Protective Effect of Liposomal Epigallocatechin-Gallate in Experimental Gentamicin-Induced Hepatotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
- control group (C): intraperitoneal (i.p.) administration of 1 mL saline solution 0.9%, daily for 7 days;
- G group: gentamicin (G) administration (1 mL i.p., 80 mg/kg b.w./day) daily for 7 days;
- G-EGCG group: gentamicin administration (1 mL i.p., 80 mg/kg b.w./day, unique dose) daily for 7 days, plus EGCG (1 mL i.p.) in a dose of 2.5 mg/0.1 kg b.w. 30 min before gentamicin administration, daily for 7 days;
- G-LEGCG group: gentamicin administration (1 mL i.p) daily for 7 days, unique dose plus liposomal EGCG (LEGCG) (1 mL i.p.), 2.5 mg/0.1 kg b.w. 30 min before gentamicin administration, daily for 7 days;
- G-Sily group: gentamicin + silymarin (100 mg/kg b.w.). Silymarin was administered i.p. once per day for 7 consecutive days, each day, 30 min before gentamicin administration [25].
2.2. Preparation and Physicochemical Characterization of EGCG-Loaded Liposomes
2.3. Blood Samples Collection and Measurement of Serum Markers
2.4. Histopathological Analysis
2.5. Statistical Analysis
3. Results
3.1. Biochemical Markers
3.2. Matrix Metalloproteinase-2 and 9
3.3. Liver Histopathology
4. Discussion
4.1. The Effects of EGCG and LEGCG on Hepatic Cells Function
4.2. The Effects of EGCG and LEGCG on Renal Function
4.3. The Effects of EGCG and LEGCG on Pancreatic Function
4.4. The Effects of EGCG and LEGCG on Nitro-Oxidative Stress/Antioxidant Balance and Inflammation
4.5. The Effects of EGCG and LEGCG on Serum Metalloproteinases
4.6. Potential Limitations and Further Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, H.-S.; Chen, J.-H.; Leong, P.-K.; Leung, H.-Y.; Chan, W.-M.; Ko, K.-M. β-Sitosterol Protects against Carbon Tetrachloride Hepatotoxicity but not Gentamicin Nephrotoxicity in Rats via the Induction of Mitochondrial Glutathione Redox Cycling. Molecules 2014, 19, 17649–17662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulboacă, A.E.; Porfire, A.; Bolboacă, S.D.; Nicula, C.A.; Feștilă, D.G.; Roman, A.; Râjnoveanu, R.M.; Râjnoveanu, A.; Dogaru, G.; Boarescu, P.-M.; et al. Protective Effects of Liposomal Curcumin on Oxidative Stress/Antioxidant Imbalance, Metalloproteinases 2 and -9, Histological Changes and Renal Function in Experimental Nephrotoxicity Induced by Gentamicin. Antioxidants 2021, 10, 325. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Hassanein, E.H.M.; Bakr, A.G.; El-Shoura, E.A.M.; El-Gamal, D.A.; Mahmoud, A.R.; Abd-Elhamid, T.H. Ursodeoxycholic acid abrogates gentamicin-induced hepatotoxicity in rats: Role of NF-kappaB-p65/TNF-alpha, Bax/Bcl-xl/Caspase-3, and eNOS/iNOS pathways. Life Sci. 2020, 254, 117760. [Google Scholar] [CrossRef] [PubMed]
- Khaksari, M.; Esmaili, S.; Abedloo, R.; Khastar, H. Palmatine ameliorates nephrotoxicity and hepatotoxicity induced by gentamicinin rats. Arch. Physiol. Biochem. 2021, 127, 273–278. [Google Scholar] [CrossRef]
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich Riceberry bran extract attenuates gentamicin-inducedhepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Q.; He, J.; Li, Y. Novel Therapeutic Targets in Liver Fibrosis. Front. Mol. Biosci. 2021, 8, 766855. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [Green Version]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gazák, R.; Hrbác, J.; Sedmera, P.; Kren, V.; Lazzaroni, R.; Duroux, J.L.; et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: A joint experimental and theoretical study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef]
- Mahi-Birjand, M.; Yaghoubi, S.; Abdollahpour-Alitappeh, M.; Keshtkaran, Z.; Bagheri, N.; Pirouzi, A.; Khatami, M.; Sineh Sepehr, K.; Peymani, P.; Karimzadeh, I. Protective effects of pharmacological agents against aminoglycoside-induced nephrotoxicity: A systematic review. Expert Opin. Drug Saf. 2020, 19, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Erseçkin, V.; Mert, H.; İrak, K.; Yildirim, S.; Mert, N. Nephroprotective effect of ferulic acid on gentamicin-induced nephrotoxicity in female rats. Drug Chem. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, J.C. Potential role of green tea catechins in various disease therapies: Progress and promise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 265–273. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 2008, 13, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Sonee, M.; Sum, T.; Wang, C.; Mukherjee, S.K. The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology 2004, 25, 885–891. [Google Scholar] [CrossRef]
- Murakami, C.; Hirakawa, Y.; Inui, H.; Nakano, Y.; Yoshida, H. Effect of tea catechins on cellular lipid peroxidation and cytotoxicity in HepG2 cells. Biosci. Biotechnol. Biochem. 2002, 66, 1559–1562. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.T.; Koo, M.W. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis 2000, 148, 67–73. [Google Scholar] [CrossRef]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Qian, Y.; Chen, F.; Chen, X.; Chen, Z.; Zheng, M. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim. Biophys. Sin. 2014, 46, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, S.; Suhail, N.; Alrashed, M.; Wasi, S.; Aljaser, F.S.; AlSubki, R.A.; Alsharidah, A.S.; Banu, N. Epigallocatechin gallate and coenzyme Q10 attenuate cisplatin-induced hepatotoxicity in rats via targeting mitochondrial stress and apoptosis. J. Biochem. Mol. Toxicol. 2021, 35, e22701. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.W.; Nam, Y.; Choi, W.S.; Kim, T.W.; Kim, G.M.; Sohn, U.D. Hepatoprotective effect of sodium hydrosulfide on hepatic encephalopathy in rats. Korean J. Physiol. Pharmacol. 2019, 23, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Barbălată, C.I.; Tomuță, I.; Achim, M.; Boșca, A.B.; Cherecheș, G.; Sorițău, O.; Porfire, A.S. Application of the QbD Approach in the Development of a Liposomal Formulation with EGCG. J. Pharm. Innov. 2021. [Google Scholar] [CrossRef]
- Mohammad, A.; Ali, N.; Reza, B.; Ali, K. Effect of ascorbic acid supplementation on nitric oxide metabolites and systolic blood pressure in rats exposed to lead. Indian J. Pharmacol. 2010, 42, 78–81. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Yagi, K. Assay for blood plasma and serum peroxides. Methods Enzymol. 1984, 105, 328–331. [Google Scholar] [CrossRef]
- Galaly, S.R.; Ahmed, O.M.; Mahmoud, A.M. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J. Physiol. Pharmacol. 2014, 65, 823–832. [Google Scholar] [PubMed]
- Prokhorova, I.; Altman, R.B.; Djumagulov, M.; Shrestha, J.P.; Urzhumtsev, A.; Ferguson, A.; Chang, C.T.; Yusupov, M.; Blanchard, S.C.; Yusupova, G. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc. Natl. Acad. Sci. USA 2017, 114, E10899–E10908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehme, M.; Voisine, C. Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis. Model Mech. 2016, 9, 823–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Moradi Sarabi, M. Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxid. Med. Cell. Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Owoloye, T.R.; Agbebi, O.J. Modulatory effects of dietary inclusion of garlic (Allium sativum) on gentamycin-induced hepatotoxicity and oxidative stress in rats. Asian Pac. J. Trop. Biomed. 2013, 3, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Bekheet, S.H.; Awadalla, E.A.; Salman, M.M.; Hassan, M.K. Prevention of hepatic and renal toxicity with bradykinin potentiating factor (BPF) isolated from Egyptian scorpion venom (Buthus occitanus) in gentamicin treated rats. Tissue Cell. 2013, 45, 89–94. [Google Scholar] [CrossRef]
- Abolfathi, A.A.; Mohajeri, D.; Rezaie, A.; Nazeri, M. Protective Effects of Green Tea Extract against Hepatic Tissue Injury in Streptozotocin-Induced Diabetic Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 740671. [Google Scholar] [CrossRef] [Green Version]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Eweis, E.A.; El-Beltagi, H.S.; Abdel-Mobdy, Y.E. Abdel-Mobdy Effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. Biomed. 2012, 2, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial Roles and Cytoprotection in Chronic Liver Injury. Biochem. Res. Int. 2012, 2012, 387626. [Google Scholar] [CrossRef] [PubMed]
- Huidobro, E.J.; Tagle, R.; Guzmán, A.M. Estimation of glomerular filtration rate with creatinine. Rev. Med. Chil. 2018, 146, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Mohan, T.; Narasimhan, K.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Srinivasan, A.; Karthikeyan, P.; Kannan, P.; Tamilarasan, B.; et al. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Mishra, G.; İlgün, S.; Samarghandian, S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother. Res. 2021, 35, 3078–3112. [Google Scholar] [CrossRef]
- Klover, P.J.; Mooney, R.A. Hepatocytes: Critical for glucose homeostasis. Int. J. Biochem. Cell Biol. 2004, 36, 753–758. [Google Scholar] [CrossRef]
- Tanikawa, K.; Torimura, T. Studies on oxidative stress in liver diseases: Important future trends in liver research. Med. Mol. Morphol. 2006, 39, 22–27. [Google Scholar] [CrossRef]
- Otto-Buczkowska, E. The clinical utility of C-peptide measurement in diabetology. Pediatr. Endocrinol. Diabetes Metab. 2015, 20, 63–68. [Google Scholar] [CrossRef]
- Dogaru, G.; Bulboacă, A.E.; Gheban, D.; Boarescu, P.M.; Rus, V.; Festila, D.; Sitar-Taut, A.V.; Stănescu, I. Effect of Liposomal Curcumin on Acetaminophen Hepatotoxicity by Down-regulation of Oxidative Stress and Matrix Metalloproteinases. In Vivo 2020, 34, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Bulboacă, A.E.; Porfire, A.S.; Barbalata, C.; Bolboacă, S.D.; Nicula, C.; Boarescu, P.M.; Stănescu, I.C.; Dogaru, G. The effect of liposomal epigallocatechingallate andmetoclopramide hydrochloride co-administration on experimental migraine. Farmacia 2019, 67, 905–911. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hamid, H.A.; Abdel-Hakeem, E.A.; Zenhom, N.M.; Toni, N. C-peptide corrects hepatocellular dysfunction in a rat model of type 1 diabetes. J. Physiol. Biochem. 2020, 76, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, N.J. C-peptide and diabetic kidney disease. J. Intern. Med. 2017, 281, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksu, B.; Umit, H.; Kanter, M.; Guzel, A.; Aktas, C.; Civelek, S.; Uzun, H. Effects of methylene blue in reducing cholestatic oxidative stress and hepatic damage after bile-duct ligation in rats. Acta Histochem. 2010, 112, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, Z.; Bardania, H.; Gheitasi, I.; Barmoudeh, Z.; Omidifar, N.; Parvin, H.; Khalvati, B.; Fouani, M.H.; Alipour, M.; Doustimotlagh, A.H. Liposome Extract of Stachys pilifera Benth Effectively Improved Liver Damage due to Bile Duct Ligation Rats. Oxid. Med. Cell. Longev. 2021, 2021, 8141563. [Google Scholar] [CrossRef]
- Sadeghi, H.; Mansourian, M.; Kokhdan, E.P.; Salehpour, Z.; Sadati, I.; Abbaszadeh-Goudarzi, K.; Asfaram, A.; Doustimotlagh, A.H. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem. 2020, 44, e13190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Shu, C.-M.; Zhang, X.-Y.; Lin, X.-T.; Guan, Q.-H.; Zhang, F.; Zhi, X.-T. Effect and mechanism of omega-3 polyunsaturated fatty acids on intestinal injury in rats with obstructive jaundice. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6077–6092. [Google Scholar] [CrossRef]
- Abdeen, A.; Samir, A.; Elkomy, A.; Aboubaker, M.; Habotta, O.A.; Gaber, A.; Alsanie, W.F.; Abdullah, O.; Elnoury, H.A.; Baioumy, B.; et al. The potential antioxidant bioactivity of date palm fruit against gentamicin-mediated hepato-renal injury in male albino rats. Biomed. Pharmacother. 2021, 143, 112154. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sichetti Munekata, P.E. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Z.; Yu, P.; Gan, W.; Ren, K.; Zhang, F.; Chen, F.; Wang, M.; Bao, J.; Wang, T. Beneficial effects of green tea on age related diseases. Front. Biosci. 2020, 12, 70–91. [Google Scholar] [CrossRef]
- Mohamadi Yarijani, Z.; Najafi, H.; Shackebaei, D.; Madani, S.H.; Modarresi, M.; Jassemi, S.V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed. Pharmacother. 2019, 112, 108635. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Yang, W.; Bao, S.; Cao, Y. Epigallocatechin Gallate Suppresses Inflammatory Responses by Inhibiting Toll-like Receptor 4 Signaling and Alleviates Insulin Resistance in the Livers of High-fat-diet Rats. J. Oleo Sci. 2020, 69, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, S.A.A.; Eldeeb, H.M.; Mohammed, H.A.; Al-Omar, M.S.; Almahmoud, S.A.; El-Readi, M.Z.; Ragab, E.A.; Sulaiman, G.M.; Aly, M.S.A.; Khan, R.A. Roles of Suaeda vermiculata Aqueous-Ethanolic Extract, Its Subsequent Fractions, and the Isolated Compounds in Hepatoprotection against Paracetamol-Induced Toxicity as Compared to Silymarin. Oxid. Med. Cell. Longev. 2021, 2021, 6174897. [Google Scholar] [CrossRef]
- Romero, F.; Pérez, M.; Chávez, M.; Parra, G.; Durante, P. Effect of uric acid on gentamicin-induced nephrotoxicity in rats—Role of matrixmetalloproteinases 2 and 9. Basic Clin. Pharmacol. Toxicol. 2009, 105, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Richelmi, P.; Cadamuro, M.; Fabris, L.; Perlini, S.; Adorini, L.; Vairetti, M. Obeticholic acid reduces biliary and hepatic matrix metalloproteinases activity in rat hepatic ischemia/reperfusion injury. PLoS ONE 2020, 15, e0238543. [Google Scholar] [CrossRef]
- Hamada, T.; Fondevila, C.; Busuttil, R.W.; Coito, A.J. Metallo-proteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008, 47, 186–198. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [Green Version]
- An, H.; Kim, J.Y.; Oh, E.; Lee, N.; Cho, Y.; Seo, J.H. Salinomycin Promotes Anoikis and Decreases the CD44+/CD24− Stem-Like Population via Inhibition of STAT3 Activation in MDA-MB-231 Cells. PLoS ONE 2015, 10, e0141919. [Google Scholar] [CrossRef] [Green Version]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Jeng, K.S.; Lu, S.J.; Wang, C.H.; Chang, C.F. Liver Fibrosis and Inflammation under the Control of ERK2. Int. J. Mol. Sci. 2020, 21, 3796. [Google Scholar] [CrossRef]
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.W.; Chung, J.; Sverdlov, D.Y.; Miyamoto, M.; Kim, Y.O.; Ogawa, S.; Arch, R.H.; et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014, 147, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Morini, S.; Ginanni Corradini, S.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Onetti Muda, A.; Berloco, P.; Rossi, M.; et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef] [PubMed]


| Group | Statistics | AST (UI/L) | ALT (UI/L) |
|---|---|---|---|
| C | median (Q1 to Q3) | 24.4 (23.4 to 24.8) | 25 (24.3 to 26.3) |
| mean (SD) | 24.2 (1.2) | 25.3 (1.6) | |
| (min to max) | (22.7 to 26.1) | (23.3 to 27.6) | |
| G | median (Q1 to Q3) | 165 (163.5 to 177.5) | 207 (200 to 212.5) |
| mean (SD) | 169.4 (8.7) | 205.6 (12.7) | |
| (min to max) | (159 to 180) | {185 to 222) | |
| G-EGCG | median (Q1 to Q3) | 134 (125.5 to 138.5) | 157 (145 to 163.5) |
| mean (SD) | 130.9 (11.4) | 156 (14.5) | |
| (min to max) | {111 to 143) | {138 to 180) | |
| G-LEGCG | median (Q1 to Q3) | 101 (98 to 106) | 133 (123 to 146) |
| mean (SD) | 101 (7.8) | 134.6 (13.2) | |
| (min to max) | {87 to 111) | {120 to 151) | |
| G-Sily | median (Q1 to Q3) | 79 (75 to 84) | 115 (108 to 118) |
| mean (SD) | 79 (6.5) | 113.3 (7.5) | |
| (min to max) | {69 to 87) | {103 to 123) | |
| p-value | C vs. G | <0.0001 | <0.0001 |
| G vs. G-EGCG | <0.0001 | <0.0001 | |
| G vs. G-LEGCG | <0.0001 | <0.0001 | |
| G vs. G-Sily | <0.0001 | <0.0001 | |
| G-EGCG vs. G-LEGCG | 0.0001 | 0.0136 | |
| G-EGCG vs. G-Sily | <0.0001 | <0.0001 | |
| G-LEGCG vs. G-Sily | 0.0001 | 0.0030 |
| Group | Statistics | Creatinine (mmol/L) | Urea (mmol/L) | BUN (mg/dL) |
|---|---|---|---|---|
| C | median (Q1 to Q3) | 12 (10.5 to 13) | 13.1 (11.8 to 13.8) | 6.1 (5.5 to 6.4) |
| mean (SD) (min to max) | 12.1 (2.2) (10 to 16) | 12.9 (1.4) (11.2 to 15) | 6 (0.6) (5.2 to 7) | |
| G | median (Q1 to Q3) | 47 (46.5 to 48.5) | 31.5 (30.7 to 32.8) | 14.7 (14.3 to 15.3) |
| mean (SD) (min to max) | 47.6 (2) (45 to 51) | 31.6 (1.8) (28.9 to 34) | 14.7 (0.8) (13.5 to 15.8) | |
| G-EGCG | median (Q1 to Q3) | 40 (39 to 40.5) | 25 (24.3 to 25.6) | 11.7 (11.3 to 11.9) |
| mean (SD) (min to max) | 39.7 (1.1) (38 to 41) | 24.9 (1.5) (22.5 to 27) | 11.6 (0.7) (10.5 to 12.6) | |
| G-LEGCG | median (Q1 to Q3) | 30 (28 to 33) | 21.7 (20.2 to 22.3) | 10.1 (9.4 to 10.4) |
| mean (SD) (min to max) | 30.6 (3.2) (27 to 35) | 21.2 (1.5) (19 to 23) | 9.9 (0.7) (8.9 to 10.7) | |
| G-Sily | median (Q1 to Q3) | 25 (22 to 26) | 15 (14 to 16.5) | 7 (6.5 to 7.7) |
| mean (SD) (min to max) | 24.6 (3.2) (21 to 30) | 15.1 (1.6) (13 to 17) | 7.1 (0.7) (6.1 to 7.9) | |
| p-value | C vs. G | <0.0001 | <0.0001 | <0.0001 |
| G vs. G-EGCG | <0.0001 | <0.0001 | <0.0001 | |
| G vs. G-LEGCG | <0.0001 | <0.0001 | <0.0001 | |
| G vs. G-Sily | <0.0001 | <0.0001 | <0.0001 | |
| G-EGCG vs. G-LEGCG | <0.0001 | 0.0006 | 0.0006 | |
| G-EGCG vs. G-Sily | <0.0001 | <0.0001 | <0.0001 | |
| G-LEGCG vs. G-Sily | 0.0042 | <0.0001 | <0.0001 |
| Group | Statistics | Basal Glycemia (mmol/L) | C-Peptide (pmol/L) |
|---|---|---|---|
| C | median (Q1 to Q3) | 4.6 (4.5 to 4.7) | 600 (585 to 612.5) |
| mean (SD) (min to max) | 4.6 (0.1) (4.5 to 4.7) | 598.6 (17.5) (575 to 620) | |
| G | median (Q1 to Q3) | 8.8 (8.7 to 9.4) | 363 (353 to 368) |
| mean (SD) (min to max) | 9 (0.7) (7.9 to 10) | 360.7 (10.8) (345 to 375) | |
| G-EGCG | median (Q1 to Q3) | 7.2 (6.9 to 7.4) | 390 (382.5 to 400) |
| mean (SD) (min to max) | 7.3 (0.4) (6.7 to 8.1) | 391.4 (12.5) (375 to 410) | |
| G-LEGCG | median (Q1 to Q3) | 5.7 (5.5 to 6) | 420 (412.5 to 425) |
| mean (SD) (min to max) | 5.7 (0.3) (5.3 to 6.1) | 419.3 (10.2) (405 to 435) | |
| G-Sily | median (Q1 to Q3) | 5.2 (5.1 to 5.6) | 460 (452.5 to 482.5) |
| mean (SD) (min to max) | 5.3 (0.3) (5 to 5.8) | 465 (18.3) (440 to 485) | |
| p-value | C vs. G | <0.0001 | <0.0001 |
| G vs. G-EGCG | 0.0001 | 0.0004 | |
| G vs. G-LEGCG | <0.0001 | <0.0001 | |
| G vs. G-Sily | <0.0001 | <0.0001 | |
| G-EGCG vs. G-LEGCG | <0.0001 | 0.0006 | |
| G-EGCG vs. G-Sily | <0.0001 | <0.0001 | |
| G-LEGCG vs. G-Sily | 0.0646 | 0.0001 |
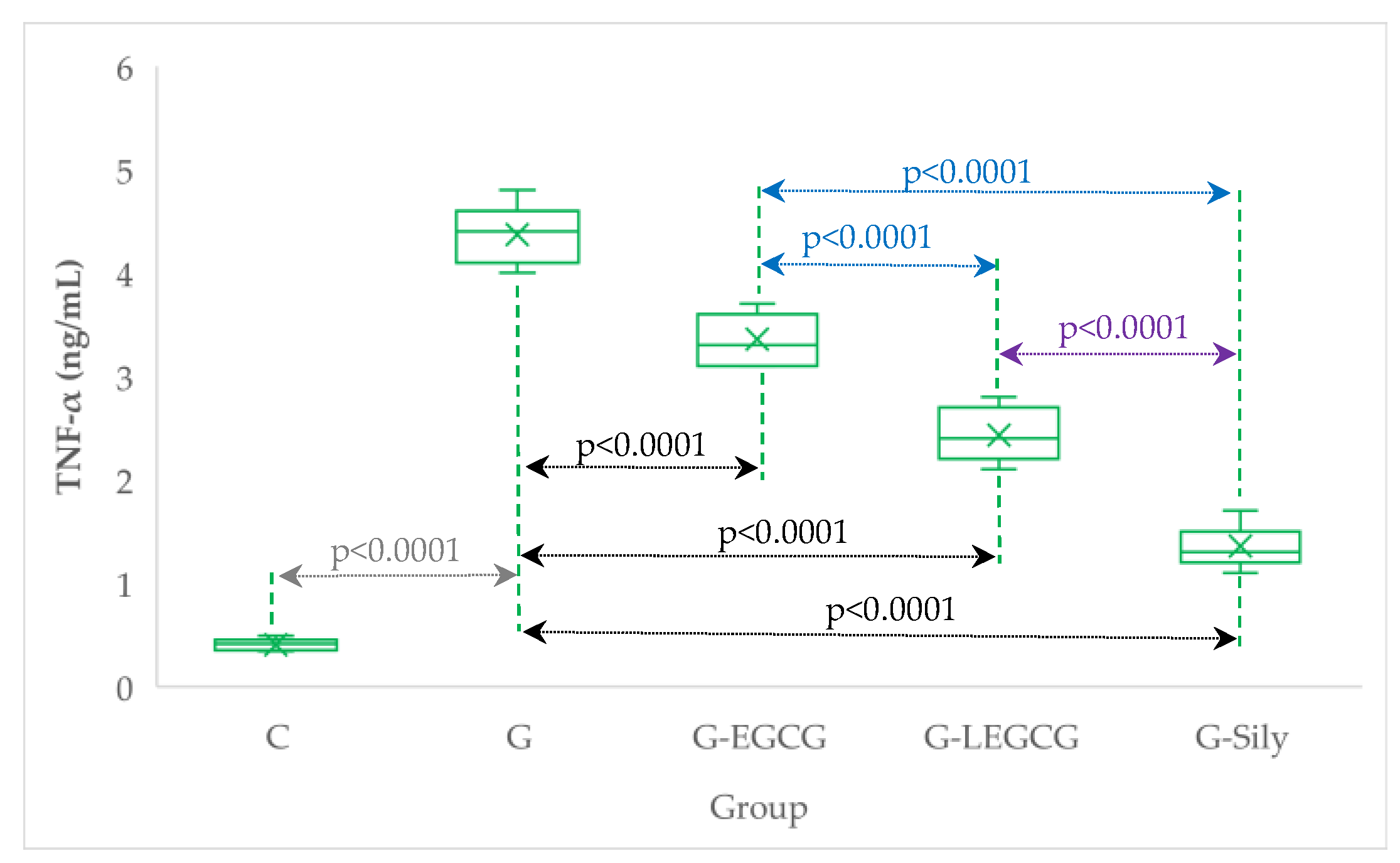
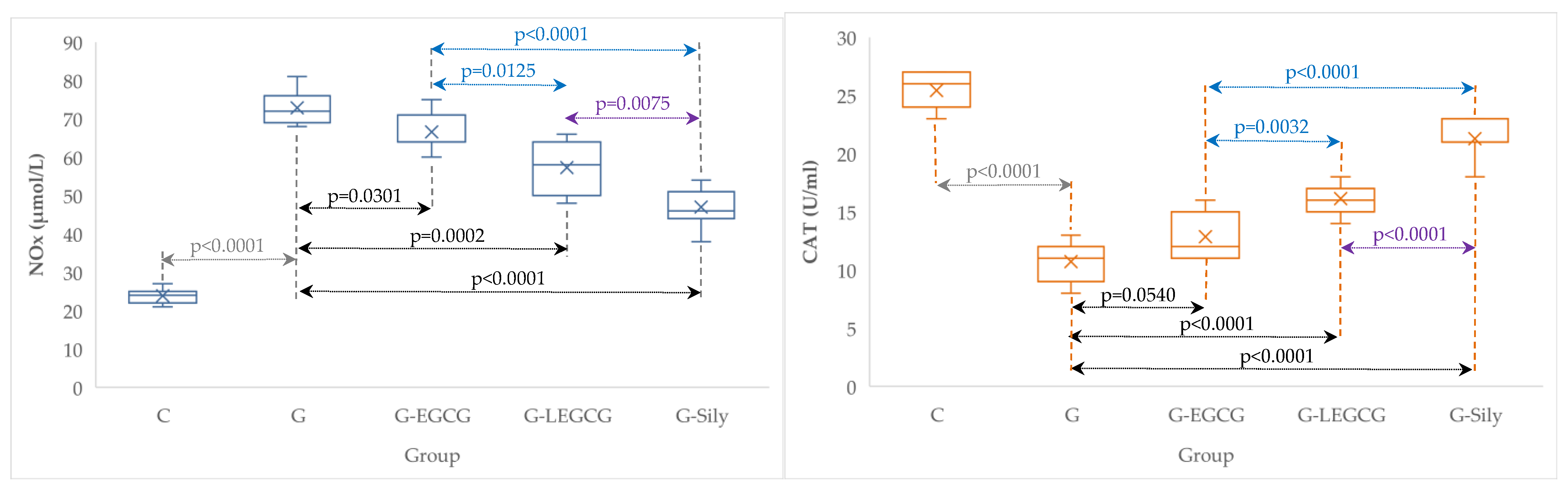
| Group | TNF-α (ng/mL) | NOx (µmol/L) | CAT (U/mL) |
|---|---|---|---|
| C | 0.4 (0.1) | 23.9 (2.0) | 25.4 (1.5) |
| G | 4.4 (0.3) | 72.9 (4.5) | 10.7 (1.8) |
| G-EGCG | 3.4 (0.2) | 66.6 (5.1) | 12.9 (2.0) |
| G-LEGCG | 2.4 (0.3) | 57.3 (6.7) | 16.1 (1.3) |
| G-Sily | 1.4 (0.2) | 47 (5.3) | 21.3 (1.7) |
| Group | MMP-2 (ng/mL) | MMP-9 (ng/mL) |
|---|---|---|
| C | 79.3 (5.3) | 18.6 (2.9) |
| G | 262.6 (11.5) | 45.6 (5.8) |
| G-EGCG | 195 (13.9) | 36 (4.8) |
| G-LEGCG | 154.9 (9) | 32 (2.4) |
| G-Sily | 116.3 (9.6) | 25.1 (2.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulboacă, A.E.; Porfire, A.S.; Rus, V.; Nicula, C.A.; Bulboacă, C.A.; Bolboacă, S.D. Protective Effect of Liposomal Epigallocatechin-Gallate in Experimental Gentamicin-Induced Hepatotoxicity. Antioxidants 2022, 11, 412. https://doi.org/10.3390/antiox11020412
Bulboacă AE, Porfire AS, Rus V, Nicula CA, Bulboacă CA, Bolboacă SD. Protective Effect of Liposomal Epigallocatechin-Gallate in Experimental Gentamicin-Induced Hepatotoxicity. Antioxidants. 2022; 11(2):412. https://doi.org/10.3390/antiox11020412
Chicago/Turabian StyleBulboacă, Adriana Elena, Alina Silvia Porfire, Vasile Rus, Cristina Ariadna Nicula, Corneliu Angelo Bulboacă, and Sorana D. Bolboacă. 2022. "Protective Effect of Liposomal Epigallocatechin-Gallate in Experimental Gentamicin-Induced Hepatotoxicity" Antioxidants 11, no. 2: 412. https://doi.org/10.3390/antiox11020412
APA StyleBulboacă, A. E., Porfire, A. S., Rus, V., Nicula, C. A., Bulboacă, C. A., & Bolboacă, S. D. (2022). Protective Effect of Liposomal Epigallocatechin-Gallate in Experimental Gentamicin-Induced Hepatotoxicity. Antioxidants, 11(2), 412. https://doi.org/10.3390/antiox11020412









