β-Sitosterol Glucoside-Loaded Nanosystem Ameliorates Insulin Resistance and Oxidative Stress in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Experimental Animals
2.4. Extraction and Isolation
2.5. Spectroscopic Characterization
2.6. Preparation of SEDDS
2.7. Infinite Dilution Capacity
2.8. Percentage Transmittance
2.9. Drug Loading
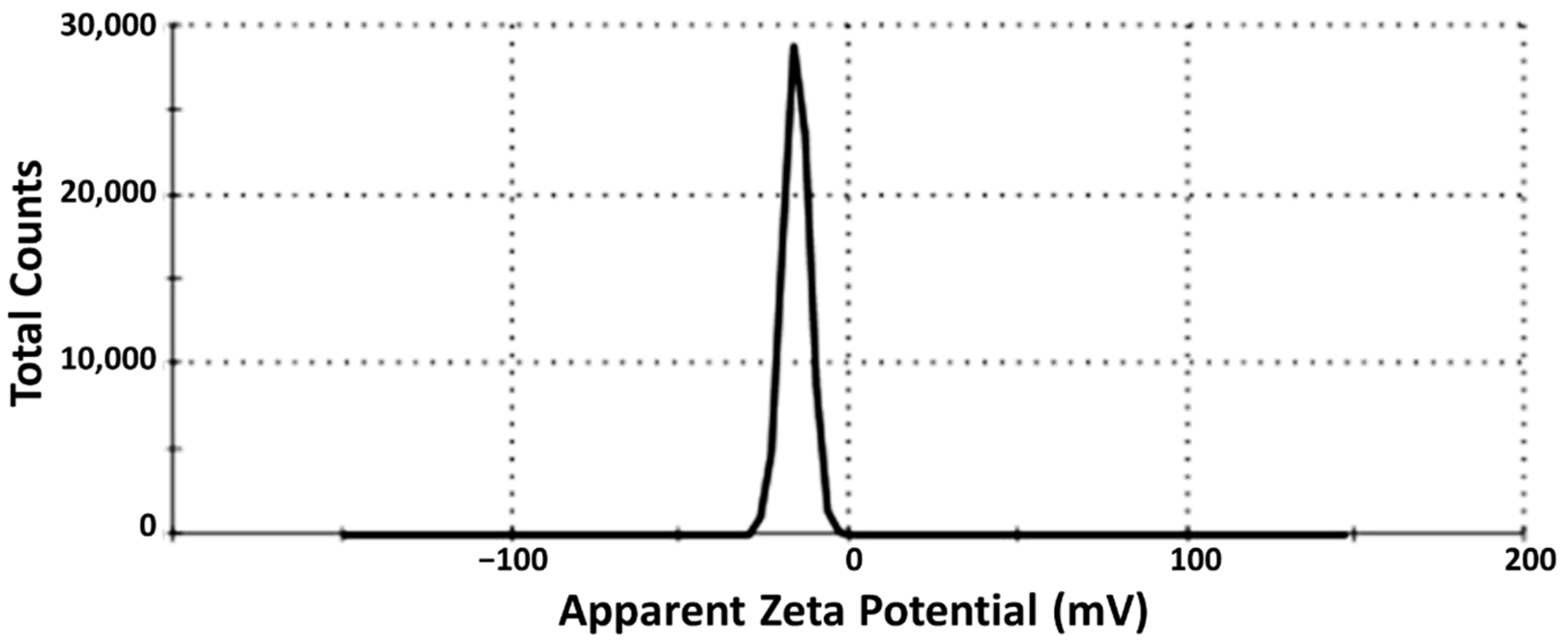
2.10. Droplet Size Determination, Polydispersity Index, and Zeta Potential
2.11. In Vitro Release Studies
2.12. Transmission Electron Microscopy (TEM)
2.13. Induction of Diabetes
2.14. Collection of Blood and Tissue Samples
2.15. Measurements of Biochemical Parameters
2.16. Determination of Serum Malondialdehyde (MDA)
2.17. Determination of Serum Catalase
2.18. Histopathological Examination of Pancreatic Tissue
2.19. Statistical Analysis
3. Results and Discussion
3.1. Structure Identification of β-Sitosterol Glucoside
3.2. Assessment of Self-Emulsification
3.3. Percentage Transmittance
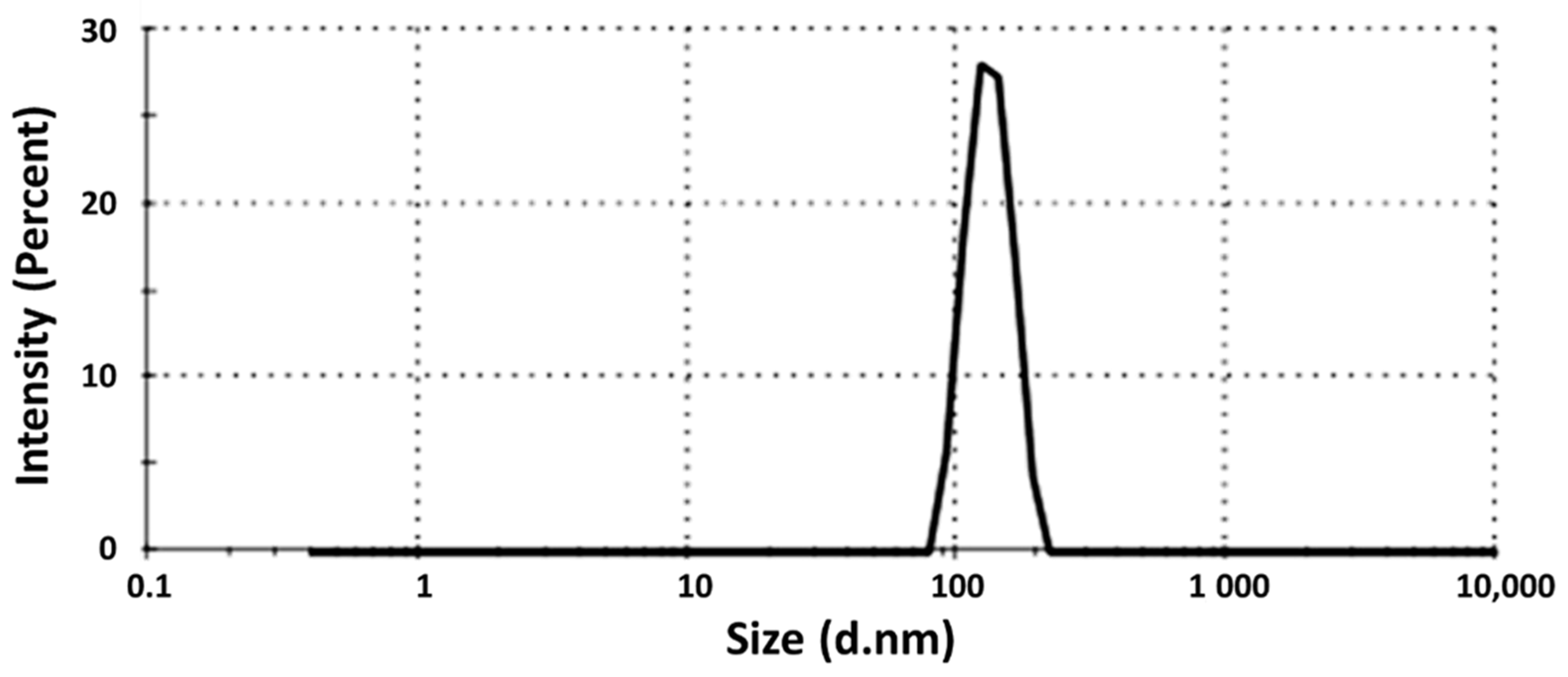
3.4. Droplet Size Determination
3.5. In Vitro Release Study
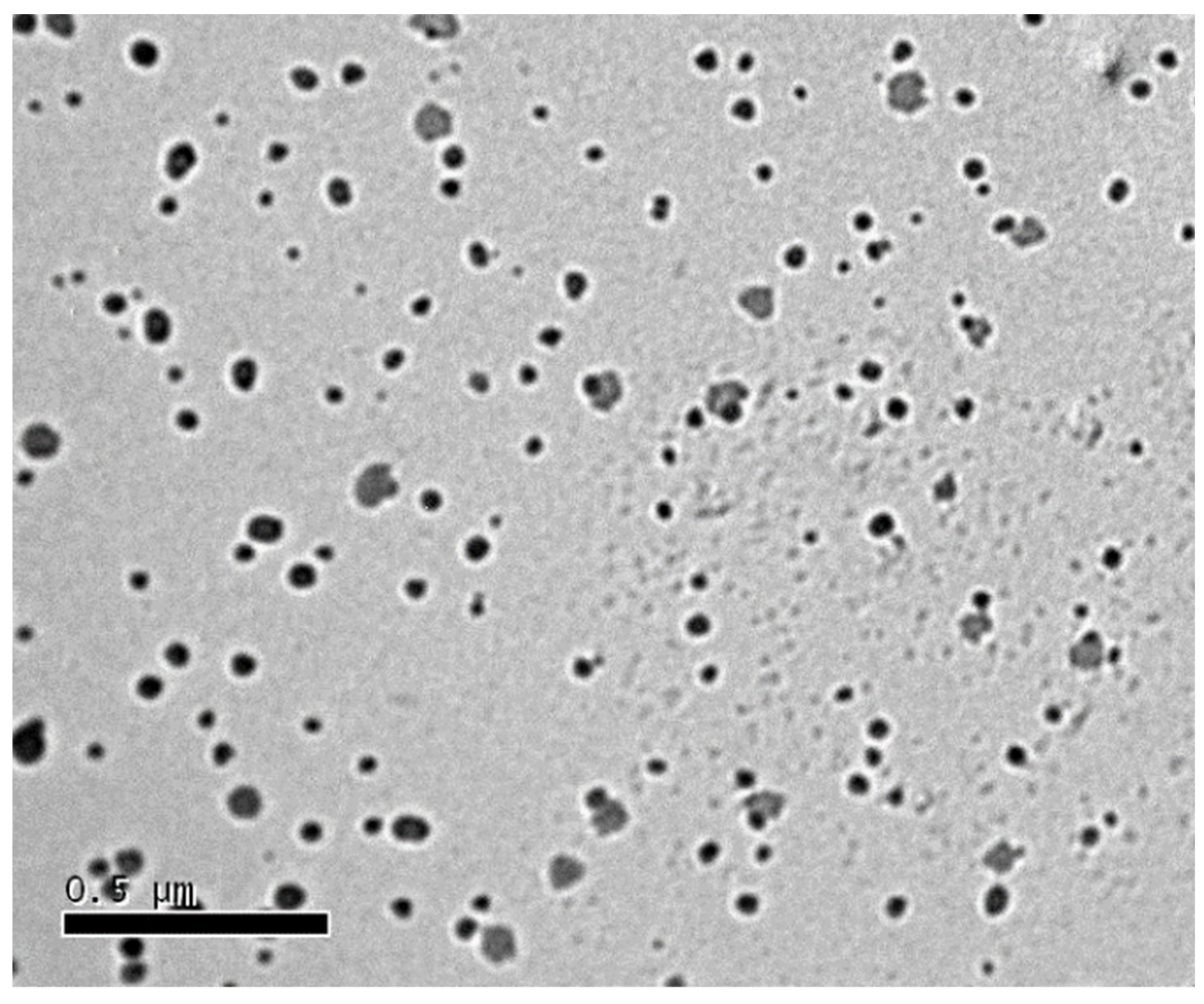
3.6. Transmission Electron Microscopy
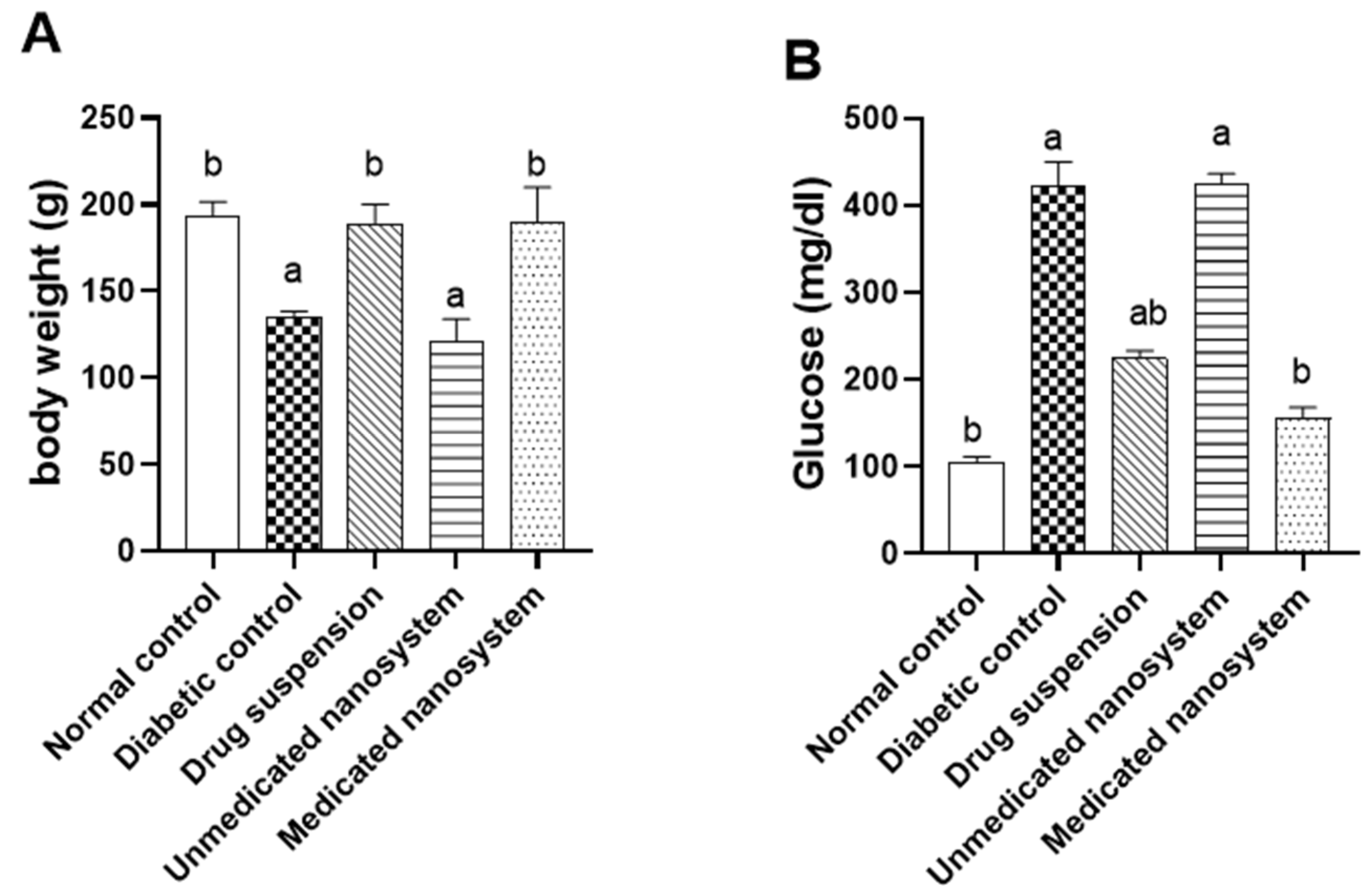
3.7. Effects of β-Sitosterol Glucoside on Body Weight and Serum Glucose
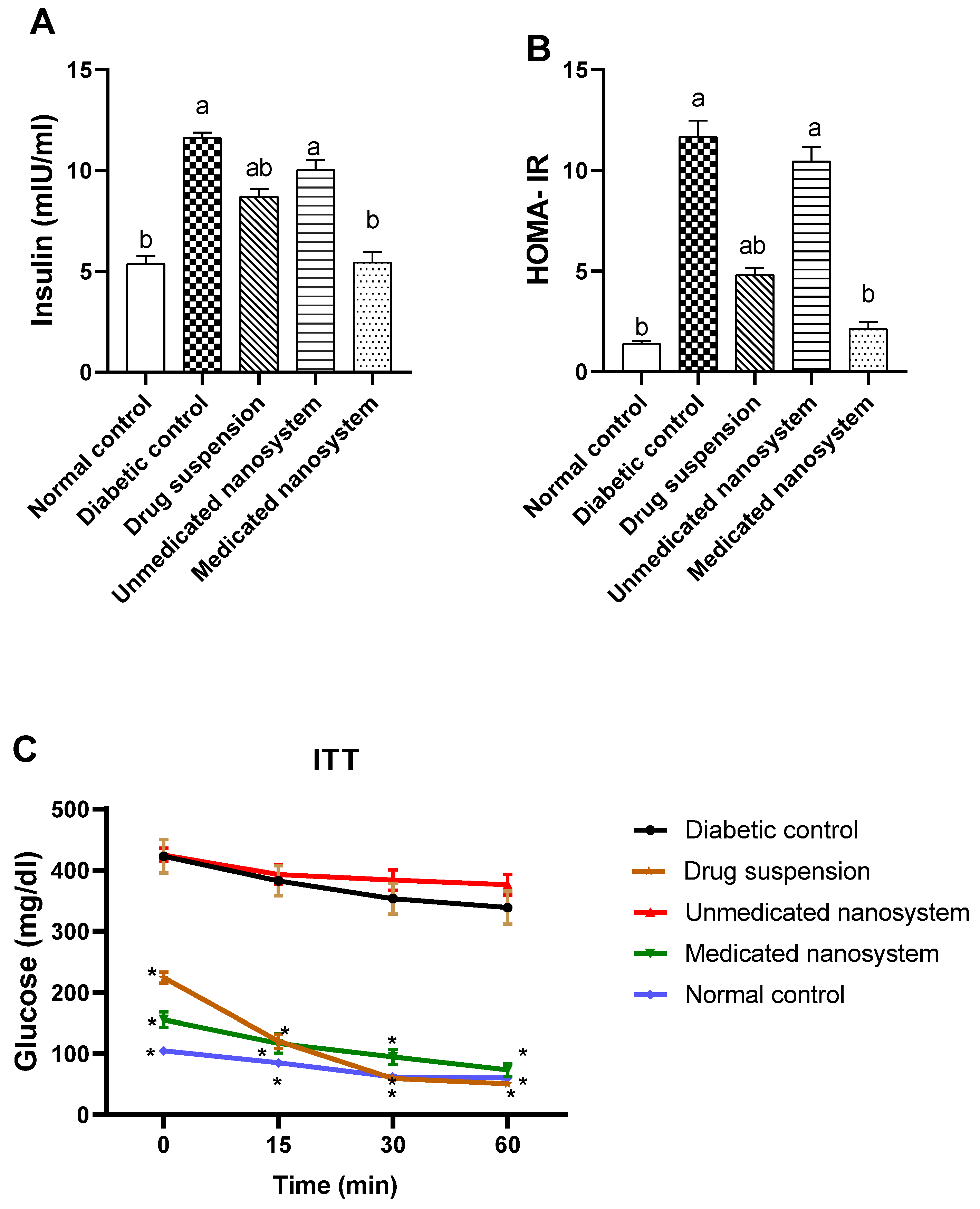
3.8. Effect of β-Sitosterol Glucoside on Serum Insulin, HOMA-IR, and Insulin Tolerance
3.9. Effects of β-Sitosterol Glucoside on Serum Malondialdehyde and Catalase Levels
3.10. Effects of β-Sitosterol Glucoside on Serum Glucagon Levels
3.11. Effects of β-Sitosterol Glucoside on Pancreatic Histological Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2019, 47, 22–27. [Google Scholar] [CrossRef]
- Afroz, A.; Zhang, W.; Wei Loh, A.J.; Jie Lee, D.X.; Billah, B. Macro- and micro-vascular complications and their determinants among people with type 2 diabetes in Bangladesh. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Miaffo, D.; Ntchapda, F.; Mahamad, T.A.; Maidadi, B.; Kamanyi, A. Hypoglycemic, antidyslipidemic and antioxydant effects of Vitellaria paradoxa barks extract on high-fat diet and streptozotocin-induced type 2 diabetes rats. Metab. Open 2021, 9, 100071. [Google Scholar] [CrossRef] [PubMed]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral hypoglycemic drugs: Pathophysiological basis of their mechanism of action oral hypoglycemic drugs: Pathophysiological basis of their mechanism of action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef] [Green Version]
- Mandlik, R.V.; Naik, S.R.; Zine, S.; Ved, H.; Doshi, G. Antidiabetic activity of Caulerpa racemosa: Role of proinflammatory mediators, oxidative stress, and other biomarkers. Planta Med. Int. Open 2022, 9, e60–e71. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2003, 88, 2399–2403. [Google Scholar] [CrossRef]
- Iamonico, D. Alien taxa of the tribe Senecioneae (Asteraceae) in Italy: A nomenclatural synopsis. Hacquetia 2017, 16, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Yao, H.; Song, J.; Zhu, Y.; Liu, C.; Chen, S. Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evol. Biol. 2010, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Funston, A.M. Taxonomic revision of Roldana (Asteraceae: Senecioneae), a genus of the southwestern USA, Mexico, and Central America. Ann. Mo. Bot. Gard. 2008, 95, 282–337. [Google Scholar] [CrossRef]
- Yan, Y.; Lei, Z.; YuFang, W.; ManLi, C.; ChangHong, H.; YuCheng, G.; QingWen, S.; Kiyota, H. Chemical and pharmacological research on plants from the genus Senecio. Chem. Biodivers. 2011, 8, 13–72. [Google Scholar]
- Portero, A.; González-Coloma, A.; Reina, M.; Díaz, C.E. Plant-defensive sesquiterpenoids from Senecio species with biopesticide potential. Phytochem. Rev. 2012, 11, 391–403. [Google Scholar] [CrossRef]
- McDonald, J.A. Alpine flora of Cerro Quiexobra, Oaxaca, Mexico. J. Bot. Res. Inst. Tex. 2013, 7, 765–769. [Google Scholar]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; González-Cortazar, M.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Identification and quantification of β-sitosterol β-D-glucoside of an ethanolic extract obtained by microwave-assisted extraction from Agave angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef] [Green Version]
- Ambavade, S.D.; Misar, A.V.; Ambavade, P.D. Pharmacological, nutritional, and analytical aspects of β-sitosterol: A review. Orient. Pharm. Exp. Med. 2014, 14, 193–211. [Google Scholar] [CrossRef]
- Neslihan Gursoy, R.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Scott Swenson, E.; Curatolo, W.J. Means to enhance penetration: (2) Intestinal permeability enhancement for proteins, peptides and other polar drugs: Mechanisms and potential toxicity. Adv. Drug Deliv. Rev. 1992, 8, 39–92. [Google Scholar] [CrossRef]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Drug Carr. Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef]
- Kamel, R.; Basha, M. Preparation and in vitro evaluation of rutin nanostructured liquisolid delivery system. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Khoo, S.-M.; Humberstone, A.J.; Porter, C.J.H.; Edwards, G.A.; Charman, W.N. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int. J. Pharm. 1998, 167, 155–164. [Google Scholar] [CrossRef]
- Yang, S.C.; Lu, L.F.; Cai, Y.; Zhu, J.B.; Liang, B.W.; Yang, C.Z. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release 1999, 59, 299–307. [Google Scholar] [CrossRef]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; di Cesare Mannelli, L.; Ghelardini, C.; Cirri, M.; Maestrelli, F. β-Sitosterol loaded nanostructured lipid carrier: Physical and oxidative stability, in vitro simulated digestion and hypocholesterolemic activity. Pharmaceutics 2020, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Runtuwene, J.; Cheng, K.-C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.R.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des Devel 2016, 10, 2193–2202. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Bancroft, J.D.G.M. Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 2008. [Google Scholar]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-hepatocellular-cancer activity exerted by β-sitosterol and β-sitosterol-glucoside from Indigofera zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-cancer activity exerted by β-sitosterol-D-glucoside from sweet potato via upregulation of microRNA-10a and via the PI3K–Akt signaling pathway. J. Agric. Food Chem. 2018, 66, 9704–9718. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Langer, R.; Shastri, V.P. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm. Res. 2003, 20, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, A.; Passalacqua, A.; Wickham, M.S.J.; Faulks, R.M.; Craig, D.Q.M.; Barker, S.A. The effect of composition and gastric conditions on the self-emulsification process of ibuprofen-loaded self-emulsifying drug delivery systems: A microscopic and dynamic gastric model study. Pharm. Res. 2011, 28, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Kommuru, T.R.; Gurley, B.; Khan, M.A.; Reddy, I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Kahlweit, M.; Busse, G.; Faulhaber, B.; Eibl, H. Preparing nontoxic microemulsions. Langmuir 1995, 11, 4185–4187. [Google Scholar] [CrossRef]
- Kamel, R.; Mostafa, D.M. Rutin nanostructured lipid cosmeceutical preparation with sun protective potential. J. Photochem. Photobiol. B Biol. 2015, 153, 59–66. [Google Scholar] [CrossRef]
- Kim, S.; Ng, W.K.; Shen, S.; Dong, Y.; Tan, R.B.H. Phase behavior, microstructure transition, and antiradical activity of sucrose laurate/propylene glycol/the essential oil of Melaleuca alternifolia/water microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 289–297. [Google Scholar] [CrossRef]
- Kallakunta, V.R.; Bandari, S.; Jukanti, R.; Veerareddy, P.R. Oral self emulsifying powder of lercanidipine hydrochloride: Formulation and evaluation. Powder Technol. 2012, 221, 375–382. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.G.; Suddek, G.M.; Salem, H.A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021, 83, 103577. [Google Scholar] [CrossRef] [PubMed]
- Swanston-Flatt, S.K.; Day, C.; Bailey, C.J.; Flatt, P.R. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990, 33, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Reaven, G.M. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: The price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol. Metab. Clin. 2005, 34, 49–62. [Google Scholar] [CrossRef]
- Kozawa, J.; Iwahashi, H.; Okita, K.; Okauchi, Y.; Imagawa, A.; Shimomura, I. Insulin tolerance test predicts the effectiveness of insulin sensitizers in japanese type 2 diabetic patients. Diabetes Ther. 2010, 1, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Veerapur, V.P.; Prabhakar, K.R.; Kandadi, M.R.; Srinivasan, K.K.; Unnikrishnan, M.K. Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: A mechanistic approach. Pharm. Biol. 2010, 48, 1137–1148. [Google Scholar] [CrossRef]
- Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and antioxidative potential of the blue congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal Gilani, S.; Nasser Bin-Jumah, M.; Al-Abbasi, F.A.; Shahid Nadeem, M.; Afzal, M.; Sayyed, N.; Kazmi, I. Fustin ameliorates hyperglycemia in streptozotocin induced type-2 diabetes via modulating glutathione/superoxide dismutase/catalase expressions, suppress lipid peroxidation and regulates histopathological changes. Saudi J. Biol. Sci. 2021, 28, 6963–6971. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Pacini, G.; Ahrén, B.; D’Argenio, D.Z. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides 2017, 88, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suanarunsawat, T.; Klongpanichapak, S.; Rungseesantivanon, S.; Chaiyabutr, N. Glycemic effect of stevioside and Stevia rebaudiana in streptozotocin-induced diabetic rats. East. J. Med. 2004, 9, 51–56. [Google Scholar]
- Kulina, G.R.; Rayfield, E.J. The role of glucagon in the pathophysiology and management of diabetes. Endocr Pract. 2016, 22, 612–621. [Google Scholar] [CrossRef]
- Færch, K.; Vistisen, D.; Pacini, G.; Torekov, S.S.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 2016, 65, 3473–3481. [Google Scholar] [CrossRef] [Green Version]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Richard, E.; Ostlund, J. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Pegel, K.H.D. The importance of sitosterol and sitosterolin in human and animal nutrition. S. Afr. J. Sci. 1997, 93, 263–268. [Google Scholar]
- Uppoor, V.R.S. Regulatory perspectives on in vitro (dissolution)/in vivo (bioavailability) correlations. J. Control. Release 2001, 72, 127–132. [Google Scholar] [CrossRef]
- Cao, X.; Deng, W.; Fu, M.; Zhu, Y.; Liu, H.; Wang, L.; Zeng, J.; Wei, Y.; Xu, X.; Yu, J. Seventy-two-hour release formulation of the poorly soluble drug silybin based on porous silica nanoparticles: In vitro release kinetics and in vitro/in vivo correlations in beagle dogs. Eur. J. Pharm. Sci. 2013, 48, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kim, S.; Park, K. In vitro–in vivo correlation: Perspectives on model development. Int. J. Pharm. 2011, 418, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Picatoste, B.; Yammine, L.; Leahey, R.A.; Soares, D.; Johnson, E.F.; Cohen, P.; McGraw, T.E. Defective insulin-stimulated GLUT4 translocation in brown adipocytes induces systemic glucose homeostasis dysregulation independent of thermogenesis in female mice. Mol. Metab. 2021, 53, 101305. [Google Scholar] [CrossRef]
- Pandey, J.; Dev, K.; Chattopadhyay, S.; Kadan, S.; Sharma, T.; Maurya, R.; Sanyal, S.; Siddiqi, M.I.; Zaid, H.; Tamrakar, A.K. β-Sitosterol-D-glucopyranoside mimics estrogenic properties and stimulates glucose utilization in skeletal muscle cells. Molecules 2021, 26, 3129. [Google Scholar] [CrossRef]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The role of JNk signaling pathway in obesity-driven insulin resistance. Diabetes Metab. Syndr. Obes. 2020, 13, 1399–1406. [Google Scholar] [CrossRef]
- Gao, P.; Huang, X.; Liao, T.; Li, G.; Yu, X.; You, Y.; Huang, Y. Daucosterol induces autophagic-dependent apoptosis in prostate cancer via JNK activation. Biosci. Trends 2019, 13, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit. Rev. Food Sci. Nutr. 2017, 57, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, H.; Salehi, P.; Amiri, M.S. Bioassay-guided purification of α-amylase, α-glucosidase inhibitors and DPPH radical scavengers from roots of Rheum turkestanicum. Ind. Crops Prod. 2018, 117, 303–309. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, J.; Yin, J.; Staszkiewicz, J.; Gawronska-Kozak, B.; Jung, D.Y.; Ko, H.J.; Ong, H.; Kim, J.K.; Mynatt, R.; et al. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through elevated energy expenditure 2. J. Biol. Chem. 2010, 285, 4637–4644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, U.J.; Chang, L.C. Chemical constituents of fermented noni (Morinda citrifolia) juice exudates and their biological activity. Nat. Prod. Sci. 2017, 23, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Nualkaew, S.; Padee, P.; Talubmook, C. Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O-β-D glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. J. Med. Plants Res. 2015, 9, 629–635. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afifi, S.M.; Ammar, N.M.; Kamel, R.; Esatbeyoglu, T.; Hassan, H.A. β-Sitosterol Glucoside-Loaded Nanosystem Ameliorates Insulin Resistance and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants 2022, 11, 1023. https://doi.org/10.3390/antiox11051023
Afifi SM, Ammar NM, Kamel R, Esatbeyoglu T, Hassan HA. β-Sitosterol Glucoside-Loaded Nanosystem Ameliorates Insulin Resistance and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants. 2022; 11(5):1023. https://doi.org/10.3390/antiox11051023
Chicago/Turabian StyleAfifi, Sherif M., Naglaa M. Ammar, Rabab Kamel, Tuba Esatbeyoglu, and Heba A. Hassan. 2022. "β-Sitosterol Glucoside-Loaded Nanosystem Ameliorates Insulin Resistance and Oxidative Stress in Streptozotocin-Induced Diabetic Rats" Antioxidants 11, no. 5: 1023. https://doi.org/10.3390/antiox11051023
APA StyleAfifi, S. M., Ammar, N. M., Kamel, R., Esatbeyoglu, T., & Hassan, H. A. (2022). β-Sitosterol Glucoside-Loaded Nanosystem Ameliorates Insulin Resistance and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants, 11(5), 1023. https://doi.org/10.3390/antiox11051023






