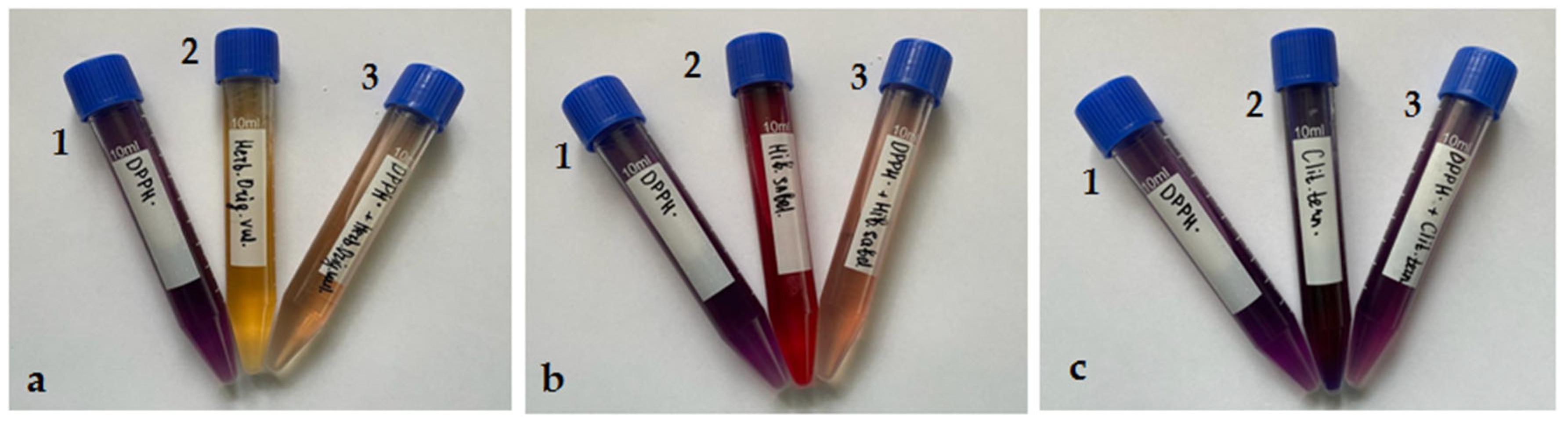
3.4. The Determination of the Antioxidant Capacity of Infusions of Medicinal Plants and Their Water-Ethanol Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Orsavová, J.; Sytařová, I.; Mlček, J.; Mišurcová, L. Phenolic Compounds, Vitamins C and E and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea L. var. kamtschatica Pojark) in Relation to Their Origin. Antioxidants 2022, 11, 433. [Google Scholar] [PubMed]
- Zeynep, A.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar]
- Beiranvand, S.; Williams, A.; Long, S.; Brooks, P.R.; Russell, F.D. Use of kinetic data to model potential antioxidant activity: Radical scavenging capacity of Australian Eucalyptus honeys. Food Chem. 2021, 342, 128332. [Google Scholar] [CrossRef]
- Campos, A.M.; Duran, N.; Lopez-Alarcon, C.; Lissi, E. Kinetic and stoichiometric evaluation of free radicals scavengers activities based on diphenyl-picryl hydrazyl (DPPH) consumption. J. Chil. Chem. Soc. 2012, 57, 1381–1384. [Google Scholar] [CrossRef] [Green Version]
- Barroso, R.P.; Berlim, L.S.; Ito, A.S.; Costa-Filho, A.J. In vitro antioxidant properties of golden grass (Syngonanthus nitens) by electron paramagnetic resonance. Food Sci. Nutr. 2019, 7, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Stasko, A.; Brezová, V.; Biskupic, S.; Misík, V. The potential pitfalls of using 1,1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents. Free Radic Res. 2007, 41, 379–390. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Snegureva, Y.; Budnikov, H. Novel approach for the voltammetric evaluation of antioxidant activity using DPPH -modified electrode. Electrochim. Acta 2017, 247, 97–106. [Google Scholar] [CrossRef]
- Tyurin, V.Y.; Moiseeva, A.A.; Shpakovsky, D.B.; Milaeva, E.R. The electrochemical approach to antioxidant activity assay of metal complexes with dipicolylamine ligand, containing 2,6-di-tert-butylphenol groups, based on electrochemical DPPH-test. J. Electroanal. Chem. 2015, 756, 212–221. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Albua, C.; Radoi, A.; Litescu, S.-C.; Radua, G.-L. Determination of the antiradical properties of olive oils using an electrochemical method based on DPPH radical. Food Chem. 2015, 166, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z. Electroanalysis: From Laboratory to Field Versions. J. Anal. Chem. 2001, 56, 303–312. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathologic Statesand Diseases; Siberian University Publishing: Novosibirsk, Russia, 2008; p. 534. [Google Scholar]
- Menschikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathological Conditions and Diseases; ARTA: Novosibirsk, Russia, 2008; p. 435. [Google Scholar]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley-Blackwell: Oxford, UK, 2018; p. 337. [Google Scholar]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. New antiradical capacity assay with the use potentiometric method. Anal. Chim. Acta 2019, 1046, 69–76. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. An integrated approach to the investigation of antioxidant properties by Potentiometry. Anal. Chim. Acta 2020, 1111, 83–91. [Google Scholar] [CrossRef]
- Gerasimova, E.; Gazizullina, E.; Radosteva, E.; Ivanova, A. Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins-Potentiometric Studies. Chemosensors 2021, 9, 112. [Google Scholar] [CrossRef]
- Gerasimova, E.L.; Gazizullina, E.R.; Borisova, M.; Igdisanova, D.I.; Nikiforov, E.A.; Moseev, T.D.; Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N.; Ivanova, A.V. Design and Antioxidant Properties of Bifunctional 2H-Imidazole-Derived Phenolic Compounds-A New Family of Effective Inhibitors for Oxidative Stress-Associated Destructive Processes. Molecules 2021, 26, 6534. [Google Scholar] [CrossRef]
- Athmouni, K.; Taheni, B.; Khaled, B.; Abdelfattah, E.F.; Habib, A. Effect of Solvent Polarity on the Content of Biomolecules and Antioxidant Activity of Thapsia Garganica (apiaceae). Alger. J. Nat. 2015, 3, 194–208. [Google Scholar]
- Shojaee, M.S.; Moeenfard, M.; Farhoosh, R. Kinetics and stoichiometry of gallic acid and methyl gallate in scavenging DPPH radical as affected by the reaction solvent. Sci. Rep. 2022, 12, 8765. [Google Scholar] [CrossRef]
- Zhuang, Q.-K.; Scholz, F.; Pragst, F. The voltammetric behaviour of solid 2,2-diphenyl-1-picrylhydrazyl (DPPH) microparticles. Electrochem. Commun. 1999, 1, 406–410. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.; Wang, D. Development of a Rapid Method for the Evaluation of DPPH Radical Scavenging Activity of Ginger (Zingiber officinale) Foods Based on Cyclic Voltammetry. Food Anal. Methods 2017, 10, 1419–1429. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Kravets, I.A.; Matern, A.I. Potentiometric determination of water-soluble antioxidants using metal complexes. J. Anal. Chem. 2015, 70, 173–177. [Google Scholar] [CrossRef]
- Angeli, L.; Imperiale, S.; Ding, Y.; Scampicchio, M.; Morozova, K. A Novel Stoichio-Kinetic Model for the DPPH• Assay: The Importance of the Side Reaction and Application to Complex Mixtures. Antioxidants 2021, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Iampanichakul, M.; Chaihongsa, N.; Poasakate, A.; Potue, P.; Rattanakanokchai, S.; Bunbupha, S.; Chiangsaen, P.; Pakdeechote, P. Butterfly Pea Flower (Clitoria ternatea Linn.) Extract Ameliorates Cardiovascular Dysfunction and Oxidative Stress in Nitric Oxide-Deficient Hypertensive Rats. Antioxidants 2021, 10, 523. [Google Scholar] [CrossRef] [PubMed]
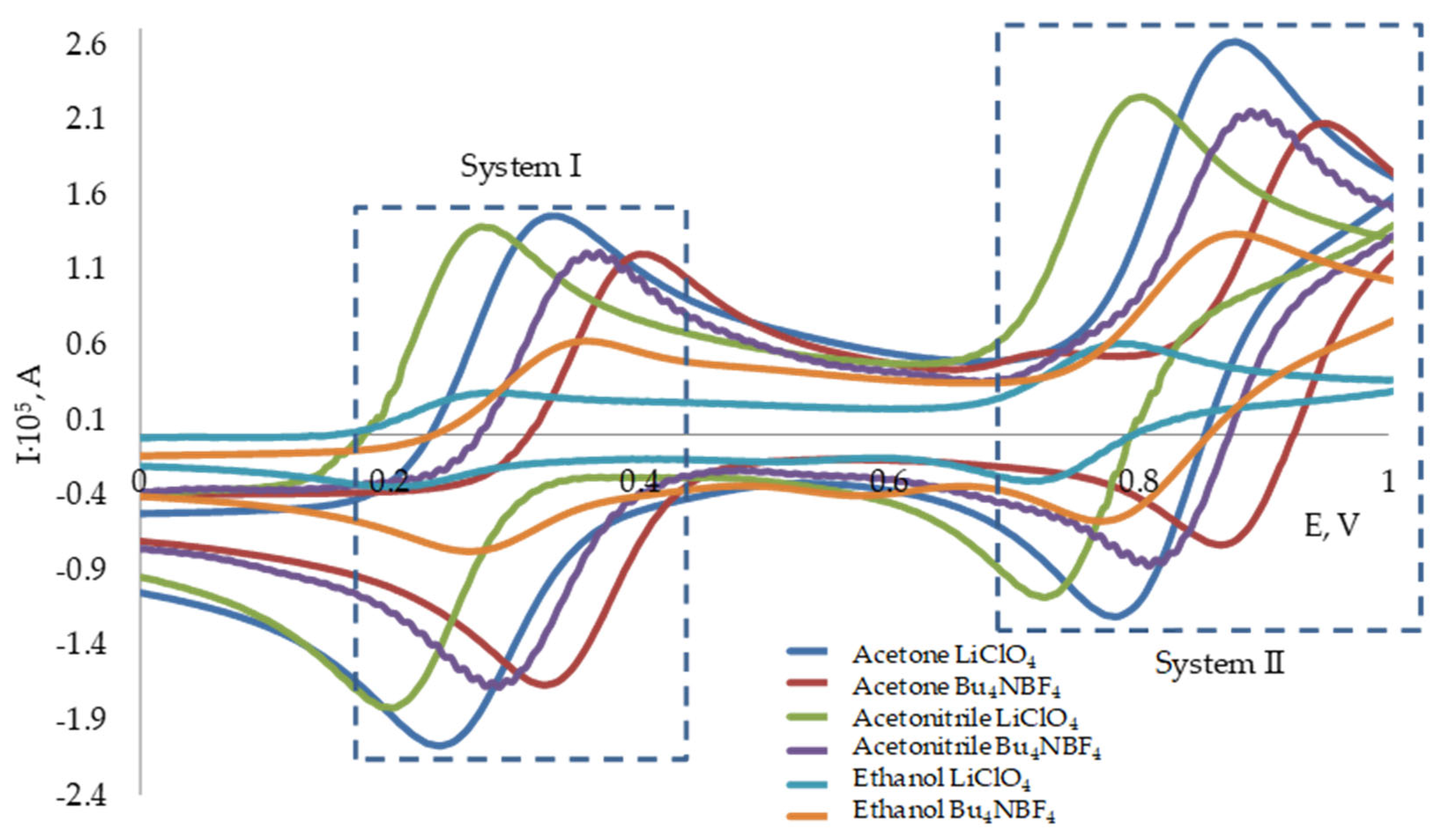



| Solvent | Electrolyte | System I | System II | IRed/IReOx | |||
|---|---|---|---|---|---|---|---|
| ERed, V | EReOx, V | ERed, V | EReOx, V | System I | System II | ||
| Ethanol | LiClO4 | 0.20 | 0.28 | 0.72 | 0.78 | 0.92 | 0.75 |
| Bu4NBF4 | 0.27 | 0.35 | 0.79 | 0.87 | 0.87 | 0.80 | |
| Acetone | LiClO4 | 0.24 | 0.32 | 0.79 | 0.87 | 1.11 | 0.95 |
| Bu4NBF4 | 0.32 | 0.40 | 0.87 | 0.94 | 1.12 | 1.05 | |
| Acetonitrile | LiClO4 | 0.20 | 0.27 | 0.72 | 0.79 | 1.13 | 0.98 |
| Bu4NBF4 | 0.28 | 0.36 | 0.81 | 0.88 | 1.11 | 0.93 | |
| AO | C(DPPH•), 10−4 mol/dm3 | Introduced, 10−5 mol/dm3 | Potentiometric Method | Spectroscopic Method | ||||
|---|---|---|---|---|---|---|---|---|
| AOCDPPH•, 10−5 mol-eq/dm3 | Sr,% | n * | Sr,% | n * | ||||
| α-tocopherol | 1 | 2 | 4.30 ± 0.61 | 0.06 | 2.2 | 4.10 ± 0.41 | 0.04 | 2.1 |
| 0.5 | 1 | 1.51 ± 0.22 | 0.05 | 1.5 | 1.71 ± 0.12 | 0.01 | 1.7 | |
| Quercetin | 1 | 2.0 | 5.10 ± 1.19 | 0.09 | 5.1 | 5.08 ± 0.79 | 0.06 | 5.1 |
| 0.5 | 1.0 | 2.40 ± 0.61 | 0.09 | 4.8 | 2.48 ± 0.16 | 0.08 | 5.0 | |
| (±)-Catechin hydrate | 1 | 1.5 | 5.67 ± 0.48 | 0.03 | 3.8 | 5.47 ± 0.18 | 0.07 | 3.6 |
| 0.5 | 1.5 | 3.02 ± 0.65 | 0.08 | 2.1 | 3.21 ± 0.09 | 0.07 | 2.1 | |
| α-Lipoic acid | 1 | 1.0 | 2.42 ± 0.55 | 0.08 | 2.4 | 2.54 ± 0.06 | 0.04 | 2.5 |
| AO | C(DPPH•), 10−3 mol/dm3 | Introduced, 10−4 mol/dm3 | AOCDPPH•, 10−4 mol-eq/dm3 | Sr,% | n * | b ** | Sr (b),% |
|---|---|---|---|---|---|---|---|
| α-Tocopherol | 0.5 | 0.5 | 3.46 ± 0.25 | 0.03 | 6.9 | 0.033 ± 0.007 | 0.08 |
| 1.0 | 2.63 ± 0.58 | 0.09 | 2.6 | 0.049 ± 0.009 | 0.11 | ||
| 2.0 | 3.53 ± 0.14 | 0.02 | 1.8 | 0.053 ± 0.004 | 0.03 | ||
| 1 | 3.0 | 6.63 ± 0.96 | 0.05 | 2.2 | 0.038 ± 0.007 | 0.07 | |
| Quercetin | 0.5 | 0.7 | 3.90 ± 0.51 | 0.05 | 5.6 | 0.052 ± 0.006 | 0.06 |
| 1.0 | 3.13 ± 0.14 | 0.06 | 3.1 | 0.051 ± 0.001 | 0.01 | ||
| 1.5 | 4.90 ± 0.05 | 0.01 | 3.2 | 0.081 ± 0.009 | 0.07 | ||
| 1 | 2.0 | 8.97 ± 0.58 | 0.02 | 4.5 | 0.028 ± 0.006 | 0.07 | |
| 3.0 | 9.40 ± 0.43 | 0.02 | 3.1 | 0.042 ± 0.005 | 0.05 | ||
| (±)-Catechin hydrate | 0.5 | 1.0 | 3.30 ± 0.25 | 0.03 | 3.3 | 0.073 ± 0.004 | 0.02 |
| 2.0 | 4.10 ± 0.61 | 0.08 | 2.1 | 0.051 ± 0.008 | 0.10 | ||
| 1 | 1.5 | 8.09 ± 1.25 | 0.07 | 5.4 | 0.037 ± 0.004 | 0.02 | |
| 2.0 | 4.10 ± 0.50 | 0.05 | 2.1 | 0.050 ± 0.006 | 0.08 | ||
| α-Lipoic acid | 1 | 1 | 3.72 ± 0.58 | 0.06 | 3.3 | 0.024 ± 0.002 | 0.02 |
| 0.5 | 1 | 2.38 ± 0.51 | 0.08 | 2.4 | 0.030 ± 0.001 | 0.01 | |
| 0.7 | 1.72 ± 0.39 | 0.08 | 2.4 | 0.030 ± 0.006 | 0.05 | ||
| 0.5 | 0.96 ± 0 | 0 | 2.0 | 0.040 ± 0 | 0 |
| Objects | Analyzed Extract | Potentiometric Method | Spectroscopic Method | ||
|---|---|---|---|---|---|
| AOCDPPH•, 10−2 mol-eq/g | Sr | mol-eq/g | Sr | ||
| Calendula (Calenduale flores) | Water | 1.1 ± 0.1 | 0.06 | 1.1 ± 0.1 | 0.06 |
| Water-ethanol | 1.2 ± 0.1 | 0.04 | 1.2 ± 0.2 | 0.08 | |
| Motherwort herb (Herba Leonuri) | Water | 0.9 ± 0.2 | 0.10 | 0.9 ± 0.1 | 0.04 |
| Water-ethanol | 2.4 ± 0.1 | 0.02 | 2.1 ± 0.3 | 0.06 | |
| Birch leaves (Folia Betulae) | Water | 1.2 ± 0.3 | 0.09 | 1.2 ± 0.1 | 0.02 |
| Water-ethanol | 2.8 ± 0.4 | 0.06 | 2.9 ± 0.2 | 0.03 | |
| St. John’s wort (Hyperici herba) | Water | 1.9 ± 0.4 | 0.09 | 1.8 ± 0.4 | 0.09 |
| Water-ethanol | 3.0 ± 0.4 | 0.05 | 2.9 ± 0.1 | 0.02 | |
| Oregano herb (Herbia Origanum vulgare) | Water | 4.5 ± 0.4 | 0.04 | 4.2 ± 0.1 | 0.02 |
| Water-ethanol | 10.2 ± 0.4 | 0.02 | 10.0 ± 0.1 | 0.02 | |
| Peppermint leaves (Menthae piperitae folia) | Water | 2.7 ± 0.1 | 0.02 | 2.7 ± 0.1 | 0.02 |
| Water-ethanol | 3.0 ± 0.1 | 0.02 | 3.1 ± 0.1 | 0.03 | |
| Hibiscus (Hibiscus sabdariffa L.) | Water | 3.4 ± 0.2 | 0.05 | 2.6 ± 0.2 | 0.06 |
| Water-ethanol | 4.2 ± 0.2 | 0.04 | 3.7 ± 0.2 | 0.03 | |
| Clitoria ternatea (Clitoria ternatea) | Water | 2.8 ± 0.2 | 0.02 | - | |
| Water-ethanol | 3.6 ± 0.2 | 0.02 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, E.; Gazizullina, E.; Kolbaczkaya, S.; Ivanova, A. The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl. Antioxidants 2022, 11, 1974. https://doi.org/10.3390/antiox11101974
Gerasimova E, Gazizullina E, Kolbaczkaya S, Ivanova A. The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl. Antioxidants. 2022; 11(10):1974. https://doi.org/10.3390/antiox11101974
Chicago/Turabian StyleGerasimova, Elena, Elena Gazizullina, Sofya Kolbaczkaya, and Alla Ivanova. 2022. "The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl" Antioxidants 11, no. 10: 1974. https://doi.org/10.3390/antiox11101974
APA StyleGerasimova, E., Gazizullina, E., Kolbaczkaya, S., & Ivanova, A. (2022). The Novel Potentiometric Approach to Antioxidant Capacity Assay Based on the Reaction with Stable Radical 2,2′-diphenyl-1-picrylhydrazyl. Antioxidants, 11(10), 1974. https://doi.org/10.3390/antiox11101974







