Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pigs, Experimental Design, and Housing
2.2. Growth Performance and Diarrhea Rate
2.3. Complete Blood Count (CBC) Test
2.4. Antioxidant Indicators Measurements
2.5. DNA Extraction and 16S rRNA Gene Sequencing
2.6. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Diarrhea and Survival Rate
3.3. CBC Test Indicators
3.4. Antioxidant Capacity Level
3.5. Microbiological Analysis of Gastrointestinal Contents
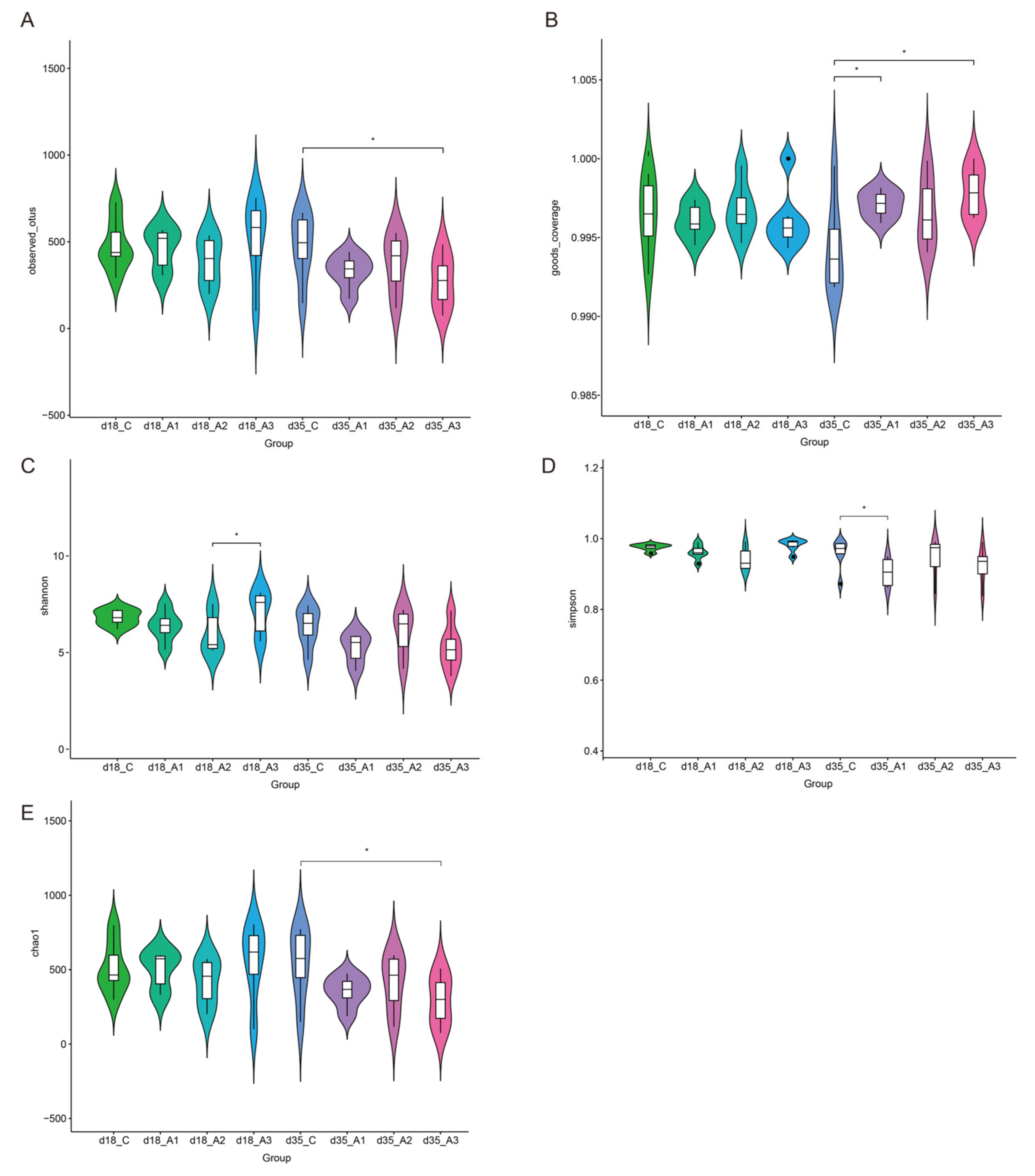
3.5.1. Alpha Diversity Analysis
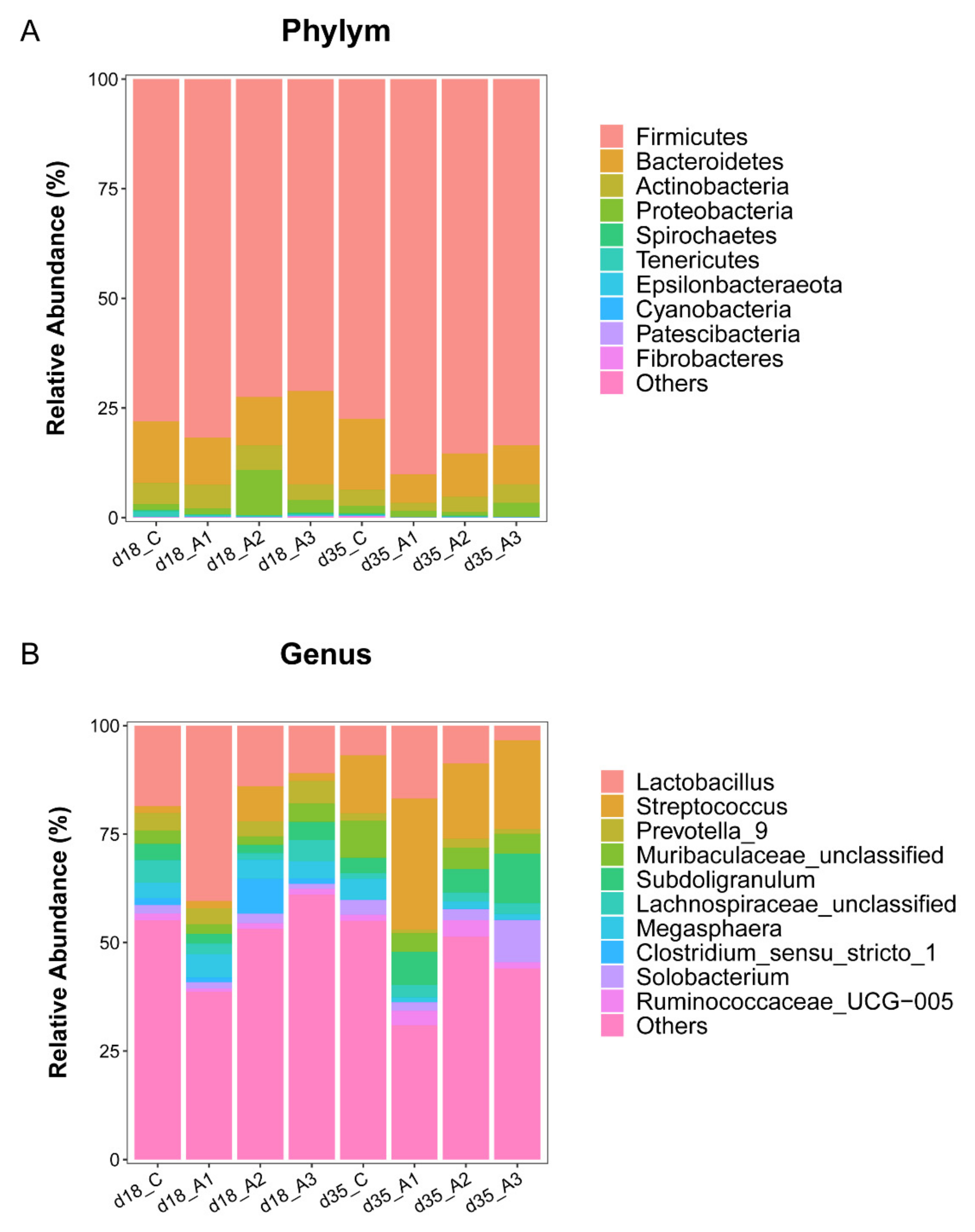
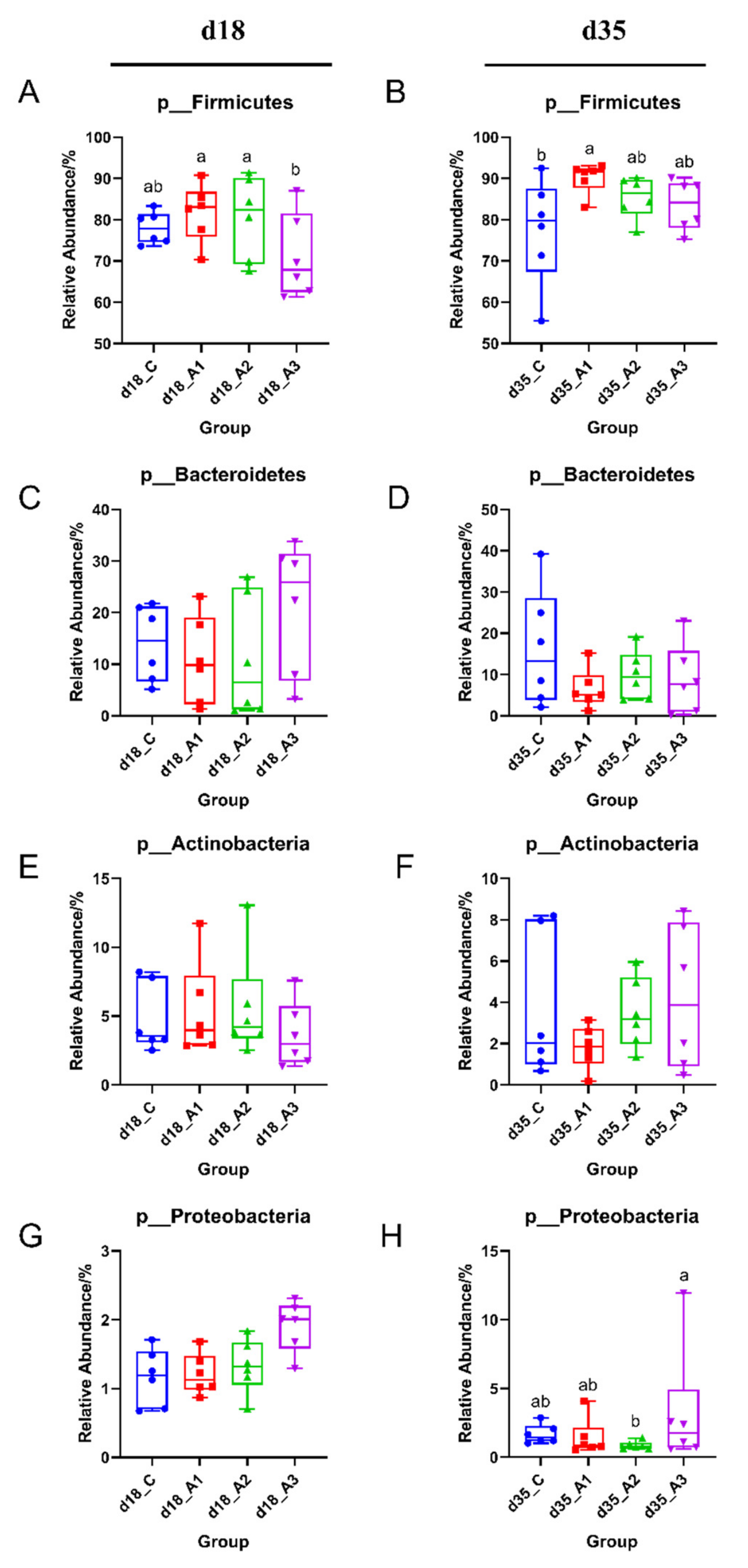
3.5.2. Analysis of Group Differences of Intestinal Flora at the Phylum Level
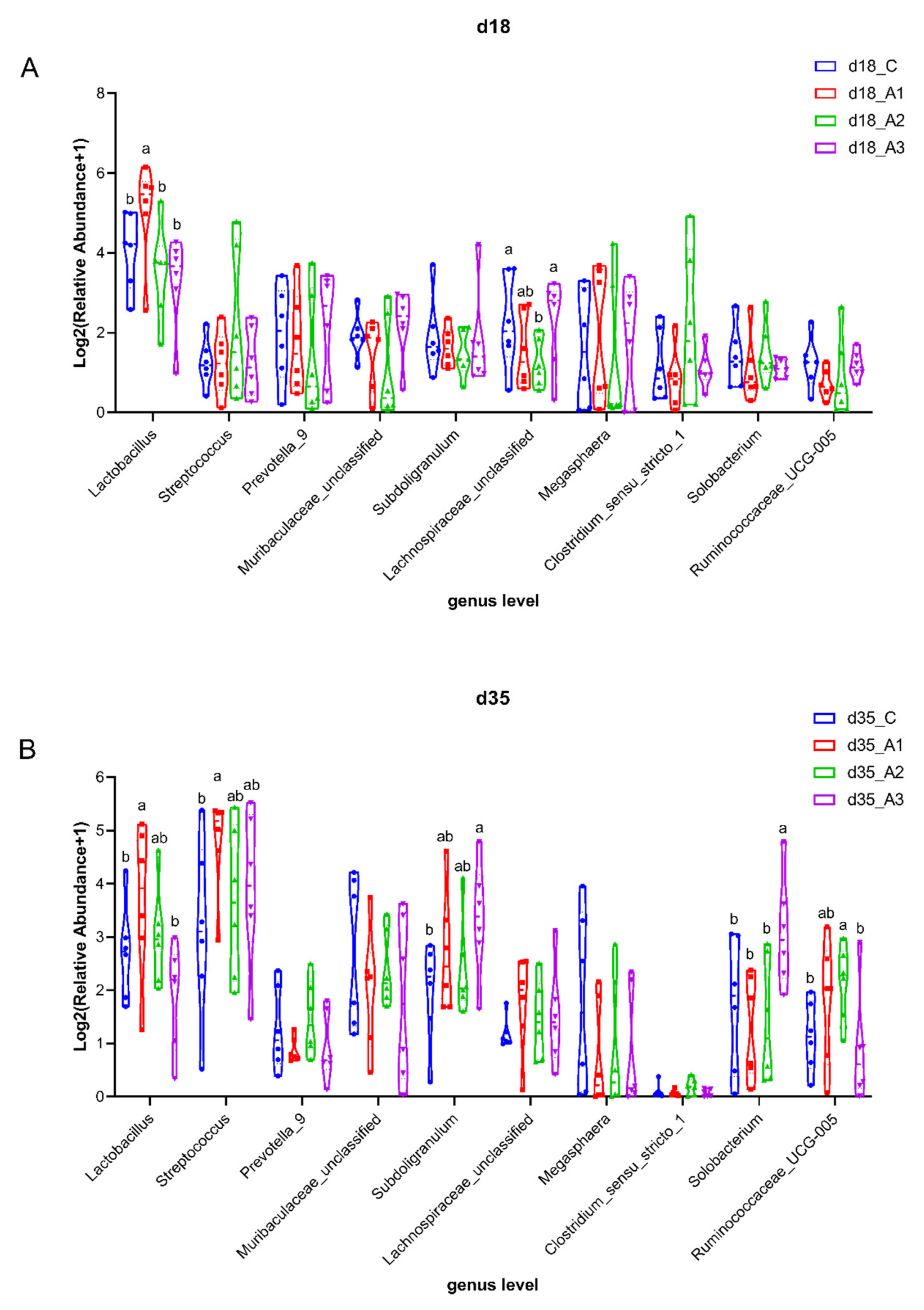
3.5.3. Analysis of Group Differences of Intestinal Flora at the Genus Level
3.6. Partial Correlation Analyses between the Differential Microbial Species and Measured Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koketsu, Y.; Tani, S.; Iida, R. Factors for improving reproductive performance of sows and herd productivity in commercial breeding herds. Porc. Health Manag. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.J.; Kuwaki, K.; Knosalla, C.; Dor, F.J.; Gollackner, B.; Wilkinson, R.A.; Arn, S.; Sachs, D.H.; Cooper, D.K.; Fishman, J.A. Early weaning of piglets fails to exclude porcine lymphotropic herpesvirus. Xenotransplantation 2005, 12, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Yang, Z.; Li, C.; Liang, H.; Wu, Z.; Pu, W. Yeast Probiotics Shape the Gut Microbiome and Improve the Health of Early-Weaned Piglets. Front. Microbiol. 2018, 9, 2011. [Google Scholar] [CrossRef]
- Hung, D.Y.; Cheng, Y.H.; Chen, W.J.; Hua, K.F.; Pietruszka, A.; Dybus, A.; Lin, C.S.; Yu, Y.H. Bacillus licheniformis-Fermented Products Reduce Diarrhea Incidence and Alter the Fecal Microbiota Community in Weaning Piglets. Animals 2019, 9, 1145. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.L.; Thymann, T.; Cilieborg, M.S.; Lykke, M.; Molbak, L.; Jensen, B.B.; Schmidt, M.; Kelly, D.; Mulder, I.; Burrin, D.G.; et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G59–G71. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, J.M.; Hampton, R.S.; Johnson, H.M.; Callaway, T.R.; Rothrock, M.J., Jr.; Azain, M.J. The Effects of Feeding Antibiotic on the Intestinal Microbiota of Weanling Pigs. Front. Vet. Sci. 2021, 8, 601394. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Hu, Y.; Huang, J.; Yang, H.; Wang, L.; Chen, S.; Chen, C.; He, S. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020, 98, skaa112. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Yu, B.; Degroote, J.; Spranghers, T.; Van Noten, N.; Majdeddin, M.; Van Poucke, M.; Peelman, L.; De Vrieze, J.; Boon, N.; et al. Antibiotic affects the gut microbiota composition and expression of genes related to lipid metabolism and myofiber types in skeletal muscle of piglets. BMC Vet. Res. 2020, 16, 392. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, Y.; Yu, B.; Chen, D.W.; Mao, X.B.; Zheng, P.; Luo, J.Q.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Luo, Y.; Li, Y.; He, J. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. 2018, 9, 4968–4978. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Yan, H.; et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Canibe, N.; Hojberg, O.; Hojsgaard, S.; Jensen, B.B. Feed physical form and formic acid addition to the feed affect the gastrointestinal ecology and growth performance of growing pigs. J. Anim. Sci. 2005, 83, 1287–1302. [Google Scholar] [CrossRef]
- Matsumoto, H.; Miyagawa, M.; Yin, Y.; Oosumi, T. Effects of organic acid, Enterococcus faecalis strain EC-12 and sugar cane extract in feed against enterotoxigenic Escherichia coli-induced diarrhea in pigs. Amb. Express 2021, 11, 68. [Google Scholar] [CrossRef]
- Dahmer, P.L.; Jones, C.K. The Impacts of Commercial Dietary Acidifiers on Growth Performance of Nursery Pigs. J. Anim. Sci. 2021, 99, 87. [Google Scholar] [CrossRef]
- Mudarra, R.A.; Tsai, T.C.C.; Bottoms, K.; Shieh, T.S.; Bradly, C.; Maxwell, C.V. Effect of Adding Bioactive Peptide in Combination of Pharmaceutical Zinc Oxide or Organic Acids on Growth Performance, Hematology Profile, and Nutrient Digestibility in Nursery Pigs. J. Anim. Sci. 2021, 99, 22. [Google Scholar] [CrossRef]
- Marden, J.P.; Julien, C.; Monteils, V.; Auclair, E.; Moncoulon, R.; Bayourthe, C. How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high-yielding dairy cows? J. Dairy Sci. 2008, 91, 3528–3535. [Google Scholar] [CrossRef] [Green Version]
- Lingbeek, M.M.; Borewicz, K.; Febery, E.; Han, Y.; Doelman, J.; van Kuijk, S.J.A. Short-chain fatty acid administration via water acidifier improves feed efficiency and modulates fecal microbiota in weaned piglets. J. Anim. Sci. 2021, 99, skab307. [Google Scholar] [CrossRef]
- Mustafa, A.; Bai, S.P.; Zeng, Q.F.; Ding, X.M.; Wang, J.P.; Xuan, Y.; Su, Z.W.; Zhang, K.Y. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express 2021, 11, 140. [Google Scholar] [CrossRef]
- De Busser, E.V.; Dewulf, J.; Nollet, N.; Houf, K.; Schwarzer, K.; De Sadeleer, L.; De Zutter, L.; Maes, D. Effect of organic acids in drinking water during the last 2 weeks prior to slaughter on Salmonella shedding by slaughter pigs and contamination of carcasses. Zoonoses Public Health 2009, 56, 129–136. [Google Scholar] [CrossRef]
- Kuley, E.; Ozyurt, G.; Ozogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Ozogul, F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Qi, L.; Lv, Z.; Wei, Q.; Shi, F. Dietary stevioside supplementation increases feed intake by altering the hypothalamic transcriptome profile and gut microbiota in broiler chickens. J. Sci. Food. Agric. 2021, 101, 2156–2167. [Google Scholar] [CrossRef]
- Wu, X.; Yang, P.; Sifa, D.; Wen, Z. Effect of dietary stevioside supplementation on growth performance, nutrient digestibility, serum parameters, and intestinal microflora in broilers. Food Funct. 2019, 10, 2340–2346. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Kim, D.H.; Cervantes, A.A.P.; Arriola, K.G.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Fate of Escherichia coli O157:H7 and bacterial diversity in corn silage contaminated with the pathogen and treated with chemical or microbial additives. J. Dairy Sci. 2017, 100, 1780–1794. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Toft, N. Intra- and inter-observer agreement when using a descriptive classification scale for clinical assessment of faecal consistency in growing pigs. Prev. Vet. Med. 2011, 98, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Nowak, P.; Zaworska-Zakrzewska, A.; Frankiewicz, A.; Kasprowicz-Potocka, M. The Effects and Mechanisms of Acids on the Health of Piglets and Weaners—A Review. Ann. Anim. Sci. 2021, 21, 433–455. [Google Scholar] [CrossRef]
- Gong, J.; Yu, H.; Liu, T.; Li, M.; Si, W.; de Lange, C.F.M.; Dewey, C. Characterization of ileal bacterial microbiota in newly-weaned pigs in response to feeding lincomycin, organic acids or herbal extract. Livest. Sci. 2008, 116, 318–322. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2019, 97, 133–143. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Rasschaert, G.; Dierick, N.; Heyndrickx, M.; De Smet, S. Effect of organic acids on Salmonella colonization and shedding in weaned piglets in a seeder model. J. Food Prot. 2012, 75, 1974–1983. [Google Scholar] [CrossRef]
- Creus, E.; Perez, J.F.; Peralta, B.; Baucells, F.; Mateu, E. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health 2007, 54, 314–319. [Google Scholar] [CrossRef]
- Tsiloyiannis, V.K.; Kyriakis, S.C.; Vlemmas, J.; Sarris, K. The effect of organic acids on the control of porcine post-weaning diarrhoea. Res. Vet. Sci. 2001, 70, 287–293. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hwang, J.A.; Hoon, J.; Mun, H.S.; Yang, C.J. Comparison of Single and Blend Acidifiers as Alternative to Antibiotics on Growth Performance, Fecal Microflora, and Humoral Immunity in Weaned Piglets. Asian Austral. J. Anim. 2014, 27, 93–100. [Google Scholar] [CrossRef]
- Mou, Q.; Yang, H.S.; Yin, Y.L.; Huang, P.F. Amino Acids Influencing Intestinal Development and Health of the Piglets. Animals 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhang, M.; Zhao, L.; Ge, K.; Wang, Z.; Jun, L.; Ren, F. Growth Performance and Post-Weaning Diarrhea in Piglets Fed a Diet Supplemented with Probiotic Complexes. J. Microbiol. Biotechnol. 2018, 28, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, F.; Harding, J.C.; Mote, B.; Ladinig, A.; Plastow, G.S.; Rothschild, M.F. Genomic investigation of piglet resilience following porcine epidemic diarrhea outbreaks. Anim. Genet. 2017, 48, 228–232. [Google Scholar] [CrossRef]
- Bosi, P.; Sarli, G.; Casini, L.; De Filippi, S.; Trevisi, P.; Mazzoni, M.; Merialdi, G. The influence of fat protection of calcium formate on growth and intestinal defence in Escherichia coli K88-challenged weanling pigs. Anim. Feed Sci. Technol. 2007, 139, 170–185. [Google Scholar] [CrossRef]
- Andersen, H.M.; Dybkjaer, L.; Herskin, M.S. Growing pigs’ drinking behaviour: Number of visits, duration, water intake and diurnal variation. Animal 2014, 8, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Shen, C.; Chen, R.; Gao, S.; Liu, X.; Schinckel, A.P.; Zhou, B. Reestablishment of Social Hierarchies in Weaned Pigs after Mixing. Animals 2019, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a Potential Mediator of Cardiovascular Risk in Metabolic Diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; An, Z.; Chen, H.; He, X.; Wang, W.; Li, X.; Zhang, H.; Li, F.; Liu, D. Ulva prolifera Extract Alleviates Intestinal Oxidative Stress via Nrf2 Signaling in Weaned Piglets Challenged With Hydrogen Peroxide. Front. Immunol. 2020, 11, 599735. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Novais, A.K.; Martel-Kennes, Y.; Roy, C.; Deschene, K.; Beaulieu, S.; Bergeron, N.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Tissue-specific profiling reveals modulation of cellular and mitochondrial oxidative stress in normal- and low-birthweight piglets throughout the peri-weaning period. Animal 2020, 14, 1014–1024. [Google Scholar] [CrossRef]
- Meng, Q.; Luo, Z.; Cao, C.; Sun, S.; Ma, Q.; Li, Z.; Shi, B.; Shan, A. Weaning Alters Intestinal Gene Expression Involved in Nutrient Metabolism by Shaping Gut Microbiota in Pigs. Front. Microbiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.J.; Berrios, R.; Stelzhammer, S.; Bracarense, A. Ingestion of organic acids and cinnamaldehyde improves tissue homeostasis of piglets exposed to enterotoxic Escherichia coli (ETEC). J. Anim. Sci. 2020, 98, skaa012. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.D.; Deng, Z.C.; Wang, Y.W.; Sun, H.; Wang, L.; Han, Y.M.; Wu, Y.Y.; Liu, J.G.; Sun, L.H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Balintova, J.; Welter, M.; Marx, A. Antibody-nucleotide conjugate as a substrate for DNA polymerases. Chem. Sci. 2018, 9, 7122–7125. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.C.; Yang, Y.H.; Zhu, Q.; Wang, Z.H.; Hu, G.J.; Shi, H.C.; Zhou, X.H. ELISA-Based Method for Variant-Independent Detection of Total Microcystins and Nodularins via a Multi-immunogen Approach. Environ. Sci. Technol. 2021, 55, 12984–12993. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ma, J.; Li, P.; Geng, Z.; Sun, C.; Wang, D.; Xu, W. Development of indirect competitive ELISA for determination of dehydroabietic acid in duck skin and comparison with the HPLC method. Poult. Sci. 2020, 99, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Martinez-Subiela, S.; Hernandez-Ruiz, J.; Tvarijonaviciute, A.; Ceron, J.J. Analytical validation of an automated assay for ferric-reducing ability of plasma in dog serum. J. Vet. Diagn. Investig. 2017, 29, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Beaumont, M.; Cauquil, L.; Bertide, A.; Ahn, I.; Barilly, C.; Gil, L.; Canlet, C.; Zemb, O.; Pascal, G.; Samson, A.; et al. Gut Microbiota-Derived Metabolite Signature in Suckling and Weaned Piglets. J. Proteome Res. 2021, 20, 982–994. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Y.; Su, Y.; Gao, Z.; Diao, X.; Li, J.; Zhu, X.; Li, D.; Li, Z.; Wang, C.; et al. Dietary Fiber Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Function Damage in Piglets by Modulation of Intestinal Microbiome. Msystems 2021, 6, e01374-20. [Google Scholar] [CrossRef]
- De Busser, E.V.; Dewulf, J.; Zutter, L.D.; Haesebrouck, F.; Callens, J.; Meyns, T.; Maes, W.; Maes, D. Effect of administration of organic acids in drinking water on faecal shedding of E. coli, performance parameters and health in nursery pigs. Vet. J. 2011, 188, 184–188. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Arfken, A.M.; Frey, J.F.; Ramsay, T.G.; Summers, K.L. Yeasts of Burden: Exploring the Mycobiome-Bacteriome of the Piglet GI Tract. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Dhakal, S.; Wang, L.; Antony, L.; Rank, J.; Bernardo, P.; Ghimire, S.; Bondra, K.; Siems, C.; Lakshmanappa, Y.S.; Renu, S.; et al. Amish (Rural) vs. non-Amish (Urban) Infant Fecal Microbiotas Are Highly Diverse and Their Transplantation Lead to Differences in Mucosal Immune Maturation in a Humanized Germfree Piglet Model. Front. Immunol. 2019, 10, 1509. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Lan, W.; Liu, G.; Ni, H.; Gu, J.D. Exploring possible associations of the intestine bacterial microbiome with the pre-weaned weight gaining performance of piglets in intensive pig production. Sci. Rep. 2019, 9, 15534. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Long, S.; Wang, Q.; Zhang, L.; Hu, J.; Yang, J.; Cheng, Z.; Piao, X. Mixed organic acids improve nutrients digestibility, volatile fatty acids composition and intestinal microbiota in growing-finishing pigs fed high-fiber diet. Asian-Australas J. Anim. Sci. 2019, 32, 856–864. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Ubiquitous and persistent Proteobacteria and other Gram-negative bacteria in drinking water. Sci. Total Environ. 2017, 586, 1141–1149. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Kim, I. Effects of organic acid and medium chain fatty acid blends on the performance of sows and their piglets. Anim. Sci. J. 2018, 89, 1673–1679. [Google Scholar] [CrossRef]
- Lei, L.F.; Wang, Z.B.; Li, J.Z.; Yang, H.S.; Yin, Y.L.; Tan, B.; Chen, J.S. Comparative Microbial Profiles of Colonic Digesta between Ningxiang Pig and Large White Pig. Animals 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Q.; Ran, Y.; Lin, L.; Chen, P.; He, J.; Chen, Y.; Wen, J. Multiomics Study Reveals Enterococcus and Subdoligranulum Are Beneficial to Necrotizing Enterocolitis. Front. Microbiol. 2021, 12, 752102. [Google Scholar] [CrossRef]
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.D.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Xu, H.J.; Zhang, Q.Y.; Wang, L.H.; Zhang, C.R.; Li, Y.; Zhang, Y.G. Growth performance, digestibility, blood metabolites, ruminal fermentation, and bacterial communities in response to the inclusion of gallic acid in the starter feed of preweaning dairy calves. J. Dairy Sci. 2022, 105, 3078–3089. [Google Scholar] [CrossRef]
- Wu, W.Y.; Chou, P.L.; Yang, J.C.; Chien, C.T. Silicon-containing water intake confers antioxidant effect, gastrointestinal protection, and gut microbiota modulation in the rodents. PLoS ONE 2021, 16, e0248508. [Google Scholar] [CrossRef]

| Items | Treatment 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | A1 | A2 | A3 | |||
| IBW, kg | 9.20 c | 10.41 a | 10.02 b | 9.05 c | 0.14 | 0.002 |
| FBW, kg | 24.81 b | 27.16 a | 26.01 a,b | 25.38 a,b | 0.42 | 0.091 |
| ADG, g | 445.29 b | 512.25 a | 478.81 a,b | 462.26 b | 12.85 | 0.040 |
| ADFI, g | 645.40 b | 743.80 a | 696.20 a,b | 653.50 b | 11.35 | 0.024 |
| F:G | 1.45 | 1.45 | 1.46 | 1.41 | 0.009 | 0.130 |
| Items | Treatment 1 | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | A1 | A2 | A3 | |||
| Diarrhea rate, % | ||||||
| d 1 to 18 | 3.11 | 2.72 | 2.95 | 2.78 | 0.30 | 0.802 |
| d 19 to 35 | 3.00 | 2.94 | 3.00 | 2.94 | 0.23 | 0.995 |
| d 1 to 35 | 3.06 | 2.83 | 2.97 | 2.86 | 0.25 | 0.905 |
| Survival rate, % | ||||||
| d 1 to 35 | 93.00 b | 96.00 a,b | 99.00 a | 95.00 a,b | 1.56 | 0.107 |
| Items 1 | Treatment 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | A1 | A2 | A3 | |||
| d18 | ||||||
| WBC, 109/L | 21.17 | 18.56 | 17.92 | 20.50 | 2.09 | 0.653 |
| Neu, 109/L | 6.59 a,b | 2.74 b | 2.96 b | 8.65 a | 1.39 | 0.007 |
| LYM, 109/L | 12.34 | 12.50 | 12.98 | 10.18 | 1.41 | 0.507 |
| Mon, 109/L | 1.79 a,b | 3.12 a | 1.84 a,b | 1.26 b | 0.55 | 0.109 |
| Eos, 109/L | 0.37 a | 0.15 b | 0.13 b | 0.31 a,b | 0.07 | 0.057 |
| Bas, 109/L | 0.09 | 0.06 | 0.07 | 0.10 | 0.02 | 0.407 |
| Neu, % | 31.52 a | 13.93 b | 14.08 b | 37.08 a | 2.48 | <0.001 |
| LYM, % | 57.66 b,c | 68.46 a,b | 73.65 a | 55.00 c | 4.42 | 0.010 |
| Mon, % | 8.69 c | 15.48 a | 11.46 b | 6.01 c | 1.18 | <0.001 |
| Eos, % | 1.77 a | 0.89 b,c | 0.56 c | 1.50 a,b | 0.27 | 0.008 |
| Bas, % | 0.36 a,b | 0.26 b | 0.26 b | 0.41 a | 0.05 | 0.042 |
| RBC, 109/L | 5.30 a | 4.35 b | 4.53 b | 5.63 a | 0.25 | <0.001 |
| HGB, g/L | 100.56 | 103.30 | 103.01 | 104.92 | 5.45 | 0.366 |
| HCT, % | 30.57 a | 19.80 b | 21.58 b | 31.89 a | 1.40 | <0.001 |
| MCV, fL | 57.66 a | 45.50 b | 46.79 b | 56.18 a | 0.85 | 0.000 |
| MCH, pg | 18.96 b | 22.20 a | 21.83 a | 18.53 b | 0.58 | <0.001 |
| MCHC, g/L | 329.06 c | 477.67 a | 462.67 b | 330.13 c | 12.99 | 0.000 |
| RDW-CV, % | 19.41 c | 36.23 a | 33.28 b | 19.23 c | 0.59 | 0.000 |
| RDW-SD, fL | 38.99 b | 57.04 a | 54.21 a | 37.59 b | 1.01 | 0.000 |
| PLT, 109/L | 247.19 | 371.25 | 384.98 | 212.00 | 65.61 | 0.003 |
| MPV, fL | 9.16 a | 7.99 b | 8.28 b | 9.25 a | 0.29 | 0.001 |
| PDW, % | 15.53 a | 14.95 c | 15.04 b,c | 15.43 a,b | 0.16 | 0.023 |
| PCT, % | 0.23 a,b | 0.18 b | 0.27 a | 0.20 a,b | 0.04 | 0.106 |
| d35 | ||||||
| WBC, 109/L | 26.02 a,b | 29.77 a | 31.40 a | 22.60 b | 2.09 | 0.017 |
| Neu, 109/L | 9.39 | 12.46 | 11.76 | 8.85 | 1.39 | 0.194 |
| LYM, 109/L | 12.93 | 11.91 | 13.77 | 9.90 | 1.41 | 0.246 |
| Mon, 109/L | 2.98 b | 4.40 a,b | 3.76 a | 3.04 b | 0.55 | 0.034 |
| Eos, 109/L | 0.58 b | 0.87 a | 0.87 a | 0.73 a,b | 0.07 | 0.019 |
| Bas, 109/L | 0.15 a | 0.13 a,b | 0.15 a | 0.09 b | 0.02 | 0.061 |
| Neu, % | 34.43 | 40.69 | 37.79 | 37.59 | 1.36 | 0.761 |
| LYM, % | 51.39 | 41.92 | 44.96 | 44.99 | 4.42 | 0.489 |
| Mon, % | 11.49 | 14.03 | 12.08 | 13.74 | 1.18 | 0.360 |
| Eos, % | 2.19 b | 2.96 a | 2.76 a,b | 3.34 a | 0.27 | 0.026 |
| Bas, % | 0.51 a | 0.41 a,b | 0.45 a,b | 0.34 b | 0.02 | 0.078 |
| RBC, 109/L | 5.78 a | 6.03 a | 5.91 a | 5.10 b | 0.10 | 0.006 |
| HGB, g/L | 105.31 a | 104.31 a | 104.75 a | 92.81 b | 5.45 | 0.098 |
| HCT, % | 34.68 a | 36.34 a | 34.51 a | 29.23 b | 1.40 | 0.003 |
| MCV, fL | 59.99 | 60.33 | 58.54 | 59.71 | 0.85 | 0.473 |
| MCH, pg | 18.24 | 17.36 | 17.76 | 18.29 | 0.58 | 0.626 |
| MCHC, g/L | 304.75 | 287.81 | 303.94 | 307.12 | 12.99 | 0.709 |
| RDW-CV, % | 20.13 | 20.62 | 20.86 | 20.07 | 0.59 | 0.740 |
| RDW-SD, fL | 42.36 | 43.53 | 43.13 | 41.84 | 1.01 | 0.646 |
| PLT, 109/L | 227.00 | 305.00 | 346.25 | 256.62 | 65.61 | 0.588 |
| MPV, fL | 9.03 b | 10.07 a | 10.68 a | 9.99 a | 0.29 | 0.001 |
| PDW, % | 16.28 | 16.13 | 15.84 | 16.19 | 0.16 | 0.229 |
| PCT, % | 0.22 b | 0.32 a,b | 0.37 a | 0.28 a,b | 0.04 | 0.048 |
| Items 1 | Treatment 2 | SEM 3 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | A1 | A2 | A3 | |||
| d18 | ||||||
| SOD, U/mL | 141.76 b | 123.87 c | 162.43 a | 140.32 b | 3.51 | <0.001 |
| CAT, U/mL | 6.28 b | 7.70 a | 8.25 a | 5.37 b | 0.37 | < 0.001 |
| GSH, μg/mL | 162.03 b | 218.24 a | 150.95 b | 215.37 a | 10.14 | <0.001 |
| GSH-Px, U/mL | 656.06 | 653.43 | 547.75 | 689.38 | 50.77 | 0.227 |
| T-AOC, U/mL | 4.08 c | 5.12 b | 5.74 a | 5.83 a | 0.19 | <0.001 |
| MDA, nmol/mL | 4.69 a | 2.67 b | 2.75 b | 2.70 b | 0.19 | <0.001 |
| d35 | ||||||
| SOD, U/mL | 118.29 a,b | 127.58 a | 115.74 b | 123.22 a,b | 3.51 | 0.087 |
| CAT, U/mL | 13.89 b | 14.80 a,b | 14.64 a,b | 14.97 a | 0.37 | 0.188 |
| GSH, μg/mL | 195.46 c | 236.22 b | 281.37 a | 227.00 b | 10.14 | <0.001 |
| GSH-Px, U/mL | 436.38 b | 583.96 a | 432.92 b | 551.07 a,b | 50.77 | 0.076 |
| T-AOC, U/mL | 4.23 b | 5.44 a | 4.60 b | 4.41 b | 0.19 | <0.001 |
| MDA, nmol/mL | 2.61 | 2.27 | 2.45 | 2.58 | 0.19 | 0.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.-L.; Liu, C.; Mo, X.-J.; Chen, M.; Zhao, X.-L.; Liu, M.-Z.; Wang, S.-B.; Zhou, B.; Zhao, C.-X. Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants 2022, 11, 809. https://doi.org/10.3390/antiox11050809
Xu Q-L, Liu C, Mo X-J, Chen M, Zhao X-L, Liu M-Z, Wang S-B, Zhou B, Zhao C-X. Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants. 2022; 11(5):809. https://doi.org/10.3390/antiox11050809
Chicago/Turabian StyleXu, Qing-Lei, Chang Liu, Xiao-Jian Mo, Meng Chen, Xian-Le Zhao, Ming-Zheng Liu, Shu-Bai Wang, Bo Zhou, and Cheng-Xin Zhao. 2022. "Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity" Antioxidants 11, no. 5: 809. https://doi.org/10.3390/antiox11050809
APA StyleXu, Q.-L., Liu, C., Mo, X.-J., Chen, M., Zhao, X.-L., Liu, M.-Z., Wang, S.-B., Zhou, B., & Zhao, C.-X. (2022). Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants, 11(5), 809. https://doi.org/10.3390/antiox11050809








