Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts
Abstract
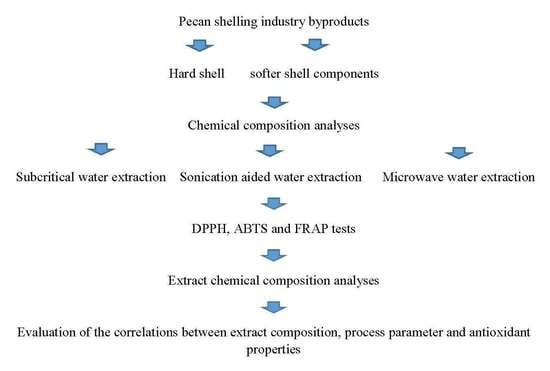
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Pecan Shell Samples
2.1.2. Reagents
2.2. Methods
2.2.1. Proximate Composition of Pecan Shells
2.2.2. Extraction
2.2.3. Chemical Composition of Shell Extracts
2.2.4. Antioxidant Capacity Tests
DPPH Radical Scavenging Capacity
Total Phenolic Content
Ferric Reducing Capacity (FRAP)
ABTS Assay
2.3. Statistical Analyses
2.4. Principal Component Analysis (PCA)
3. Results and Discussion
3.1. Chemical Composition of Byproduct Streams
3.2. Antioxidant Properties of Pecan Nut Shell Extracts
3.3. Composition of Phenolic Compounds in Pecan Nut Shell Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. Qualified Health Claims: Letter of Enforcement Discretion-Nuts and Coronory Heart Disease. Available online: http://www.cfsan.fda.gov/~dms/qhcnuts2.html (accessed on 3 June 2021).
- FDA. Dietary Guidelines for Americans 2015–2020. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 7 January 2022).
- do Prado, A.C.P.; Aragão, A.M.; Fettand, R.; Block, J.M. Antioxidant properties of Pecan nut [Carya illinoinensis (Wangenh.) C. Koch] Shell infusion. Grasas Y Aceites 2009, 60, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Sevimli-Gur, C.; Gezgin, Y.; Oz, A.; Sharqi, S.A.; Gumus, Z.P.; Dunford, N.T. Biological Activity of the Extracts from Pecan Shelling Industry Byproducts. Trans. ASABE 2021, 64, 869–877. [Google Scholar] [CrossRef]
- Robbins, K.S.; Gong, Y.; Wells, M.L.; Greenspan, P.; Pegg, R.B. Investigation of the antioxidant capacity and phenolic constituents of U.S. pecans. J. Funct. Foods 2015, 15, 11–22. [Google Scholar] [CrossRef]
- Benvegnu, D.; Barcelos, R.C.S.; Boufleur, N.; Reckziegel, P.; Pase, C.S.; Muller, L.G.; Martins, N.M.B.; Vareli, C.; Burger, M.E. Protective Effects of a By-Product of the Pecan Nut Industry (Carya illinoensis) on the Toxicity Induced by Cyclophosphamide in Rats Carya illinoensis Protects Against Cyclophosphamide- Induced Toxicity. J. Environ. Pathol. Toxicol. Oncol. 2010, 29, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.G.; Pase, C.S.; Reckziegel, P.; Barcelos, R.C.S.; Boufleur, N.; Prado, A.C.P.; Fett, R.; Block, J.M.; Pavanato, M.A.; Bauermann, L.F.; et al. Hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Exp. Toxicol. Pathol. 2013, 65, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Salama, M.M.; Abd-elrahman, E.H.; El-Maraghy, S.A. Antidiabetic activity of phenolic compounds from Pecan bark in streptozotocin-induced diabetic rats. Phytochem. Lett. 2011, 4, 337–341. [Google Scholar] [CrossRef]
- Sims, K.A. Mechanization of Post-Harvest Pecan Processing. In Pecan Technology; Santerre, C.R., Ed.; Springer: Boston, MA, USA, 1994; pp. 68–86. [Google Scholar] [CrossRef]
- NFTA. Nitrogen Determination by Combustion Method; National Forage Testing Association: Lincoln, NE, USA, 2007. [Google Scholar]
- AACC. American Association of Cereal Chemists. Approved Methods; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Agricultural Chemists: Rockville, MD, USA, 2005. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cason, C.; Yemmireddy, V.K.; Moreira, J.; Adhikari, A. Antioxidant Properties of Pecan Shell Bioactive Components of Different Cultivars and Extraction Methods. Foods 2021, 10, 713. [Google Scholar] [CrossRef]
- Hilbig, J.; Policarpi, P.d.B.; Grinevicius, V.M.A.d.S.; Mota, N.S.R.S.; Toaldo, I.M.; Luiz, M.T.B.; Pedrosa, R.C.; Block, J.M. Aqueous extract from pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell show activity against breast cancer cell line MCF-7 and Ehrlich ascites tumor in Balb-C mice. J. Ethnopharmacol. 2018, 211, 256–266. [Google Scholar] [CrossRef]
- Prado, A.C.P.d.; Manion, B.A.; Seetharaman, K.; Deschamps, F.C.; Barrera Arellano, D.; Block, J.M. Relationship between antioxidant properties and chemical composition of the oil and the shell of pecan nuts [Carya illinoinensis (Wangenh) C. Koch]. Ind. Crops Prod. 2013, 45, 64–73. [Google Scholar] [CrossRef]
- Prado, A.C.P.; Aragão, A.M.; Fett, R.; Block, J.M. Phenolic compounds and antioxidant activity of pecan [Carya illinoinensis (Wangenh.) C. Koch] shell extracts. Braz. J. Food Technol. 2009, 12, 323–332. [Google Scholar] [CrossRef]
- do Prado, A.C.P.; da Silva, H.S.; da Silveira, S.M.; Barreto, P.L.M.; Vieira, C.R.W.; Maraschin, M.; Ferreira, S.R.S.; Block, J.M. Effect of the extraction process on the phenolic compounds profile and the antioxidant and antimicrobial activity of extracts of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell. Ind. Crops Prod. 2014, 52, 552–561. [Google Scholar] [CrossRef]
- Reckziegel, P.; Boufleur, N.; Barcelos, R.C.S.; Benvegnú, D.M.; Pase, C.S.; Muller, L.G.; Teixeira, A.M.; Zanella, R.; Prado, A.C.P.; Fett, R.; et al. Oxidative stress and anxiety-like symptoms related to withdrawal of passive cigarette smoke in mice: Beneficial effects of pecan nut shells extract, a by-product of the nut industry. Ecotoxicol. Environ. Saf. 2011, 74, 1770–1778. [Google Scholar] [CrossRef]
- Ribas, L.E.; Baravalle, M.E.; Gasser, F.B.; Renna, M.S.; Addona, S.; Ortega, H.H.; Savino, G.H.; Van de Velde, F.; Hein, G.J. Extraction of phenolic compounds from the shells of pecan nuts with cytotoxic activity through apoptosis against the colon cancer cell line HT-29. J. Food Sci. 2021, 86, 5409–5423. [Google Scholar] [CrossRef] [PubMed]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Firestone, D. Physical and Chemical Characteristics of Oils, Fats and Waxes; AOCS Press: Champaign, IL, USA, 1999; p. 152. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between Free Radical Scavenging and Antioxidant Activity in Foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Analysis of Botanicals and Dietary Supplements for Antioxidant Capacity: A Review. J. AOAC Int. 2000, 83, 950–956. [Google Scholar] [CrossRef] [Green Version]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Tyrakowska, B.; Soffers, A.E.M.F.; Szymusiak, H.; Boeren, S.; Boersma, M.G.; Lemańska, K.; Vervoort, J.; Rietjens, I.M.C.M. TEAC antioxidant activity of 4-hydroxybenzoates. Free Radic. Biol. Med. 1999, 27, 1427–1436. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]




| Time (min) | A (%) | B (%) | C (%) |
|---|---|---|---|
| 0 | 96 | 2 | 2 |
| 40 | 50 | 25 | 25 |
| 45 | 40 | 30 | 30 |
| 50 | 0 | 50 | 50 |
| 52 | 0 | 50 | 50 |
| 55 | 96 | 2 | 2 |
| Compound | Retention Time (min) | CE | R2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Ferulic Acid | 28.574 | y = 13.899x − 2.0306 | 0.9999 | 0.27 | 0.90 |
| Vanilic Acid | 21.167 | y = 32.748x − 0.8276 | 0.9999 | 0.15 | 0.49 |
| Ellagic Acid | 31.205 | y = 10.283x − 2.2246 | 0.9999 | 0.20 | 0.66 |
| Gallic Acid | 9.146 | y = 51.982x − 11.556 | 0.9999 | 0.17 | 0.57 |
| Caffeic Acid | 21.873 | y = 17.314x + 2.1885 | 0.9999 | 0.27 | 0.92 |
| Thymol | 51.379 | y = 13.732x + 1.3354 | 0.9997 | 0.25 | 0.83 |
| Taxifolin | 29.443 | y = 37.837x − 13.664 | 0.9998 | 0.17 | 0.56 |
| Catechin | 18.603 | y = 9.4667x + 1.9891 | 0.9999 | 0.27 | 0.91 |
| Syringic Acid | 22.102 | y = 55.622x + 8.7233 | 0.9999 | 0.11 | 0.35 |
| p-coumaric acid | 27.073 | y = 54.957x + 6.2524 | 0.9999 | 0.17 | 0.58 |
| Protocatechuic acid | 14.074 | y = 24.798x − 1.9328 | 0.9998 | 0.20 | 0.66 |
| Extraction/Solvent | Water | Aqueous Ethanol | Reference |
|---|---|---|---|
| TPC (mg GAE/g extract) | 37.3–444.1 | 153.5–581.9 | [3,5,7,8,15,16,17,18,19,20,21] |
| DPPH (% inhibition) | 46.1–86.8 | 79.4–90.8 | [19], This work |
| DPPH (Trolox Equivalent, μmol/g extract) | 346.6–1268.0 | 524.8–1287.1 | [15,16,20] |
| ABTS (Trolox Equivalent, μmol/g extract) | 368.3–5390.4 | 1562.5–2573.0 | [3,7,18,19] This work |
| FRAP (Trolox Equivalent, μmol/g extract) | 4267.2–5800.2 | - | This work |
| Sample | Oil (as Is) | Oil (Cleaned) | Protein (as is) | Protein (Cleaned) | Ash (as Is) | Ash (Cleaned) | Moisture (as Is) | Moisture (Cleaned) |
|---|---|---|---|---|---|---|---|---|
| Native-F (Bristow, OK) | 12.9 ± 0.4 a | 7.1 ± 0.4 a | 3.5 ± 0.2 a | 2.4 ± 0.3 a | 1.3 ± 0.07 a | 1.5 ± 0.04 a | 16.01 ± 0.06 a | 15.13 ± 0.06 a |
| Native-S (Bristow, OK) | 2.8 ± 0.1 b | 0.72 ± 0.07 b | 1.9 ± 0.1 b | 1.81 ± 0.01 b | 1.73 ± 0.02 b | 1.801 ± 0.007 b | 12.82 ± 0.03 b | 13.1 ± 0.2 b |
| Sample | DPPH (% Inhibition) | TPC (mg GAE/g Extract) | ABTS (Trolox Equivalent, μmol/g Extract) | FRAP (Trolox Equivalent, μmol/g Extract) |
|---|---|---|---|---|
| F150 | 46.1 ± 0.3 f | 259.8 ± 2.7 h | 4920.6 ± 0.9 i | 4494.0 ± 3.8 j |
| F125 | 76.9 ± 1.3 cd | 349.0 ± 3.2 c | 5205.0 ± 3.5 e | 5217.6 ± 1.9 d |
| F100 | 81.4 ± 1.6 abc | 385.5 4.3 b | 5345.6 ± 0.9 b | 5800.2 ± 5.0 a |
| F80 | 86.8 ± 0.8 a | 444.1 ± 4.3 a | 5390.4 ± 2.3 a | 5653.0 ± 1.1 b |
| S150 | 33.7 ± 1.3 g | 284.2 ± 3.4 fg | 5053.8 ± 1.8 g | 5495.2 ± 2.1 c |
| S125 | 66.7 ± 2.1 e | 223.3 ± 4.8 i | 5131.9 ± 2.7 f | 5018.8 ± 0.1 e |
| S100 | 76.8 ± 0.4 cd | 294.3 ± 4.2 f | 5195.6 ± 4.4e | 4907.0 ± 1.1 f |
| S80 | 77.6 ± 0.2 bcd | 276.8 ± 3.8 g | 5253.8 ± 1.8 d | 4754.2 ± 2.9 h |
| FSON | 82.4 ± 1.1 abc | 333.7 ± 3.2 d | 5338.1 ± 0.8 b | 4880.6 ± 0.9 g |
| SSON | 74.1 ± 4.1 d | 310.4 ± 0.2 e | 5041.2 ± 3.6 h | 4267.2 ± 2.2 k |
| FMIC | 83.5 ± 0.8 ab | 362.1 1.7 c | 5288.1 ± 0.9 c | 4693.1 ± 4.4 i |
| SMIC | 78.3 ± 1.3 bcd | 287.8 ± 1.7 fg | 4069.5 ± 2.5 j | 4198.8 ± 3.5 l |
| Sample | Protocatechuic | Gallic | Catechin | Vanillic | Caffeic | Syringic | Ferulic | Taxifolin | Elagic | Thymol |
|---|---|---|---|---|---|---|---|---|---|---|
| F150 | 3.7 ± 0.3 f | 1.6 ± 0.6 h | 1.5 ± 0.1 f | 4.4 ± 0.2 e | 2.9 ± 0.3 f | 1.6 ± 0.1 gh | 8.8 ± 0.2 e | n.d. | 6.5 ± 1.9 i | n.d. |
| F125 | 3.87 ± 0.02 f | 20.3 ± 0.5 bc | 25.5 ± 0.3 a | 4.4 ± 0.2 e | 5.7 ± 0.2 b | 0.6 ± 0.1 j | 19.1 ± 0.5 b | 8.25 ± 0.04 b | 87.3 ± 0.1 b | 0.15 ± 0.01 cd |
| F100 | 7.7 ± 0.1 d | 12.6 ± 0.1 e | 25.2 ± 0.8 a | 4.401 ± 0.001 e | 3.5 ± 0.1 ef | 1.070 ± 0.001 i | n.d. | 4.9 ± 0.2 g | 96.0 ± 1.4 a | 0.28 ± 0.03 bc |
| F80 | 4.5 ± 0.2 f | 12.8 ± 0.2 e | 21.4 ± 0.5 b | 4.1 ± 0.1 ef | 4.4 ± 0.1 d | 0.490 ± 0.001 j | 7.85 ± 0.08 e | 5.49 ± 0.02 f | 74.7 ± 0.5 c | n.d. |
| FSON | 0.83 ± 0.05 h | 9.1± 0.5 g | 16.269 ± 0.001 cd | 3.998 ± 0.001 ef | 3.3 ± 0.2 ef | 1.268 ± 0.001 hi | n.d. | 6.20 ± 0.02 e | 50.5 ± 5.2 ef | n.d. |
| FMIC | 1.72 ± 0.06 g | 15.8 ± 0.3 d | 21.7 ± 0.6 b | 3.8 ± 0.1 f | 4.5 ± 0.2 cd | 1.786 ± 0.001 g | n.d. | 6.4 ± 0.1 d | 57.5 ± 1.2 de | n.d. |
| S150 | 17.8 ± 0.2 a | 41.6 ± 1.1 a | 24.8 ± 0.1 a | 12.031 ± 0.001 a | 3.706 ± 0.001 e | 10.7 ± 0.2 a | 8.1 ± 0.2 e | 2.21 ± 0.01 i | 51.8 ± 1.2 e | n.d. |
| S125 | 7.92 ± 0.09 d | 14.35 ± 0.01 de | 17.7 ± 0.4 c | 6.6 ± 0.2 c | 5.3 ± 0.2 b | 4.430 ± 0.001 d | 6.21 ± 0.04 f | 2.82 ± 0.03 h | 43.7 ± 1.0 f | n.d. |
| S100 | 10.1 ± 0.5 b | 21.9 ± 0.3 b | 21.6 ± 0.7 b | 7.7 ± 0.1 b | 5.3 ± 0.1 b | 8.2 ± 0.1 b | 14.4 ± 0.3 c | 7.61 ± 0.05 c | 59.7 ± 0.6 d | n.d. |
| S80 | 6.10 ± 0.05 e | 10.9 ± 0.1 f | 17.4 ± 0.4 cd | 6.8 ± 0.2 c | 5.274 ± 0.001 b | 2.6 ± 0.1 f | 13.1 ± 0.6 d | 4.98 ± 0.01 g | 35.3 ± 0.2 g | n.d. |
| SSON | 6.6 ± 0.2 e | 10.51 ± 0.07 fg | 11.3 ± 0.2 ed | 6.1 ± 0.1 d | 7.1 ± 0.3 a | 3.010 ± 0.001 e | 20.8 ± 0.3 a | 9.30 ± 0.01 a | 25.6 ± 0.2 h | 0.5 ± 0.2 a |
| SMIC | 8.93 ± 0.01 c | 19.4 ± 0.3 c | 15.6 ± 0.7 d | 8.2 ± 0.2 b | 5.1 ± 0.1 bc | 5.3 ± 0.1 c | 15.2 ± 0.4 c | 9.16 ± 0.05 a | 53.2 ± 0.2d e | 0.41 ± 0.01 ab |
| Phenolic Compound | DPPH | ABTS | FRAP |
|---|---|---|---|
| Gallic | 0.406 | 0.025 | 0.001 |
| Protocatechuic | 0.501 | −0.016 | −0.227 |
| Catechin | 0.499 | 0.350 | 0.533 |
| Vanillic | 0.341 | −0.207 | −0.452 |
| Caffeic | 0.602 | 0.06 | −0.342 |
| Syringic | 0.274 | −0.104 | −0.373 |
| Ferulic | 0.329 | −0.239 | −0.613 |
| Taxifolin | 0.347 | −0.191 | 0.020 |
| Thymol | 0.361 | −0.343 | −0.147 |
| Ellagic | 0.414 | 0.255 | 0.714 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunford, N.T.; Gumus, Z.P.; Gur, C.S. Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants 2022, 11, 1127. https://doi.org/10.3390/antiox11061127
Dunford NT, Gumus ZP, Gur CS. Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants. 2022; 11(6):1127. https://doi.org/10.3390/antiox11061127
Chicago/Turabian StyleDunford, Nurhan Turgut, Zinar Pinar Gumus, and Canan Sevimli Gur. 2022. "Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts" Antioxidants 11, no. 6: 1127. https://doi.org/10.3390/antiox11061127
APA StyleDunford, N. T., Gumus, Z. P., & Gur, C. S. (2022). Chemical Composition and Antioxidant Properties of Pecan Shell Water Extracts. Antioxidants, 11(6), 1127. https://doi.org/10.3390/antiox11061127