Tea Tree Oil Mediates Antioxidant Factors Relish and Nrf2-Autophagy Axis Regulating the Lipid Metabolism of Macrobrachium rosenbergii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Materials, Design, and Growth Evaluation
- WGR (%) = (Final weight − Initial weight)/initial weight × 100
- SGR(%/day) = (Ln final weight − Ln initial weight) × 100/days
- FCR = Dry feed intake (g)/weight gain (g)
- HSI (%) = (Hepatopancreas weight/ final weight) × 100
2.3. Samples Collection
2.4. Sample Analysis
2.4.1. Antioxidant, Peroxide, and Lipid Parameters Analysis
2.4.2. ROS Levels
2.4.3. Oil Red O Staining, H&E Stainings, and Transmission Electron Microscopy
2.4.4. Haemolymph Biochemical Parameters
2.5. Real-Time Quantitative PCR (qPCR)
2.6. Western Blot Analysis
2.7. Data Analysis
3. Results
3.1. Growth Evaluation
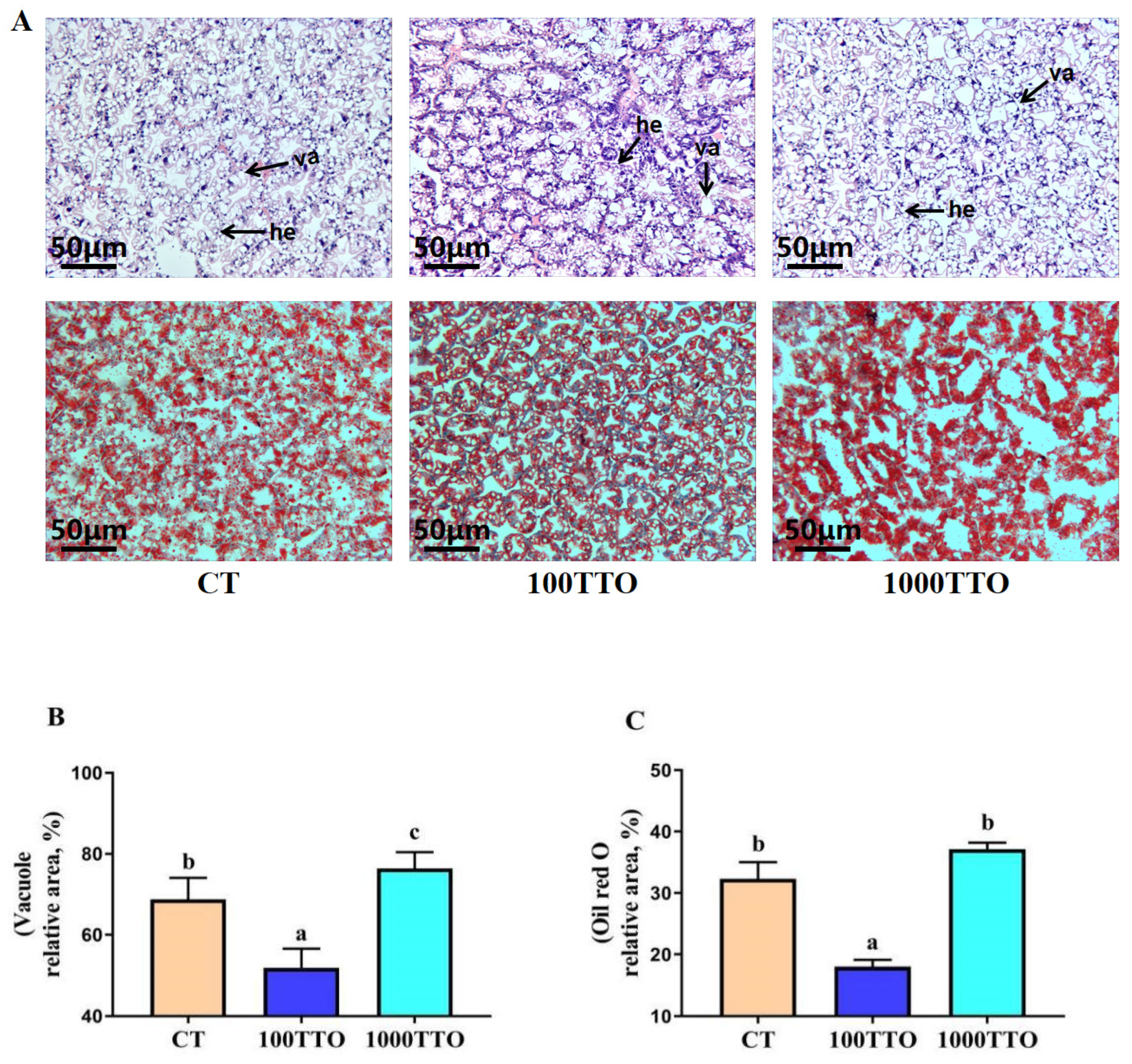
3.2. Morphological Characteristics of Hepatopancreas
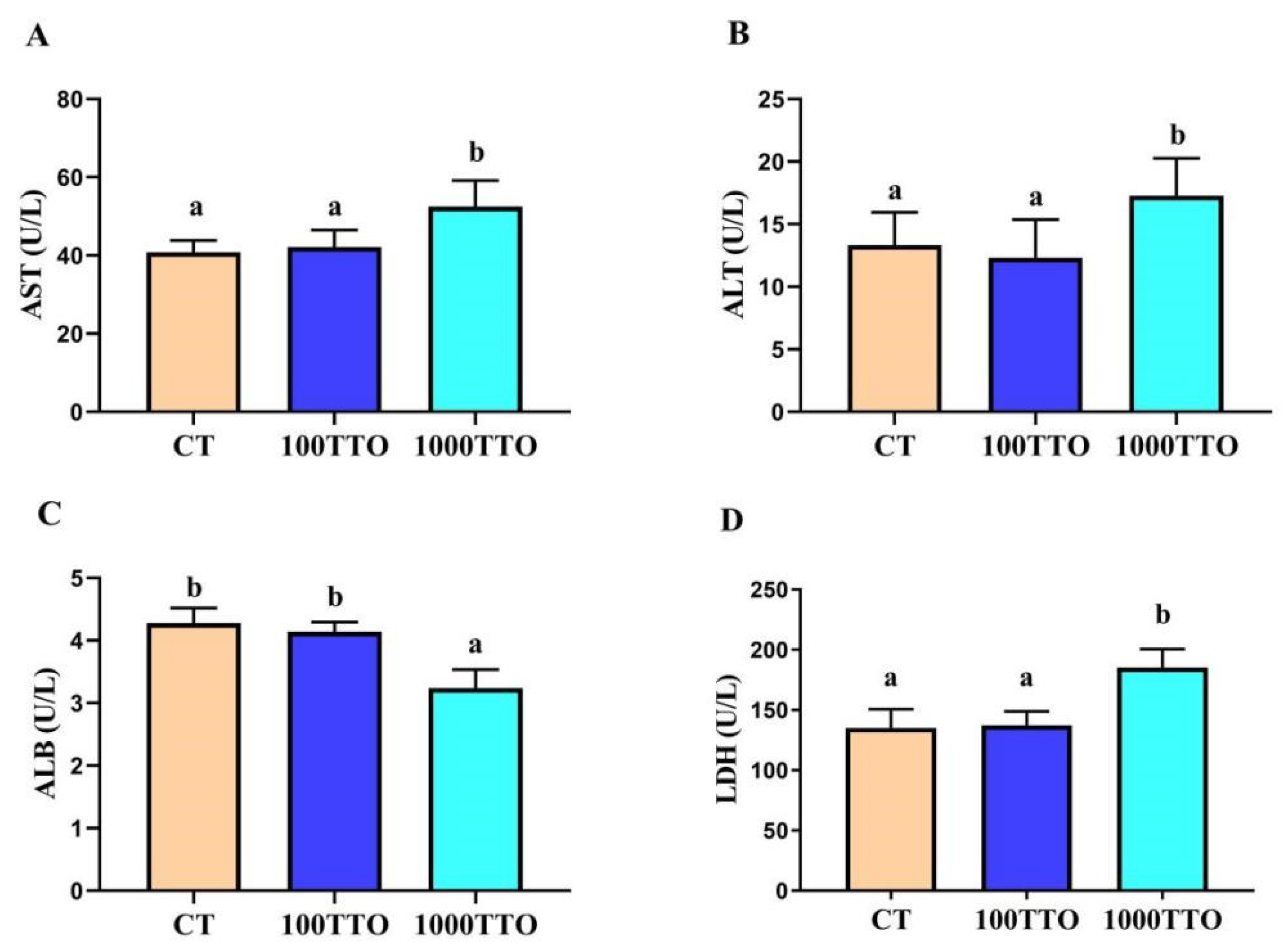
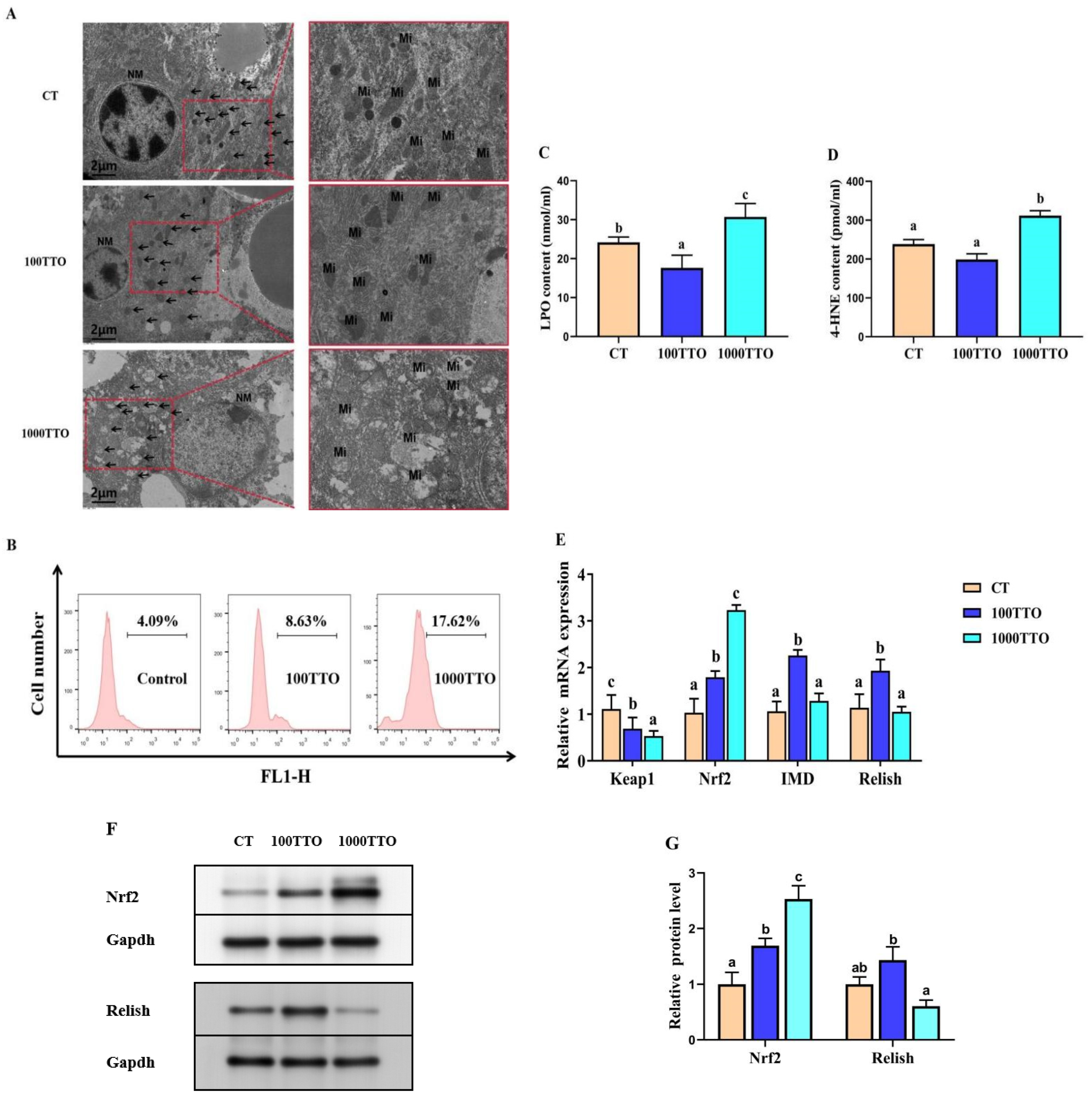
3.3. Hepatopancreatic Cell Function
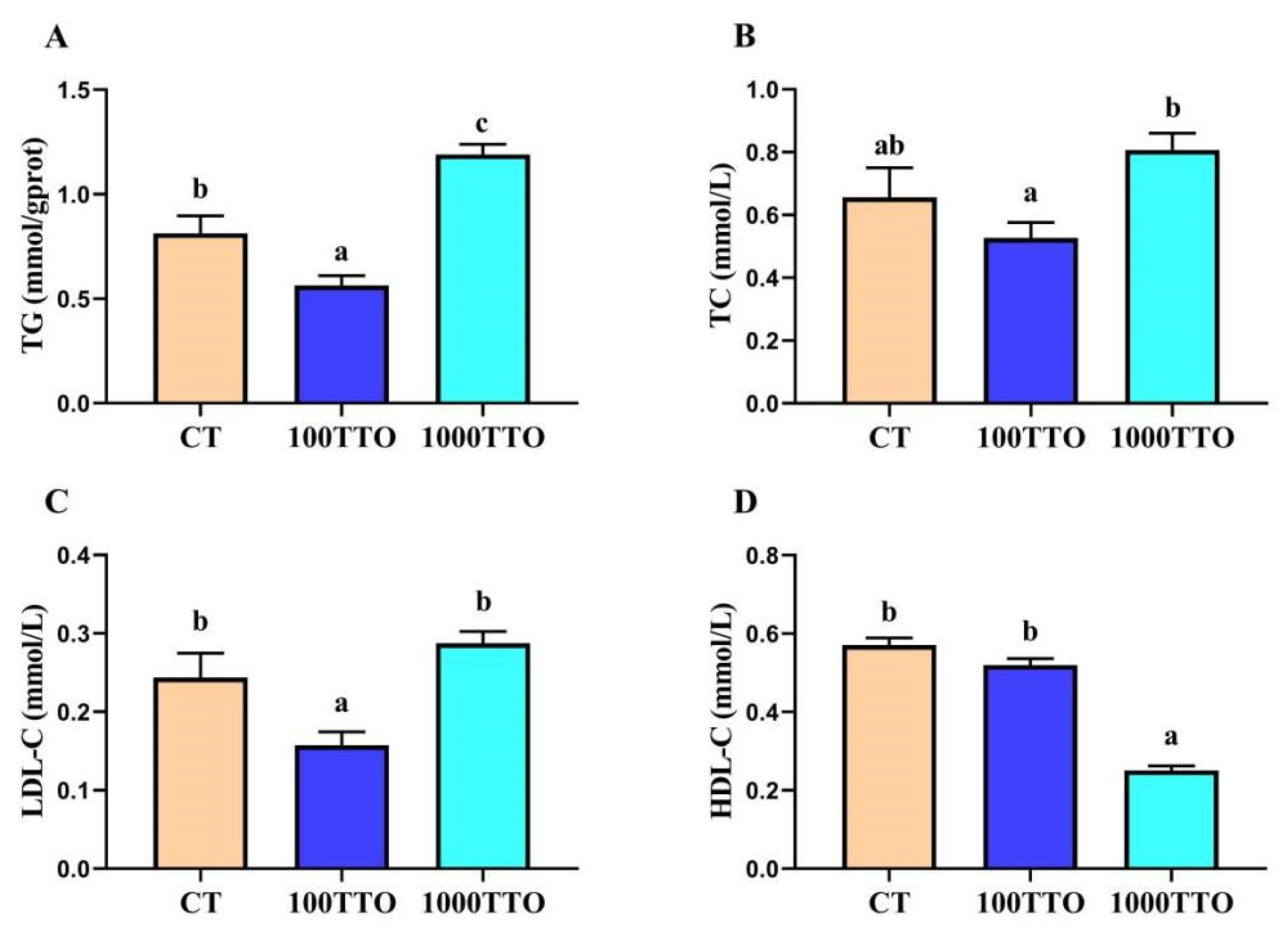
3.4. Lipid Contents in Hepatopancreas
3.5. Lipid Metabolism Enzymes Activities and Gene Expression
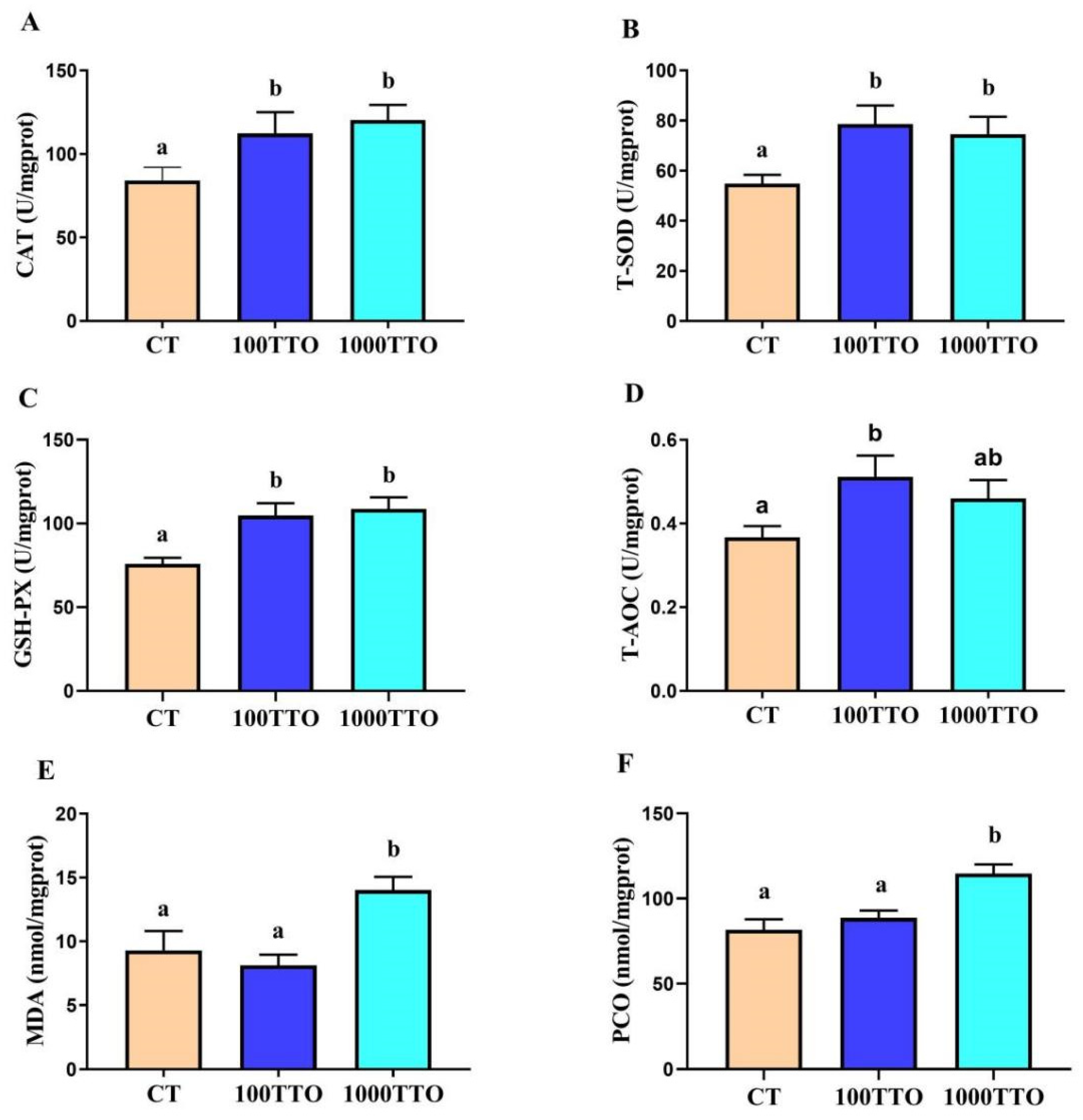
3.6. Antioxidant Enzyme Activity and Peroxidase Levels in Hepatopancreas
3.7. Different TTO Levels Affect Oxidative Stress in the Hepatopancreas
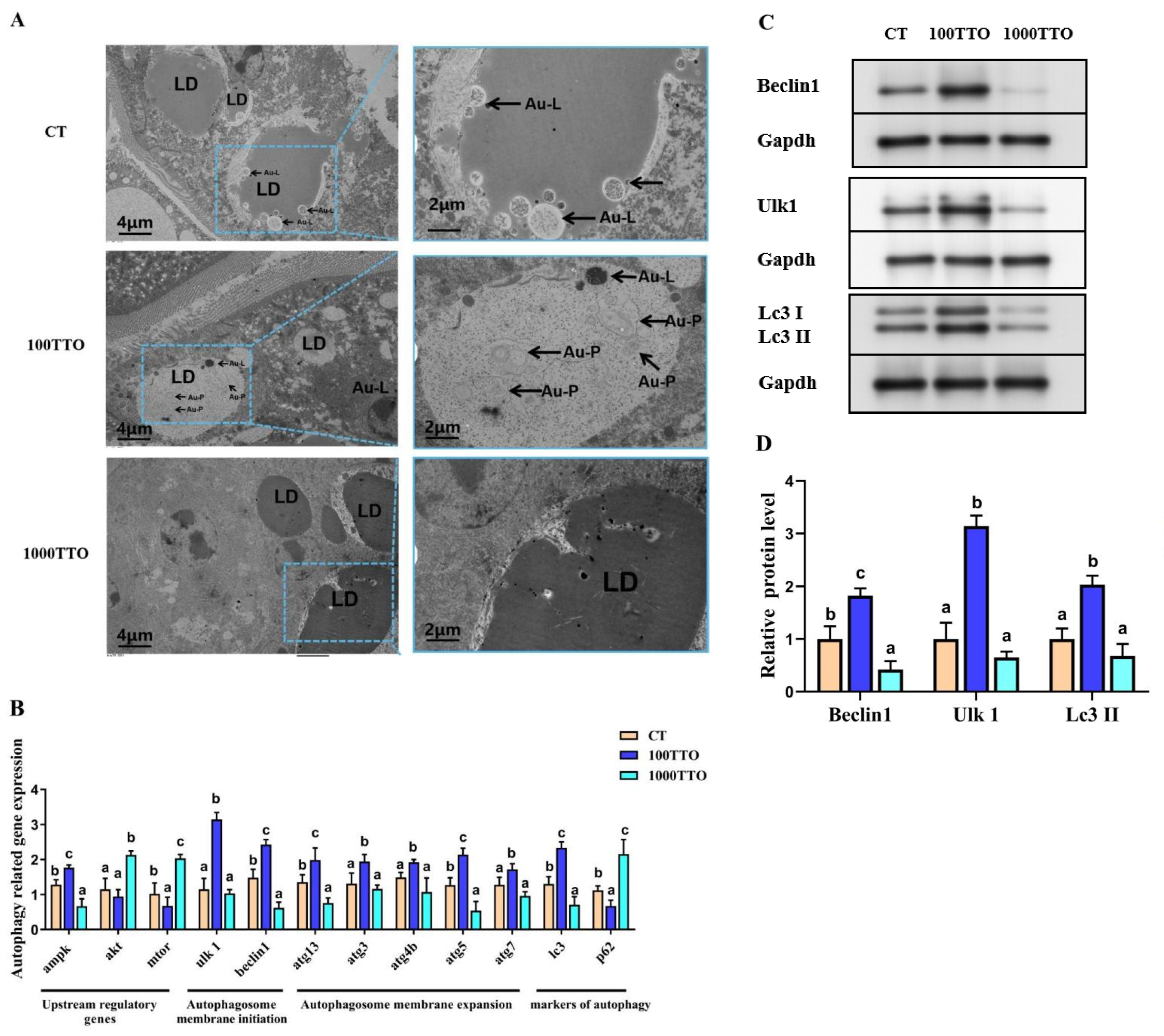
3.8. Autophagy-Related Genes and EM Observation
3.9. Correlation Analysis
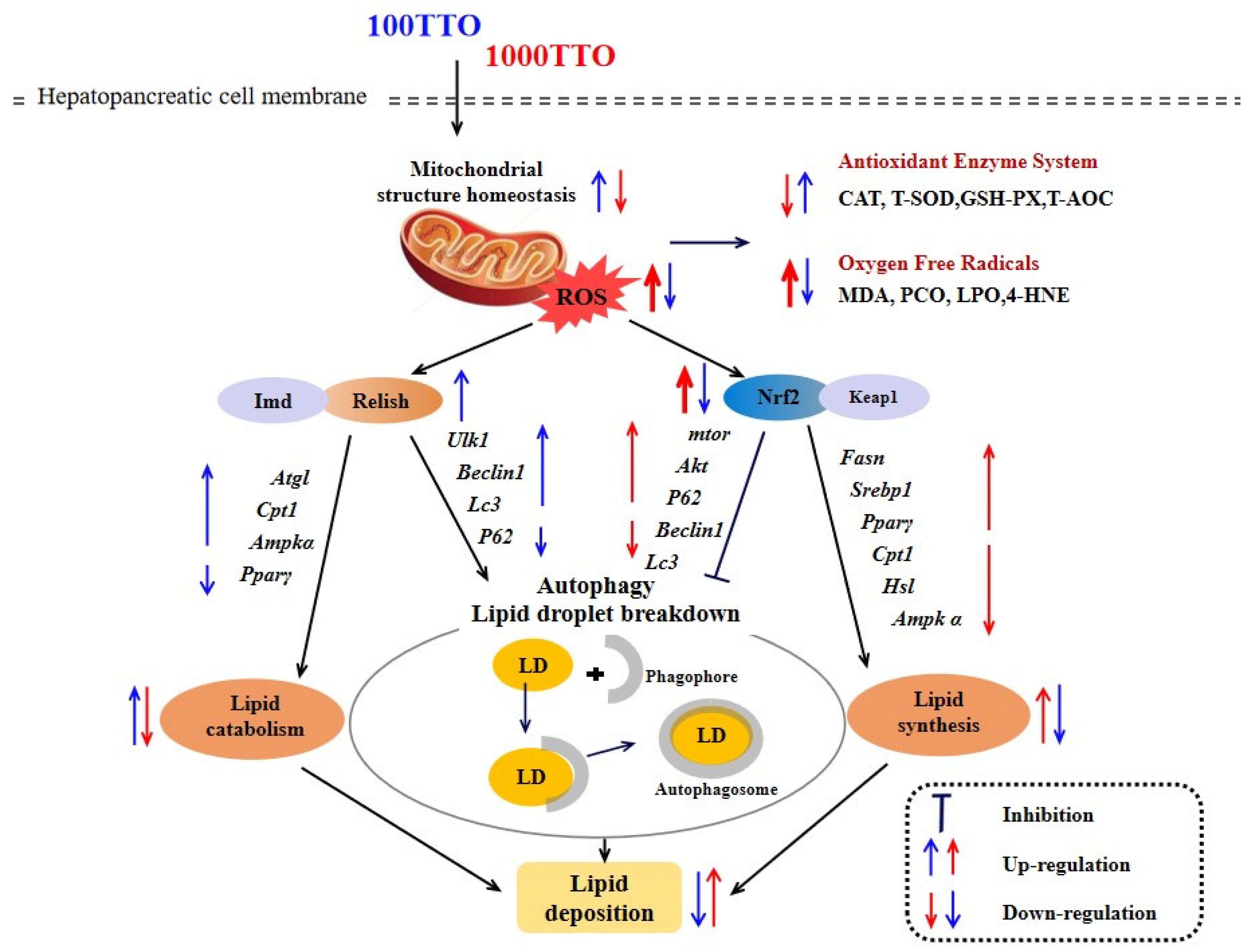
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, L.; Naylor, R.; Henriksson, P.; Leadbitter, D.; Metian, M.; Troell, M.; Zhang, W.B. China’s aquaculture and the world’s wild fisheries. Science 2015, 347, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Catacutan, M.R.; Coloso, R.M. Growth of juvenile Asian seabass, Lates calcarifer, fed varying carbohydrate and lipid levels. Aquaculture 1997, 149, 137–144. [Google Scholar] [CrossRef]
- Ruan, G.L.; Li, S.X.; He, N.J.; Liu, F.; Wang, Q. Short-term adaptability to non-hyperthermal stress: Antioxidant, immune and gut microbial responses in the red swamp crayfish, Procambarus clarkii. Aquaculture 2022, 560, 738497. [Google Scholar] [CrossRef]
- Huang, Q.C.; You, J.F.; Wang, X.D.; Song, Y.; Zhang, C.; Du, Z.Y.; Shi, Q.C.; Qin, J.G.; Chen, L.Q. Effect of vitamin A supplement on the growth performance, antioxidant status, and lipid accumulation of Chinese mitten crab Eriocheir Sinensis fed different lipid levels. Aquaculture 2022, 554, 738123. [Google Scholar] [CrossRef]
- Vogt, G. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zoology 2021, 147, 125945. [Google Scholar] [CrossRef]
- Ishida, N.; Yamada, H.; Hirose, M. Euphausia pacifica (North Pacific Krill): Review of Chemical Features and Potential Benefits of 8-HEPE against Metabolic Syndrome, Dyslipidemia, NAFLD, and Atherosclerosis. Nutrients 2021, 13, 3765. [Google Scholar] [CrossRef]
- Shi, B.; Lu, J.J.; Hu, X.Y.; Betancor, M.B.; Zhao, M.M.; Tocher, D.R. Dietary copper improves growth and regulates energy generation by mediating lipolysis and autophagy in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2021, 537, 736505. [Google Scholar] [CrossRef]
- Yu, D.; Chen, G.; Pan, M.; Zhang, J.; He, W.; Liu, Y.; Nian, X.; Sheng, L.; Xu, B. High fat diet-induced oxidative stress blocks hepatocyte nuclear factor 4 alpha and leads to hepatic steatosis in mice. J. Cell Physiol. 2018, 236, 4770–4782. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, K.; Hogstrand, C.; Xu, Y.H.; Chen, G.H.; Wei, C.C.; Luo, Z. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell. Mol. Life Sci. 2020, 77, 1987–2003. [Google Scholar] [CrossRef]
- Gummersbach, C.; Hemmrich, K.; Kröncke, K.D.; Suschek, C.V.; Fehsel, K.; Pallua, N. New aspects of adipogenesis: Radicals and oxidative stress. Differentiation 2009, 77, 115–120. [Google Scholar] [CrossRef]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo–controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011, 34, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Meng, S.; Li, Y.; Ghebre, Y.T.; Cooke, J.P. Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 2016, 15, 919. [Google Scholar] [CrossRef] [Green Version]
- Kapuy, O.; Papp, D.; Vellai, T.; Bánhegyi, G.; Korcsmáros, T. Systems-Level Feedbacks of NRF2 Controlling Autophagy upon Oxidative Stress Response. Antioxidants 2018, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Stewart, D.; Killeen, E.; Naquin, R.; Alam, S.; Alam, J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003, 278, 2396–2402. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Liu, B.; Shan, F.; Liu, B.; Gu, Z.M.; Song, C.Y.; Sun, C.X.; Zhou, Q.L. Effects of oxidized fish oil on digestive enzyme activity and antioxidant system in Macrobrachium rosenbergii post-larvae. Aquacult. Rep. 2022, 23, 101062. [Google Scholar] [CrossRef]
- Huang, Y.X.; Li, Q.; Yuan, Y.H.; Zhang, Z.Q.; Jiang, B.J.; Yang, S.P.; Jian, J.C. Silencing of Nrf2 in Litopenaeus vannamei, decreased the antioxidant capacity, and increased apoptosis and autophagy. Fish Shellfish Immunol. 2022, 122, 257–267. [Google Scholar] [CrossRef]
- Meng, H.M.; Song, J.; Fan, B.Q.; Li, Y.Q.; Zhang, J.J.; Yu, J.P.; Zheng, Y.; Wang, M. Monascus vinegar alleviates high-fat-diet-induced inflammation in rats by regulating the NF-KB and PI3K/AKT/mTOR pathways. Food Sci. Hum. Wellness 2022, 11, 943–953. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2010, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- An, P.P.; Yang, X.B.; Yu, J.; Qi, J.R.; Ren, X.Y.; Kong, Q.J. α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Liu, M.Y.; Gao, Q.; Sun, C.; Liu, B.; Liu, X.; Zhou, Q.; Liu, B.; Xu, P. Effects of dietary tea tree oil on the growth, physiological and non-specific immunity response in the giant freshwater prawn (Macrobrachium rosenbergii) under high ammonia stress. Fish Shellfish Immunol. 2022, 120, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, J.; Zuo, A.; Yao, L. Research on the Effects of Rosemary (Rosmarinus officinalisL.) on the Blood Lipids and Anti-lipid Peroxidation in Rats. J. Essent. Oil Res. 2011, 23, 26–34. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gomis, J.; Peres Rubio, C.; Martínez Conesa, C.; Otal Salaverri, J.; Cerón, J.J.; Tortosa, D.E.; Pablo, M.J.C. Effects of Dietary Supplementation of Garlic and Oregano Essential Oil on Biomarkers of Oxidative Status, Stress and Inflammation in Postweaning Piglets. Animals 2020, 10, 2093. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.; Li, J.; Liu, F.; Xu, Y.; Huang, X.; Ni, H. Superiority of Microencapsulated Essential Oils Compared With Common Essential Oils and Antibiotics: Effects on the Intestinal Health and Gut Microbiota of Weaning Piglet. Front. Nutr. 2022, 8, 808106. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, J.K.; Park, S.Y. AMPK Activation Mediated by Hinokitiol Inhibits Adipogenic Differentiation of Mesenchymal Stem Cells through Autophagy Flux. Int. J. Endocrinol. 2018, 2018, 2014192. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Carroll, B.; Buganim, Y.; Maetzel, D.; Ng, A.H.M.; Cassady, J.P.; Cohen, M.A.; Chakraborty, S.; Wang, H.Y.; Spooner, E.; et al. Impaired Autophagy in the Lipid-Storage Disorder Niemann-Pick Type C1 Disease. Cell Rep. 2013, 5, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.; Sun, C.; Xu, P.; Liu, B.; Zheng, X.C.; Liu, B.; Zhou, Q.L. Effects of dietary tea tree (Melaleuca alternifolia) oil and feeding patterns on the zootechnical performance and nonspecific immune response of the giant freshwater prawn (Macrobrachium rosenbergii). J. World Aquacult. Soc. 2021, 53, 542–557. [Google Scholar] [CrossRef]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Sun, C.X.; Shan, F.; Liu, M.Y.; Liu, B.; Zhou, Q.L.; Zheng, X.C.; Xu, X.D. High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-kappa B/NO Signal Pathway and the Amelioration of Vitamin E. Antioxidants 2022, 11, 228. [Google Scholar] [CrossRef]
- Liu, M.Y.; Sun, C.X.; Zheng, X.C.; Zhou, Q.L.; Liu, B.; Zhou, Y.F.; Xu, P.; Liu, B. Comparative Proteomic Analysis Revealed the Mechanism of Tea Tree Oil Targeting Lipid Metabolism and Antioxidant System to Protect Hepatopancreatic Health in Macrobrachium rosenbergii. Front. Immunol. 2022, 13, 906435. [Google Scholar] [CrossRef]
- Dai, Y.J.; Liu, W.B.; Abasubong, K.P.; Zhang, D.D.; Li, X.F.; Xiao, K.; Wang, X.; Jiang, G.Z. The Mechanism of Lipopolysaccharide Escaping the Intestinal Barrier in Megalobrama amblycephala Fed a High-Fat Diet. Front. Nutr. 2022, 9, 853409. [Google Scholar] [CrossRef]
- Li, G.; Chang, L.; Zhang, G.; Song, Z.; Wan, D.; Xie, C.; Wang, H.; Fan, Z.Y. Oral administration of dibutyryl adenosine cyclophosphate improved growth performance in weaning piglets by enhancing lipid fatty acids metabolism. Anim. Nutr. 2018, 4, 260–264. [Google Scholar] [CrossRef]
- Tripolino, C.; Irace, C.; Cutruzzolà, A.; Parise, M.; Barone, M.; Scicchitano, C.; Cortese, C.; Gnasso, A. Hepatic Steatosis Index Is Associated with Type 1 Diabetes Complications. Diabetes Metab. Syndr. Obes. 2019, 12, 2405–2410. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cai, C.; Cao, X.; Zhu, J.; He, J.; Wu, P.; Ye, Y. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture 2018, 483, 230–237. [Google Scholar] [CrossRef]
- Tian, J.; Lei, C.; Ji, H.; Kaneko, G.; Zhou, J.; Yu, H.; Li, Y.; Yu, E.M.; Xie, J. Comparative analysis of effects of dietary arachidonic acid and EPA on growth, tissue fatty acid composition, antioxidant response and lipid metabolism in juvenile grass carp, Ctenopharyngodon idellus. Br. J. Nutr. 2017, 118, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Li, J.N.; Zhang, Y.Y.; Wu, D.; Zheng, X.H.; Wang, C.; Wang, L.S. Excessive Dietary Lipid Affecting Growth Performance, Feed Utilization, Lipid Deposition, and Hepatopancreas Lipometabolism of Large-Sized Common Carp (Cyprinus carpio). Front. Nutr. 2021, 8, 694426. [Google Scholar] [CrossRef] [PubMed]
- Nadella, R.K.; Prakash, R.R.; Dash, G.; Ramanathan, S.K.; Kuttanappilly, L.V.; Mothadaka, M.P. Histopathological changes in giant freshwater prawn Macrobrachium rosenbergii (de Man 1879) fed with probiotic Bacillus licheniformis upon challenge with Vibrio alginolyticus. Aquac. Res. 2017, 49, 81–92. [Google Scholar] [CrossRef]
- Yao, J.; Hu, P.; Zhu, Y.; Xu, Y.; Tan, Q.; Liang, X. Lipid-Lowering Effects of Lotus Leaf Alcoholic Extract on Serum, Hepatopancreas, and Muscle of Juvenile Grass Carp via Gene Expression. Front. Physiol. 2020, 11, 584782. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Fu, L.; Du, H.; Jin, C.; Xu, Z. Changes in Growth Performance, Metabolic Enzyme Activities, and Content of Fe, Cu, and Zn in Liver and Kidney of Tilapia (Oreochromis niloticus) Exposed to Dietary Pb. Biol. Trace Elem. Res. 2008, 128, 176–183. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Song, X.; Teng, P.; Ji, H.; Peng, L.; Qiu, Y.; Guo, D.; Jiang, S. Effect of maduramicin on crayfish (Procambius clarkii): Hematological parameters, oxidative stress, histopathological changes and stress response. Ecotox. Environ. Saf. 2021, 211, 111896. [Google Scholar] [CrossRef]
- Vincent, J.L. Relevance of albumin in modern critical care medicine. Best Pract. Res. Clin. Anaesthesiol. 2009, 23, 183–191. [Google Scholar] [CrossRef]
- Wilentz, R.E.; Witters, L.A.; Pizer, E.S. Lipogenic Enzymes Fatty Acid Synthase and Acetyl-Coenzyme A Carboxylase Are Coexpressed with Sterol Regulatory Element Binding Protein and Ki-67 in Fetal Tissues. Pediatr. Devel. Pathol. 2000, 3, 525–531. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijon, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.; Glode, L.M.; Eckel, R.H. Lipid Catabolism via CPT1 as a Therapeutic Target for Prostate Cancer. Mol. Cancer Ther. 2014, 13, 2361–2371. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Nemoto, M.; Gelman, L.; Geffroy, S.; Najib, J.; Fruchart, J.C. The Human Fatty Acid Transport Protein-1 (SLC27A1; FATP-1) cDNA and Gene: Organization, Chromosomal Localization, and Expression. Genomics 2000, 66, 296–304. [Google Scholar] [CrossRef]
- Xu, W.F.; Hou, L.; Li, P.; Li, L. Effect of nicotinamide N-methyltransferase on lipid accumulation in 3T3-L1 adipocytes. Bioengineered 2022, 13, 12421–12434. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, D.F. Effect of polyene phosphatidyl choline on hepatocyte steatosis via PPARalpha/CPT-1A pathway. Chin. J. Hepatol. 2016, 24, 291–296. [Google Scholar] [CrossRef]
- Diskin, T.; Tal-Or, P.; Erlich, S.; Mizrachy, L.; Alexandrovich, A.; Shohami, E. Closed Head Injury Induces Upregulation of Beclin 1 at the Cortical Site of Injury. J. Neurotraum. 2005, 22, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Chatterjee, C. Purinergic Signaling, Dyslipidemia and Inflammatory Disease. Cell. Physiol. Biochem. 2012, 30, 1333–1339. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Signorelli, P.; Pivari, F.; Barcella, M.; Merelli, I.; Zulueta, A.; Dei Cas, M.; Rosso, L.; Ghidoni, R.; Caretti, A.; Paroni, R. Myriocin modulates the altered lipid metabolism and storage in cystic fibrosis. Cell. Signal. 2021, 81, 109928. [Google Scholar] [CrossRef]
- Tatsumi, T.; Takayama, K.; Ishii, S.; Yamamoto, A.; Hara, T.; Minami, N.; Miyasaka, N.; Kubota, T.; Matsuura, A.; Itakura, E.; et al. Forced lipophagy reveals that lipid droplets are required for early embryonic development in mouse. Development 2018, 145, dev161893. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Cederbaum, A. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules 2015, 5, 2659–2674. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Zhang, X.; Liu, M.; Liu, X.; Dong, M.; Cheng, J.; Zhang, X.Y.; Zhai, C.G.; Song, Y.; Lu, H.X.; et al. Corrigendum: Ginsenoside Rb1 Enhances Atherosclerotic Plaque Stability by Improving Autophagy and Lipid Metabolism in Macrophage Foam Cells. Front. Pharmacol. 2018, 8, 964. [Google Scholar] [CrossRef]
- Allavena, G.; Boyd, C.; Oo, K.S.; Maellaro, E.; Zhivotovsky, B.; Kaminskyy, V.O. Suppressed translation and ULK1 degradation as potential mechanisms of autophagy limitation under prolonged starvation. Autophagy 2016, 12, 2085–2097. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.J.; Wang, H.; Dong, Q.T.; Wang, T.J.; Qian, H.Y.; Xu, H. Autophagy Activation: A Novel Mechanism of Atorvastatin to Protect Mesenchymal Stem Cells from Hypoxia and Serum Deprivation via AMP-Activated Protein Kinase/Mammalian Target of Rapamycin Pathway. Stem Cells Devel. 2012, 21, 1321–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Kim, D.; Wise, J.T.F.; Shi, X.; Zhang, Z.; DiPaola, R.S. p62 as a therapeutic target for inhibition of autophagy in prostate cancer. Prostate 2018, 78, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.X.; Xu, M.X.; Qin, Y.T.; Gu, T.T.; Feng, J.; Lv, J.X.; Wang, S.J.; Ma, Y.J.; Lou, D.S.; Li, Q.; et al. Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: A mechanism involving Toll-like receptor 4 and oxidative stress. Free Radical Biol. Med. 2019, 134, 23–41. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef]
- Brand, C.; Ferrante, A.; Prager, R.H. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef]
- Haribabu, A.; Reddy, V.S.; Pallavi, C.; Bitla, A.R.; Sachan, A.; Pullaiah, P.; Suresh, V.; Rao, P.; Suchitra, M.M. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine 2012, 44, 152–157. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Du, X.; Yao, J.; He, F.; Niu, X.; Wang, G.Q.; Zhang, D.M. Effects of dietary astaxanthin on the growth, innate immunity and antioxidant defence system of Paramisgurnus dabryanus. Aquacult. Nutr. 2020, 26, 1453–1462. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmström, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. BBA-Gen. Subj. 2015, 1850, 794–801. [Google Scholar] [CrossRef]
- Pan, Y.X.; Luo, Z.; Zhuo, M.Q.; Wei, C.C.; Chen, G.H.; Song, Y.F. Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat. Toxicol. 2018, 199, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.S.; Wu, X.Z. Characterization of a Rel\NF-κB homologue in a gastropod abalone, Haliotis diversicolor supertexta. Dev. Comp. Immunol. 2007, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, L.; Zhang, H.; Zheng, P.; Zhao, J.; Qiu, L.; Zhang, Y.; Song, L. Molecular cloning and expression of a Relish gene in Chinese mitten crab Eriocheir sinensis. Int. J. Immunogenet. 2010, 37, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Fernandes-Ferreira, M.; Piper, P.; Sousa, M.J. Mentha piperitaessential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial ROS-mediated damage. FEMS Yeast Res. 2014, 14, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapeinos, C.; Larrañaga, A.; Sarasua, J.R.; Pandit, A. Functionalised collagen spheres reduce H2O2 mediated apoptosis by scavenging overexpressed ROS. Nanomed-Nanotechnol. 2018, 14, 2397–2405. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Singh, R. Autophagy and lipid droplets in the liver. Annu. Rev. Nutr. 2015, 35, 215–237. [Google Scholar] [CrossRef]
- Huang, J.; Tabbi-Anneni, I.; Gunda, V.; Wang, L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1211–G1221. [Google Scholar] [CrossRef] [Green Version]
- Nigro, D.; Menotti, F.; Cento, A.S.; Serpe, L.; Chiazza, F.; Dal Bello, F.; Romaniello, F.; Medana, C.; Aragno, M. Chronic administration of saturated fats and fructose differently affect SREBP activity resulting in different modulation of Nrf2 and Nlrp3 inflammasome pathways in mice liver. J. Nutr. Biochem. 2017, 42, 160–171. [Google Scholar] [CrossRef]
- Dong, L.; Han, X.; Tao, X.; Xu, L.; Xu, Y.; Fang, L.; Yin, L.H.; Qi, Y.; Li, H.; Peng, J.Y. Protection by the Total Flavonoids from Rosa laevigata Michx Fruit against Lipopolysaccharide-Induced Liver Injury in Mice via Modulation of FXR Signaling. Foods 2018, 7, 88. [Google Scholar] [CrossRef]
- Wakai, E.; Aritake, K.; Urade, Y.; Fujimori, K. Prostaglandin D-2 enhances lipid accumulation through suppression of lipolysis via DP2 (CRTH2) receptors in adipocytes. Biochem. Biophys. Res. Commun. 2017, 490, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.F.; Tang, K.K.; Wang, Z.Y.; Wang, L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology 2021, 464, 152999. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Z.Q.; Li, X.; Yang, X.R.; Zhao, J.; Guo, J.; Lu, W.F.; Liu, H.Y.; Wang, J. FTO Alleviates CdCl2-Induced Apoptosis and Oxidative Stress via the AKT/Nrf2 Pathway in Bovine Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 4948. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.Y.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.H.; Chen, K.; Li, B.; Chen, J.W.; Zheng, X.F.; Wang, Y.R.; Jiang, S.D.; Jiang, L.S. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER–ERK–mTOR pathway. Apoptosis 2013, 18, 1363–1375. [Google Scholar] [CrossRef]
- Nandy, A.; Lin, L.; Velentzas, P.D.; Wu, L.P.; Baehrecke, E.H.; Silverman, N. The NF-kappa B Factor Relish Regulates Atg1 Expression and Controls Autophagy. Cell Rep. 2018, 25, 2110. [Google Scholar] [CrossRef] [Green Version]
- Vucicevic, L.; Misirkic-Marjanovic, M.; Paunovic, V.; Kravic-Stevovic, T.; Martinovic, T.; Ciric, D.; Maric, N.; Petricevic, S.; Harhaji-Trajkovic, L.; Bumbasirevic, V.; et al. Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy 2014, 10, 2362–2378. [Google Scholar] [CrossRef]
- Wan, Q.; Kang, W.Q.; Zhu, Q.; Zhang, P.J.; Liu, Y. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKα. Exp. Mol. Med. 2007, 39, 535–543. [Google Scholar]
- Feng, Y.M.; Pathria, G.; Heynen-Genel, S.; Jackson, M.; James, B.; Yin, J.; Scott, D.A.; Ronai, Z.A. Identification and Characterization of IMD-0354 as a Glutamine Carrier Protein Inhibitor in Melanoma. Mol. Cancer Ther. 2021, 20, 816–832. [Google Scholar] [CrossRef]
- Jung, T.Y.; Ryu, J.E.; Jang, M.M.; Lee, S.Y.; Jin, G.R.; Kim, C.W.; Lee, C.Y.; Kim, H.; Kim, E.; Park, S.; et al. Naa20, the catalytic subunit of NatB complex, contributes to hepatocellular carcinoma by regulating the LKB1-AMPK-mTOR axis. Exp. Mol. Med. 2020, 52, 1831–1844. [Google Scholar] [CrossRef]
- Gadang, V.; Gilbert, W.; Hettiararchchy, N.; Horax, R.; Katwa, L.; Devareddy, L. Dietary Bitter Melon Seed Increases Peroxisome Proliferator-Activated Receptor-γ Gene Expression in Adipose Tissue, Down-Regulates the Nuclear Factor-κB Expression, and Alleviates the Symptoms Associated with Metabolic Syndrome. J. Med. Food 2010, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (g kg−1) | CT | 100TTO | 1000TTO |
|---|---|---|---|
| Fish meal 1 | 750.00 | 750.00 | 750.00 |
| Chellocken meal 1 | 150.00 | 150.00 | 150.00 |
| Blood globulin powder 1 | 60.00 | 60.00 | 60.00 |
| Shrimp meal 1 | 240.00 | 240.00 | 240.00 |
| Soybean meal 2 | 570.00 | 570.00 | 570 |
| Repeseed meal 2 | 300.00 | 300.00 | 300 |
| Shrimp meal 1 | 240.00 | 240.00 | 240.00 |
| Squid extract 1 | 90.00 | 90.00 | 90.00 |
| Soybean oil 2 | 60.00 | 60.00 | 60.00 |
| Fish oil 2 | 60.00 | 60.00 | 60.00 |
| α-starch 1 | 600.00 | 600.00 | 600.00 |
| Soy lecithin oil 1 | 30.00 | 30.00 | 30.00 |
| Ecdysone 1 | 0.30 | 0.30 | 0.30 |
| MCP | 60.00 | 60.00 | 60.00 |
| Premix 3 | 30.00 | 30.00 | 30.00 |
| Choline chloride 4 | 30.00 | 30.00 | 30.00 |
| Bentonite 4 | 29.70 | 29.40 | 26.70 |
| 10% Tea Tree Oil | 0.00 | 3.00 | 30.00 |
| Total | 3000.00 | 3005.40 | 3054.00 |
| Proximate analysis (%) | |||
| Moisture | 10.40 | 9.48 | 9.68 |
| Crude protein | 40.91 | 39.46 | 40.29 |
| Crude lipid | 9.76 | 9.06 | 9.54 |
| Ash | 17.43 | 16.52 | 16.87 |
| Index | Groups | ||
|---|---|---|---|
| CT | 100TTO | 1000TTO | |
| Survival rate (%) | 82.50 b ± 3.23 | 85.00 b ± 5.50 | 72.50 a ± 4.62 |
| Initial weight (g) | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.00 |
| Final weight (g) | 2.84 ± 0.10 a | 3.05 ± 0.14 ab | 3.44 ± 0.44 b |
| Weight gain rate (%) | 1839.82 ± 51.39 a | 1942.06 ± 117.08 ab | 2130.23 ± 107.98 b |
| Specific growth rate (%/day) | 6.18 ± 0.05 a | 6.27 ± 0.12 a | 6.42 ± 0.26 b |
| Feed conversion ratio | 1.33 ± 0.04 b | 1.15 ± 0.01 a | 1.26 ± 0.04 b |
| Hepatopancreas index (%) | 7.23 ± 1.06 b | 5.73 ± 0.86 a | 10.73 ± 0.42 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zheng, X.; Sun, C.; Zhou, Q.; Liu, B.; Xu, P. Tea Tree Oil Mediates Antioxidant Factors Relish and Nrf2-Autophagy Axis Regulating the Lipid Metabolism of Macrobrachium rosenbergii. Antioxidants 2022, 11, 2260. https://doi.org/10.3390/antiox11112260
Liu M, Zheng X, Sun C, Zhou Q, Liu B, Xu P. Tea Tree Oil Mediates Antioxidant Factors Relish and Nrf2-Autophagy Axis Regulating the Lipid Metabolism of Macrobrachium rosenbergii. Antioxidants. 2022; 11(11):2260. https://doi.org/10.3390/antiox11112260
Chicago/Turabian StyleLiu, Mingyang, Xiaochuan Zheng, Cunxin Sun, Qunlan Zhou, Bo Liu, and Pao Xu. 2022. "Tea Tree Oil Mediates Antioxidant Factors Relish and Nrf2-Autophagy Axis Regulating the Lipid Metabolism of Macrobrachium rosenbergii" Antioxidants 11, no. 11: 2260. https://doi.org/10.3390/antiox11112260






