Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
2.2. Cell Lines and Reagents
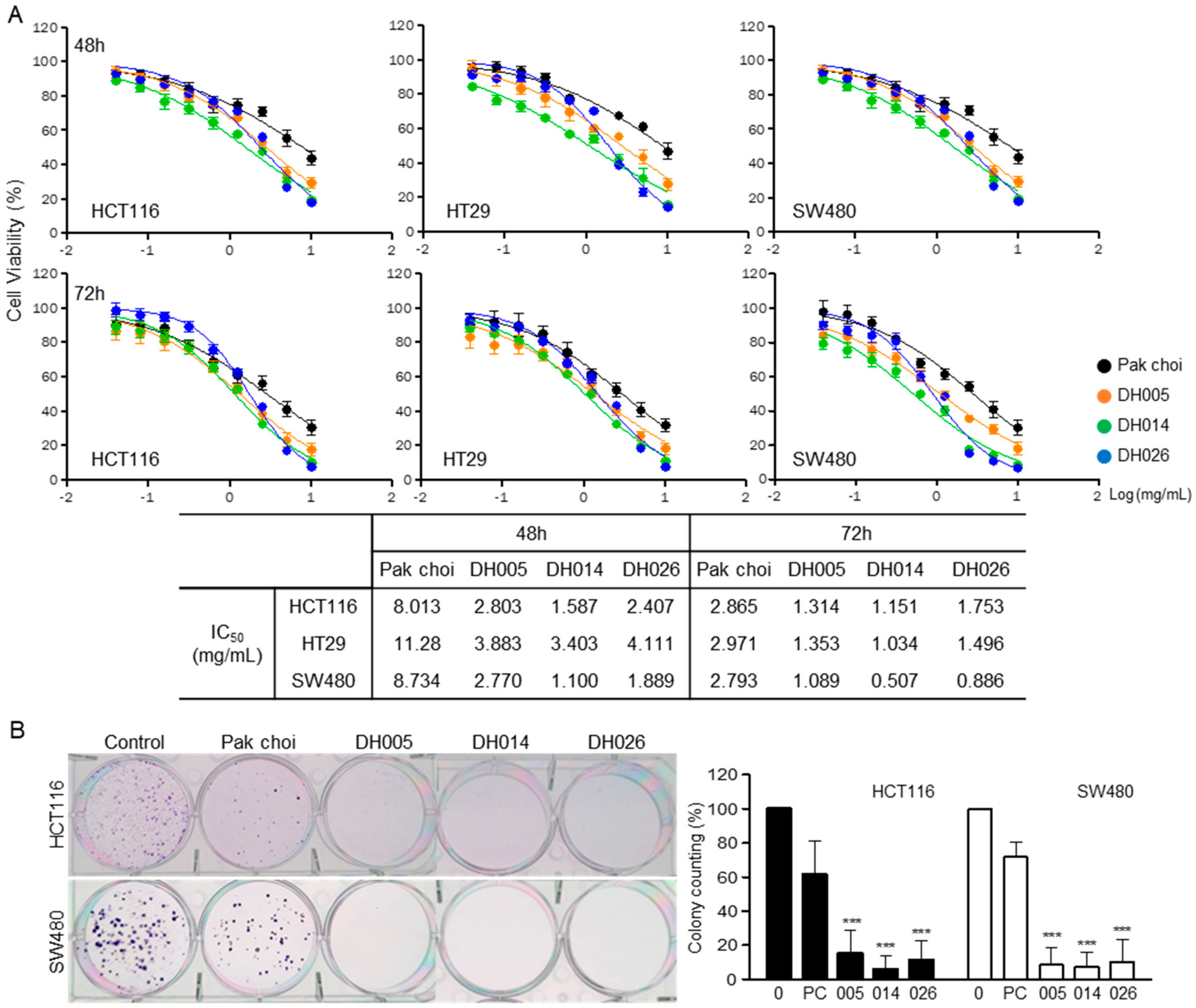
2.3. Cell Viability Assay
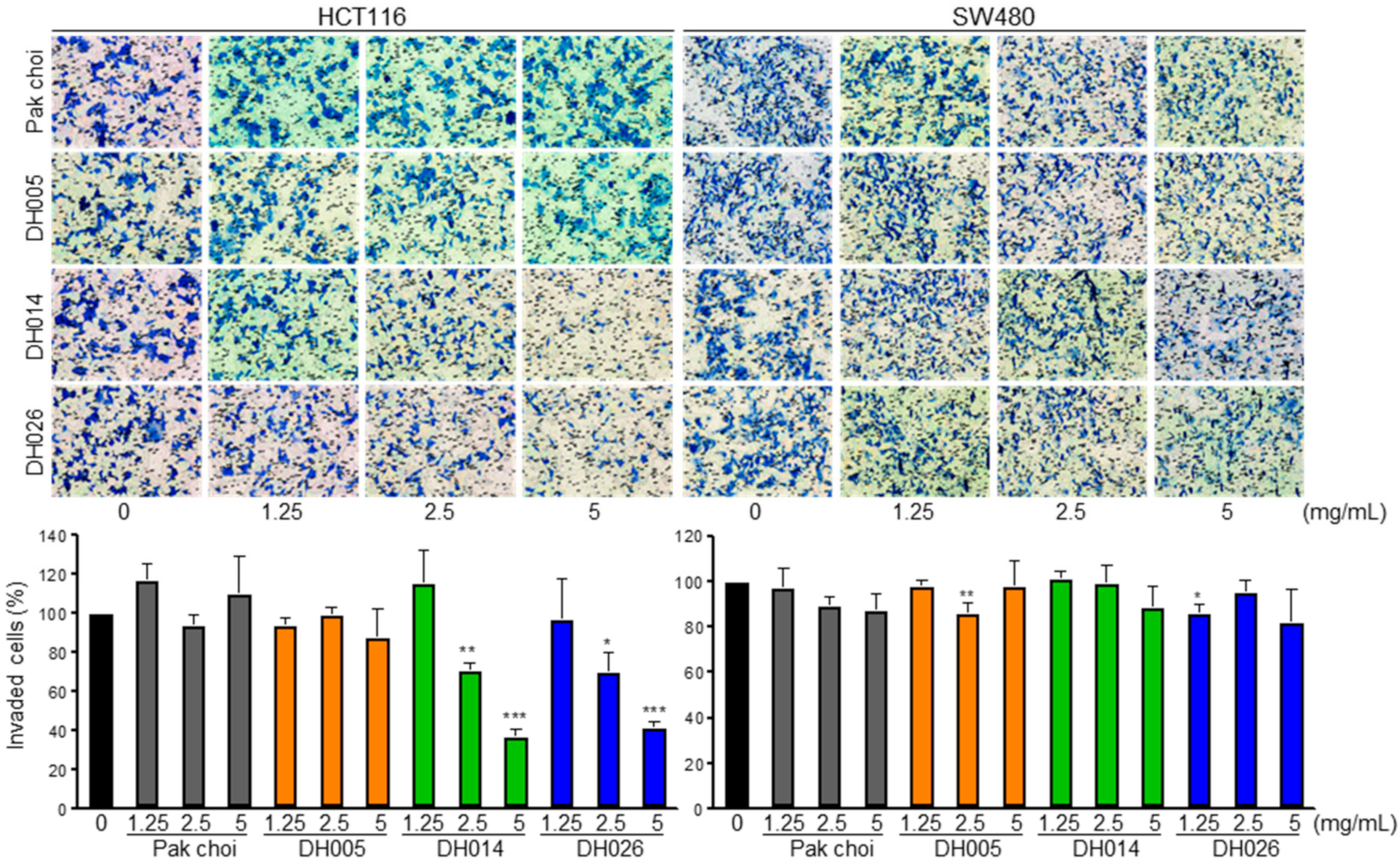
2.4. Invasion Analysis
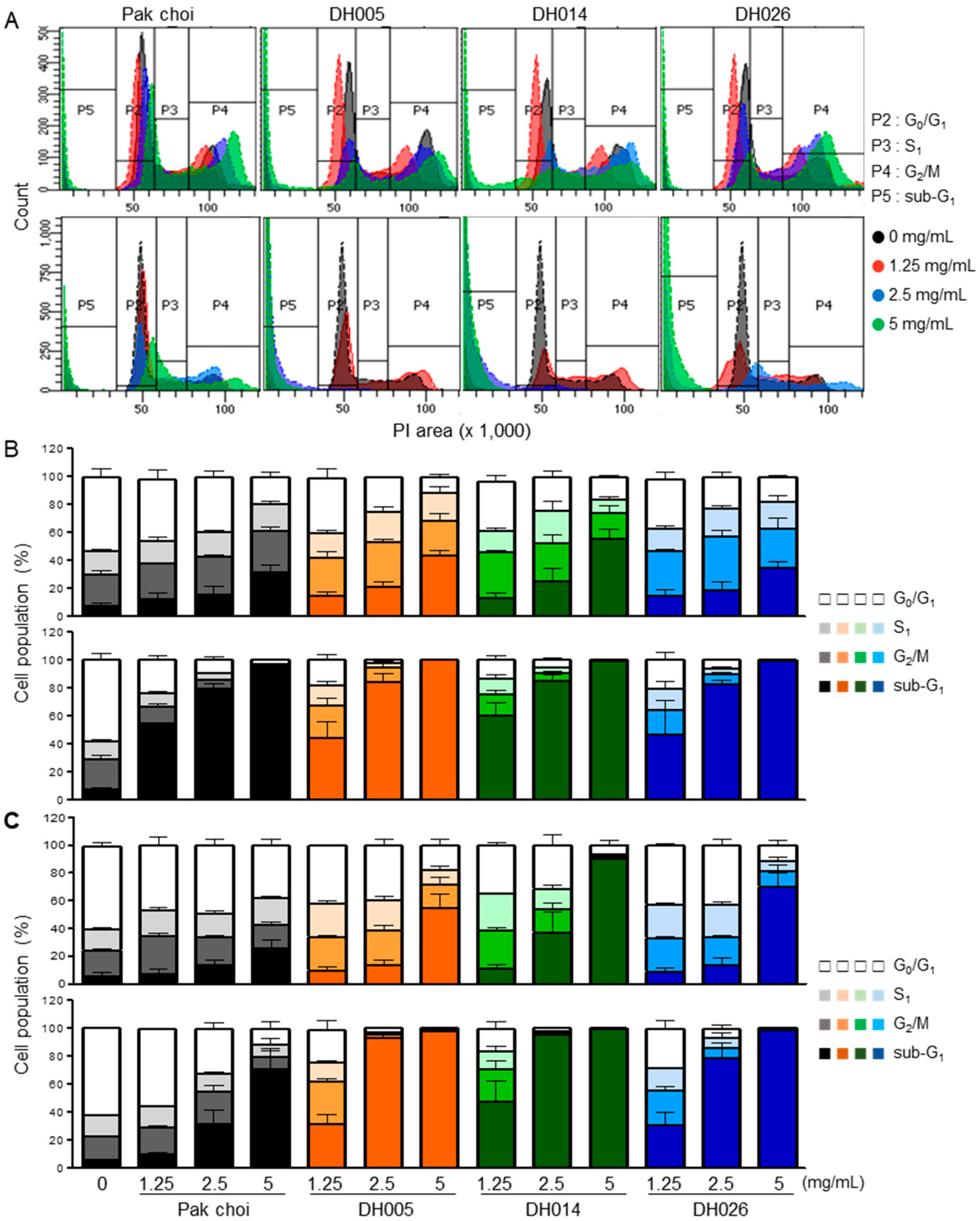
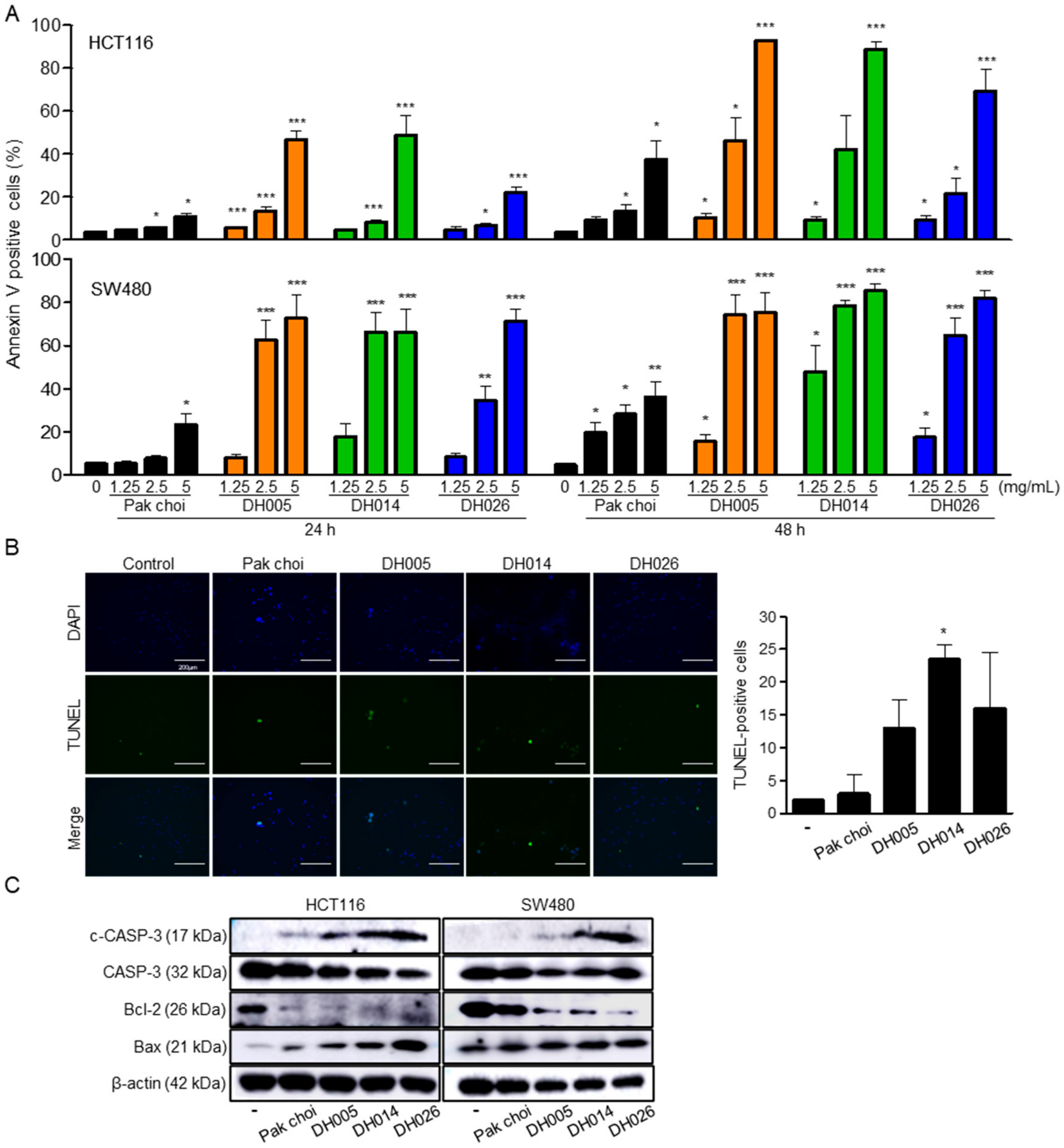
2.5. Cell Cycle and Apoptosis Analysis
2.6. Western Blot Analysis
2.7. Analysis of Subcellular Localization of p65
2.8. Analysis of Reactive Oxygen Species (ROS) Generation
3. Results
3.1. Anti-Proliferation Effects of HGSL DHL Extracts in Human CRC Cells
3.2. Effects of HGSL DHL Extracts on the Invasion of Human CRC Cells
3.3. Induction of Apoptosis by HGSL DHL Extracts in Human CRC Cells
3.4. Induction of ROS by HGSL DHL Extracts in Human CRC Cells
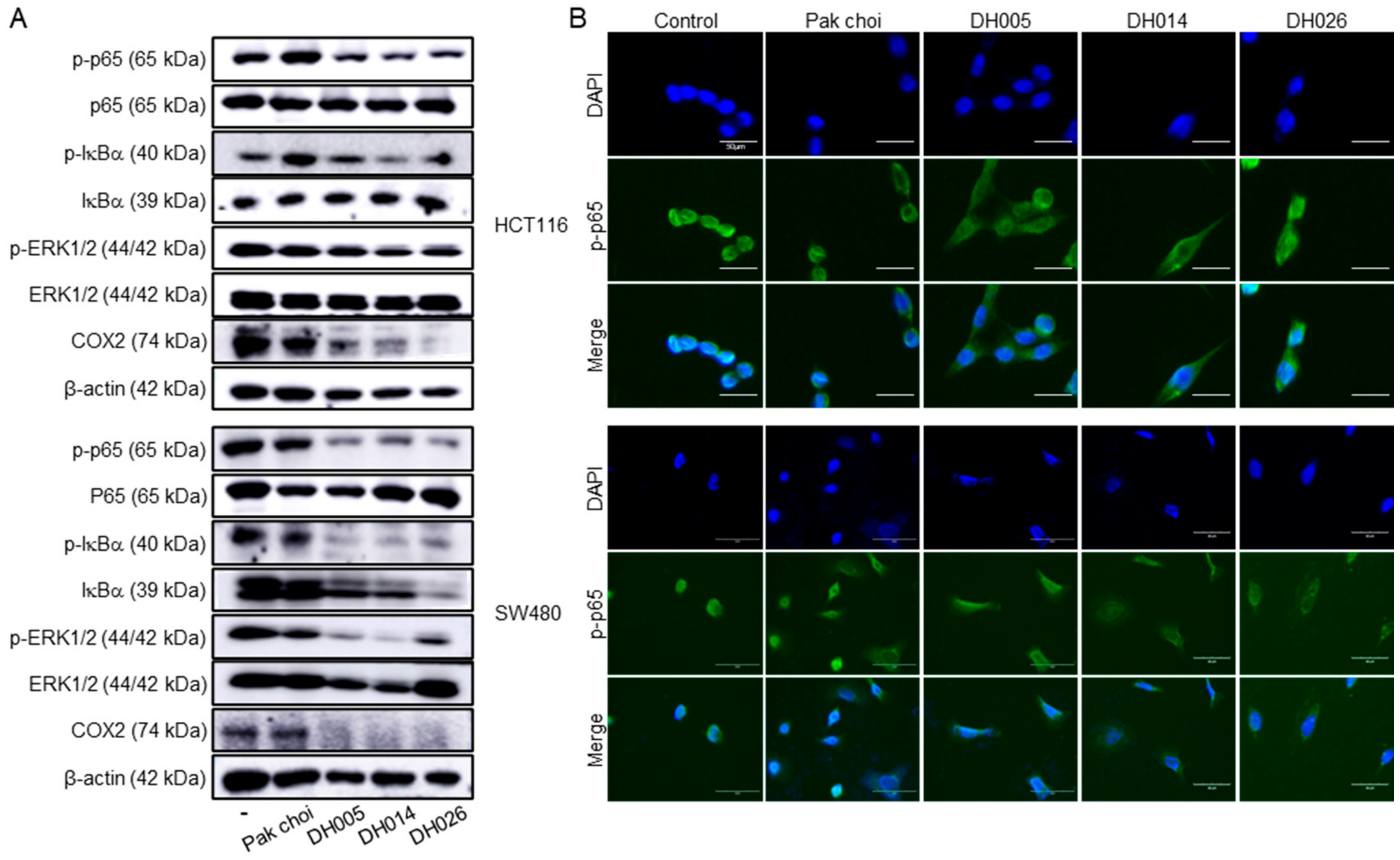
3.5. Inhibition of the NF-κB Signaling Pathway by HGSL DHL Extracts in Human CRC Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; et al. Global, Regional, and National Burden of Colorectal Cancer and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Dang, Y.-Q.; Ji, G. Role of Epigenetics in Transformation of Inflammation into Colorectal Cancer. World J. Gastroenterol. 2019, 25, 2863–2877. [Google Scholar] [CrossRef] [PubMed]
- Åhlberg, M.K. A Profound Explanation of Why Eating Green (Wild) Edible Plants Promote Health and Longevity. Food Front. 2021, 2, 240–267. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, A.; Muthamilarasan, M.; Parida, S.K.; Prasad, M. Breeding and Biotechnological Interventions for Trait Improvement: Status and Prospects. Planta 2020, 252, 54. [Google Scholar] [CrossRef]
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in Plants: Old Ideas, New Techniques. Plant Physiol. 2016, 173, 65–78. [Google Scholar] [CrossRef]
- Kalloo, G.; Rana, M.K. 10—Chinese cabbage: Brassica pekinensis, B. chinensis in B: Cole Crops. In Genetic Improvement of Vegetable Crops, 1st ed.; Kalloo, G., Bergh, B.O., Eds.; Pergamon Press: Oxford, UK, 1993; pp. 179–186. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2019, 169, 112100. [Google Scholar] [CrossRef]
- Seo, M.-S.; Jin, M.; Chun, J.-H.; Kim, S.-J.; Park, B.-S.; Shon, S.-H.; Kim, J.S. Functional Analysis of Three BrMYB28 Transcription Factors Controlling the Biosynthesis of Glucosinolates in Brassica Rapa. Plant Mol. Biol. 2016, 90, 503–516. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Kang, H.J.; Hong, Y.B.; Kim, H.J.; Wang, A.; Bae, I. Bioactive Food Components Prevent Carcinogenic Stress via Nrf2 Activation in BRCA1 Deficient Breast Epithelial Cells. Toxicol. Lett. 2012, 209, 154–160. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1–Nrf2 System in Cancers: Stress Response and Anabolic Metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 Cell Defence Pathway: Keap1-Dependent and -Independent Mechanisms of Regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An Update. Free Radic. Bio. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Kang, H.J.; Yi, Y.W.; Hong, Y.B.; Kim, H.J.; Jang, Y.-J.; Seong, Y.-S.; Bae, I. HER2 Confers Drug Resistance of Human Breast Cancer Cells through Activation of NRF2 by Direct Interaction. Sci. Rep. 2014, 4, 7201. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-ΚB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Soundararajan, P.; Park, S.-G.; Won, S.Y.; Moon, M.-S.; Park, H.W.; Ku, K.-M.; Kim, J.S. Influence of Genotype on High Glucosinolate Synthesis Lines of Brassica Rapa. Int. J. Mol. Sci. 2021, 22, 7301. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, H.J.; Lee, H.-J.; Lee, K.; Hong, D.; Lim, H.; Cho, K.; Jung, N.; Yi, Y.W. Application of a Non-Hazardous Vital Dye for Cell Counting with Automated Cell Counters. Anal. Biochem. 2016, 492, 8–12. [Google Scholar] [CrossRef]
- Lee, S.-G.; Jeon, H.-Y.; Su, Z.-Z.; Richards, J.E.; Vozhilla, N.; Sarkar, D.; Maerken, T.V.; Fisher, P.B. Astrocyte Elevated Gene-1 Contributes to the Pathogenesis of Neuroblastoma. Oncogene 2009, 28, 2476–2484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, S.-L.; Park, S.-Y.; Kang, S.; Park, D.; Kim, S.-H.; Um, J.-Y.; Jang, H.-J.; Lee, J.-H.; Jeong, C.-H.; Jang, J.-H.; et al. Morusin Induces Cell Death through Inactivating STAT3 Signaling in Prostate Cancer Cells. Am. J. Cancer Res. 2014, 5, 289–299. [Google Scholar] [PubMed]
- Kim, H.; Han, S.; Song, K.; Lee, M.Y.; Park, B.; Ha, I.J.; Lee, S.-G. Ethyl Acetate Fractions of Papaver Rhoeas, L. and Papaver Nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities. Antioxidants 2021, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2019, 150, 663–671. [Google Scholar] [CrossRef]
- Seo, M.-S.; Kim, J.S. Understanding of MYB Transcription Factors Involved in Glucosinolate Biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Burow, M.; Rowe, H.C.; Kliebenstein, D.J.; Halkier, B.A. A Complex Interplay of Three R2R3 MYB Transcription Factors Determines the Profile of Aliphatic Glucosinolates in Arabidopsis. Plant Physiol. 2010, 153, 348–363. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Sugiyama, K.; Sawada, Y.; Tohge, T.; Obayashi, T.; Suzuki, A.; Araki, R.; Sakurai, N.; Suzuki, H.; Aoki, K.; et al. Omics-Based Identification of Arabidopsis Myb Transcription Factors Regulating Aliphatic Glucosinolate Biosynthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6478–6483. [Google Scholar] [CrossRef]
- Augustine, R.; Majee, M.; Gershenzon, J.; Bisht, N.C. Four Genes Encoding MYB28, a Major Transcriptional Regulator of the Aliphatic Glucosinolate Pathway, Are Differentially Expressed in the Allopolyploid Brassica Juncea. J. Exp. Bot. 2013, 64, 4907–4921. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of Glucosinolates in Insect-Plant Relationships and Multitrophic Interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Ku, K.-M.; Becker, T.M.; Juvik, J.A. Chemopreventive Glucosinolate Accumulation in Various Broccoli and Collard Tissues: Microfluidic-Based Targeted Transcriptomics for by-Product Valorization. PLoS ONE 2017, 12, e0185112. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Han, S.; Kim, H.; Won, S.Y.; Park, H.W.; Choi, H.; Choi, M.; Lee, M.Y.; Ha, I.J.; Lee, S.-G. Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants 2022, 11, 2463. https://doi.org/10.3390/antiox11122463
Kim JS, Han S, Kim H, Won SY, Park HW, Choi H, Choi M, Lee MY, Ha IJ, Lee S-G. Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants. 2022; 11(12):2463. https://doi.org/10.3390/antiox11122463
Chicago/Turabian StyleKim, Jung Sun, Sanghee Han, Hail Kim, So Youn Won, Hyun Woo Park, Hyunjin Choi, Minji Choi, Min Young Lee, In Jin Ha, and Seok-Geun Lee. 2022. "Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells" Antioxidants 11, no. 12: 2463. https://doi.org/10.3390/antiox11122463
APA StyleKim, J. S., Han, S., Kim, H., Won, S. Y., Park, H. W., Choi, H., Choi, M., Lee, M. Y., Ha, I. J., & Lee, S.-G. (2022). Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants, 11(12), 2463. https://doi.org/10.3390/antiox11122463








