Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diets
2.3. Experimental Fish and Feeding Management
2.4. Hypoxia Stress Test
2.5. Sampling Procedures
2.6. Biochemical Parameters
2.7. Tissue Total RNA Extraction and Real-Time Fluorescence Quantitative PCR
2.8. TUNEL Staining
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Growth Results and Body Composition
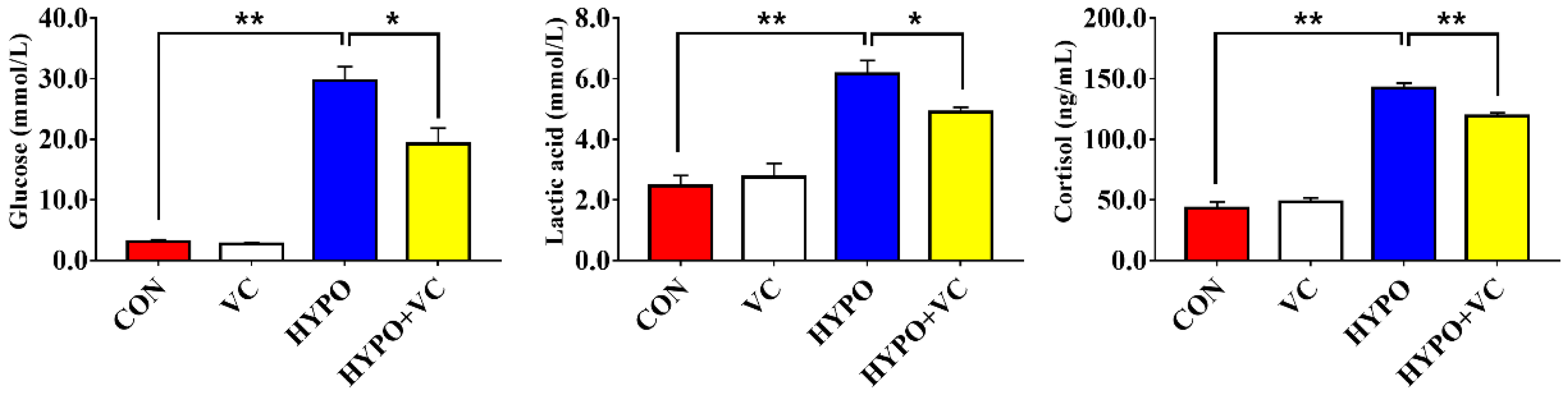
3.2. Plasma Metabolites
3.3. Changes of HIF-1α Protein in the Liver
3.4. Expression of Nrf2/Keap1 Signaling Pathway and Antioxidant-Related Genes in the Liver
3.5. Antioxidant Enzyme Parameters in the Liver
3.6. Expression of Inflammation-Related Genes in the Liver
3.7. Expression of ER Stress Key Proteins and Related Genes in the Liver
3.8. Expression of Autophagy and Apoptosis-Related Genes in the Liver
3.9. Determination of Casp 3 and 9 Activities in the Liver
3.10. TUNEL Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.; Shim, M.K.; Jin, M.; Rhyu, M.R.; Lee, Y. Methyl syringate, a TRPA1 agonist represses hypoxia-induced cyclooxygenase-2 in lung cancer cells. Phytomedicine 2016, 23, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.G.; Molina, V.M.; Carrasco, R.A.; Zepeda, A.B.; Figueroa, E.; Letelier, P.; Castillo, R.L. Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Chang, C.Y.; Jeon, S.B.; Yoon, H.J.; Ahn, Y.H.; Kim, H.S.; Kim, I.H.; Jeon, S.H.; Johnson, R.S.; Park, E.J. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat. Commun. 2015, 6, 6340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Han, C.; Dai, H.; Hou, J.; Dong, Y.; Cui, X.; Xu, L.; Zhang, M.; Xia, Q. Hypoxia-inducible factor-2α limits natural killer T cell cytotoxicity in renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2016, 27, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.L.; Lai, U.H.; Zhu, L.L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Masson, N.; Singleton, R.S.; Sekirnik, R.; Trudgian, D.C.; Ambrose, L.J.; Miranda, M.X.; Tian, Y.M.; Kessler, B.M.; Schofield, C.J.; Ratcliffe, P.J. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012, 13, 251–257. [Google Scholar] [CrossRef]
- Cecerska-Heryc, E.; Surowska, O.; Heryc, R.L.; Serwin, N.; Napiontek-Balinska, S.; Dolegowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Peng, Y.B.; Ren, Y.; Zhu, H.; An, Y.; Chang, B.S.; Sun, T.L. Ultrasmall copper nanoclusters with multi-enzyme activities. Rsc. Adv. 2021, 11, 14517–14526. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative stress and cancer: The role of nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71. [Google Scholar] [CrossRef]
- Cong, P.; Wang, T.; Tong, C.; Liu, Y.; Shi, L.; Mao, S.; Shi, X.; Jin, H.; Liu, Y.; Hou, M. Resveratrol ameliorates thoracic blast exposure-induced inflammation, endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-κB signaling pathway. Injury 2021, 52, 2795–2802. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Y.; Liu, B.; Xu, P.; Ge, X.P.; Zhang, H.M. Emodin ameliorates metabolic and antioxidant capacity inhibited by dietary oxidized fish oil through PPARs and Nrf2-Keap1 signaling in Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2019, 94, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Li, L.Y.; Xu, W.J.; Dong, B.; Geng, H.C.; Jing, J.Y.; Han, D.; Liu, H.K.; Yang, Y.X.; Xie, S.Q. Emodin alleviates acute hypoxia-induced apoptosis in gibel carp (Carassius gibelio) by upregulating autophagy through modulation of the AMPK/mTOR pathway. Aquaculture 2022, 548, 737689. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Bulnes, P.; Saiz, M.L.; Lopez-Larrea, C.; Rodriguez, R.M. Crosstalk between hypoxia and ER stress response: A key regulator of macrophage polarization. Front. Immunol. 2020, 10, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouschop, K.M.A.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C status and cognitive function: A systematic review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Su, M.; Chao, G.; Liang, M.Q.; Song, J.H.; Wu, K. Anticytoproliferative effect of vitamin C on rat hepatic stellate cell. Am. J. Transl. Res. 2016, 8, 2820–2825. [Google Scholar]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef]
- Luo, K.; Li, X.; Wang, L.; Rao, W.; Wu, Y.; Liu, Y.; Pan, M.; Huang, D.; Zhang, W.; Mai, K. Ascorbic acid regulates the immunity, anti-oxidation and apoptosis in abalone Haliotis discus hannai Ino. Antioxidants 2021, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2003. [Google Scholar]
- Li, H.Y.; Xu, W.J.; Jin, J.Y.; Zhu, X.M.; Yang, Y.X.; Han, D.; Liu, H.K.; Xie, S.Q. Effects of dietary carbohydrate and lipid concentrations on growth performance, feed utilization, glucose, and lipid metabolism in two strains of gibel carp. Front. Vet. Sci. 2019, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Sahoo, P.K. High dietary vitamin C affects growth, non-specific immune responses and disease resistance in asian catfish, Clarias batrachus. Mol. Cell. Biochem. 2005, 280, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Xie, J.; Xu, P.; Ge, X.; Liu, W.; Ye, J. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol. 2012, 32, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.H.; Mai, K.S.; Tan, B.P.; Xu, W.; Zhang, W.B.; Ma, H.M.; Liufu, Z.G. Effects of dietary vitamin C on survival, growth, and immunity of large yellow croaker, Pseudosciaena crocea. Aquaculture 2006, 261, 327–336. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; He, K.; Yan, T.; Zhou, J.; Zhao, L.L.; Sun, J.L.; Lian, W.Q.; Zhang, D.M.; Du, Z.J.; et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Sci. Total Environ. 2019, 666, 1071–1079. [Google Scholar] [CrossRef]
- Lee, D.C.; Sohn, H.A.; Park, Z.Y.; Oh, S.H.; Kang, Y.K.; Lee, K.M.; Kang, M.H.; Jang, Y.J.; Yang, S.J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, S.L.; Chen, Y.N.; Kuo, C.M. Physiological responses, desaturase activity, and fatty acid composition in milkfish (Chanos chanos) under cold acclimation. Aquaculture 2003, 220, 903–918. [Google Scholar] [CrossRef]
- Sun, R.L.; Meng, X.; Pu, Y.Q.; Sun, F.X.; Man, Z.D.; Zhang, J.; Yin, L.H.; Pu, Y.P. Overexpression of HIF-1a could partially protect K562 cells from 1,4-benzoquinone induced toxicity by inhibiting ROS, apoptosis and enhancing glycolysis. Toxicol. Vitr. 2019, 55, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Cui, C.; Liu, Q.; Sun, J.L.; He, K.; Adam, A.A.; Luo, J.; Li, Z.Q.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Husak, V.V.; Luzhna, L.I.; Lushchak, O.V.; Storey, K.B. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int. J. Biochem. Cell Biol. 2005, 37, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.M.; Groothuis, G.M.M.; Olinga, P.; Boersema, M. Targeting oxidative stress for the treatment of liver fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar] [PubMed]
- Chen, Y.Y.; Luo, G.Y.; Yuan, J.; Wang, Y.Y.; Yang, X.Q.; Wang, X.Y.; Li, G.P.; Liu, Z.G.; Zhong, N.S. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.P.; Li, Y.; Hou, Y.M.; Qiu, H.; Zhou, Q.C. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidracoRichardson). Aquacult. Res. 2015, 48, 149–160. [Google Scholar] [CrossRef]
- Quinonez-Flores, C.M.; Gonzalez-Chavez, S.A.; Pacheco-Tena, C. Hypoxia and its implications in rheumatoid arthritis. J. Biomed. Sci. 2016, 23, 62. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.X.; Korenori, Y.; Tanigawa, S.; Yamada-Kato, T.; Nagai, M.; He, X.; He, J.H. Dynamics of Nrf2 and Keap1 in ARE-Mediated NQO1 expression by easabi 6-(methylsulfinyl)hexyl isothiocyanate. J. Agric. Food Chem. 2011, 59, 11975–11982. [Google Scholar] [CrossRef]
- Strom, J.; Xu, B.B.; Tian, X.Q.; Chen, Q.M. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.Y.; Yu, J.G.; Guo, L.L.; Ma, H. TRIM16 protects from OGD/R-induced oxidative stress in cultured hippocampal neurons by enhancing Nrf2/ARE antioxidant signaling via downregulation of Keap1. Exp. Cell Res. 2020, 391, 111998. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.; Liang, X.L.; Xu, X.X.; Wu, X.M.; Yang, B. Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate-induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environ. Toxicol. Pharmacol. 2019, 65, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Flores-Santibanez, F.; Medel, B.; Bernales, J.I.; Osorio, F. Understanding the role of the unfolded protein response sensor ire1 in the biology of antigen presenting cells. Cells 2019, 8, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; De, S.; Mukherjee, S.; Das, S.; Ghosh, A.N.; Sengupta, S. Withaferin A induced impaired autophagy and unfolded protein response in human breast cancer cell-lines MCF-7 and MDA-MB-231. Toxicol. Vitr. 2017, 44, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: Therapeutic and molecular approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubra, K.T.; Akhter, M.S.; Uddin, M.A.; Barabutis, N. Unfolded protein response in cardiovascular disease. Cell. Signal. 2020, 73, 109699. [Google Scholar] [CrossRef]
- Lin, C.H.; Tseng, H.F.; Hsieh, P.C.; Chiu, V.; Lin, T.Y.; Lan, C.C.; Tzeng, I.S.; Chao, H.N.; Hsu, C.C.; Kuo, C.Y. Nephroprotective role of chrysophanol in hypoxia/reoxygenation-induced renal cell damage via apoptosis, ER stress, and ferroptosis. Biomedicines 2021, 9, 1283. [Google Scholar] [CrossRef]
- Schaaf, M.B.E.; Cojocari, D.; Keulers, T.G.; Jutten, B.; Starmans, M.H.; de Jong, M.C.; Begg, A.C.; Savelkouls, K.G.M.; Bussink, J.; Vooijs, M.; et al. The autophagy associated gene, ULK1, promotes tolerance to chronic and acute hypoxia. Radiother. Oncol. 2013, 108, 529–534. [Google Scholar] [CrossRef]
- Rzymski, T.; Milani, M.; Pike, L.; Buffa, F.; Mellor, H.R.; Winchester, L.; Pires, I.; Hammond, E.; Ragoussis, I.; Harris, A.L. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 2010, 29, 4424–4435. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Poornima, P.; Priya, L.B.; Huang, C.Y.; Padma, V.V. Neferine prevents autophagy induced by hypoxia through activation of Akt/mTOR pathway and Nrf2 in muscle cells. Biomed. Pharmacother. 2016, 83, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Al-Asmari, A.F.; Ansari, M.N.; Al-Anazi, W.A.; Bahashwan, S.; Almutairi, M.M.; Alshammari, M.; et al. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep. 2018, 70, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Xia, Q.Q.; Zhou, Y.Y.; Li, J. Endoplasmic reticulum stress and autophagy contribute to cadmium-induced cytotoxicity in retinal pigment epithelial cells. Toxicol. Lett. 2019, 311, 105–113. [Google Scholar] [CrossRef] [PubMed]










| Ingredients | CON | VC |
|---|---|---|
| White fish meal 1 | 15 | 15 |
| Rapeseed meal 2 | 20 | 20 |
| Soybean meal 2 | 25 | 25 |
| Wheat flour | 25.6 | 25.6 |
| Oil mixture 3 | 5.5 | 5.5 |
| Vitamin C | 0 | 0.12 |
| Vitamin premix 4 | 0.39 | 0.39 |
| Choline chloride | 0.11 | 0.11 |
| Mineral premix 5 | 5 | 5 |
| Carboxy methyl cellulose sodium | 3 | 3 |
| Cellulose | 0.40 | 0.28 |
| Proximate analysis (dry matter) | ||
| Crude protein (%) | 37.21 | 37.42 |
| Crude lipid (%) | 6.77 | 6.63 |
| Moisture (%) | 9.08 | 9.17 |
| Ash (%) | 10.02 | 10.34 |
| Gross energy (kJ g−1) | 19.27 | 19.22 |
| Gene Name | Sense and Antisense Primer (5’–3’) | Gene Bank | Product Length |
|---|---|---|---|
| Accession No. | (bp) | ||
| Tumor necrosis factor-α (tnf-α) | TTGAGCAGGAGATGGGAACCG | XM_026282152.1 | 115 |
| AGAGCCTCAGGGCAACGGAAA | |||
| Interleukin-2 (il-2) | GACCACAAAGGTAGACCCATCC | MN338056 | 212 |
| GAGGTTTGTGCGGAATGGAC | |||
| Interleukin-6 (il-6) | TGTTCTCAGGGCATTCGCTT | XM_026289280.1 | 161 |
| GGAGTTGTAGTGCCCTTGGT | |||
| Interleukin-12 (il-12) | CTTCAGAAGCAGCTTTGTTGTTG | LN592213.1 | 77 |
| CAGTTTTTGAGAGCTCACCAATATC | |||
| Interleukin-1β (il-1β) | TTTGTGAAGATGCGCTGCTC | AB757758.1 | 133 |
| CCAATCTCGACCTTCCTGGTG | |||
| Interleukin-4 (il-4) | CGATTGTAGCCGTTACTGGGT | KX574595 | 166 |
| TGGCAAATGTGTTCCTCCG | |||
| Transforming growth factorβ (tgf-β) | ATGAGGGTGGAGAGTTTAT | EU086521.1 | 155 |
| AGTCGTAGTTTGCTGAGAA | |||
| Nuclear factor of kappa light polypeptide gene | TTGCGAATCCAAAGGGGACA | XM_026291433.1 | 196 |
| enhancer in B-cells inhibitor, alpha (iκbα) | TCTGTGATGACGGCGAGATG |
| Category | Parameter | Group | |
|---|---|---|---|
| CON | VC | ||
| Growth performance | Initial body weight (g) | 6.60 ± 0.00 | 6.67 ± 0.03 |
| Final body weight (g) | 26.40 ± 0.21 a | 31.40 ± 1.36 b | |
| WGR 1 (%) | 219.80 ± 12.98 | 261.38 ± 17.86 | |
| FE 2 (%) | 44.19 ± 0.42 | 50.79 ± 2.81 | |
| SGR 3 (% d−1) | 4.93 ± 0.03 a | 5.54 ± 0.16 b | |
| Body composition | Crude protein (%) | 15.18 ± 0.16 | 14.91 ± 0.1 |
| Crude lipid (%) | 7.35 ± 0.19 | 7.22 ± 0.04 | |
| Ash (%) | 2.68 ± 0.12 | 2.60 ± 0.13 | |
| Moisture (%) | 71.95 ± 0.54 | 71.81 ± 0.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. https://doi.org/10.3390/antiox11050935
Wu L, Xu W, Li H, Dong B, Geng H, Jin J, Han D, Liu H, Zhu X, Yang Y, et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants. 2022; 11(5):935. https://doi.org/10.3390/antiox11050935
Chicago/Turabian StyleWu, Liyun, Wenjie Xu, Hongyan Li, Bo Dong, Hancheng Geng, Junyan Jin, Dong Han, Haokun Liu, Xiaoming Zhu, Yunxia Yang, and et al. 2022. "Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio)" Antioxidants 11, no. 5: 935. https://doi.org/10.3390/antiox11050935






