Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability
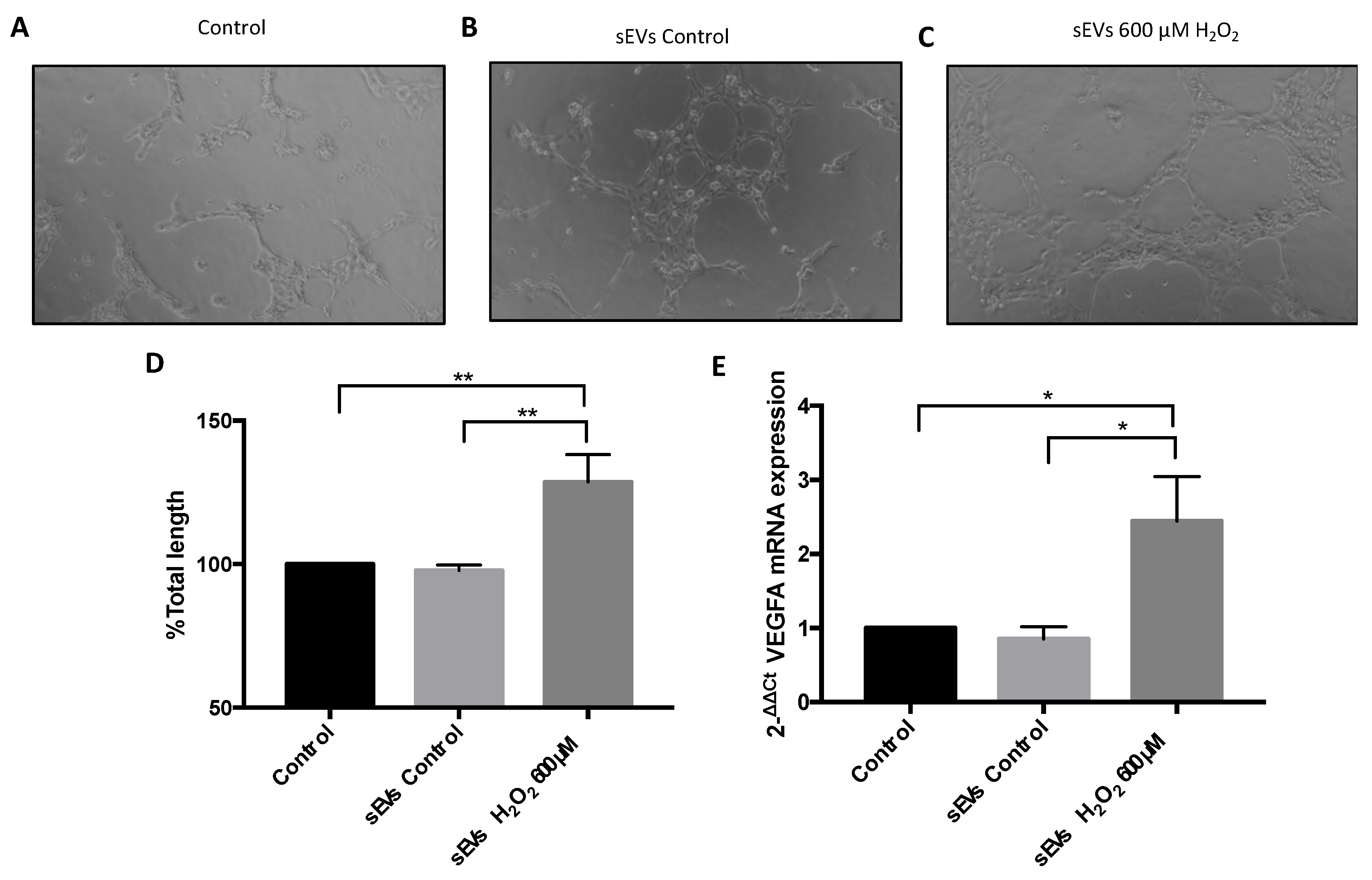
2.3. Vasculogenesis Assay
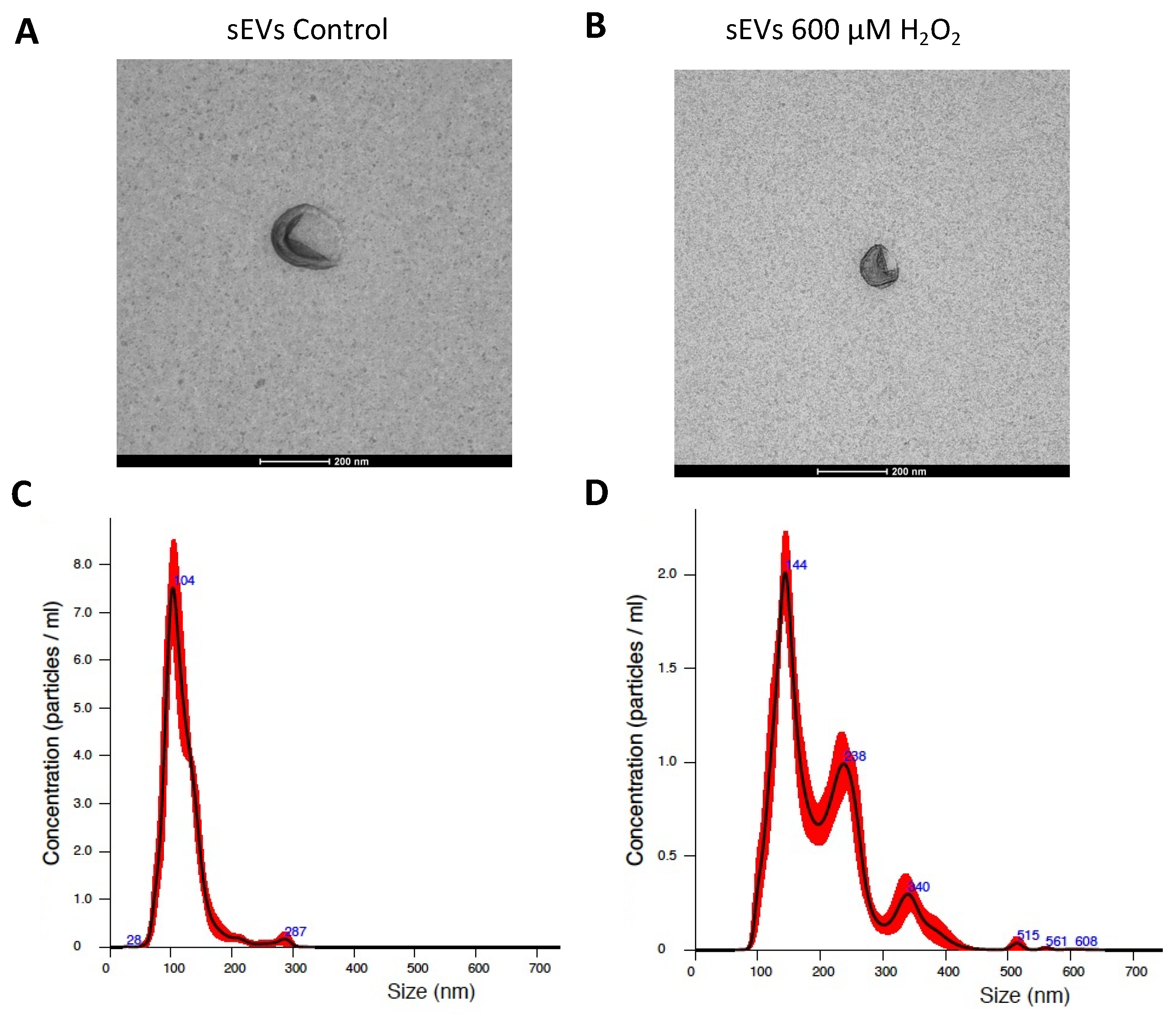
2.4. sEVs Isolation
2.5. sEV Characterization
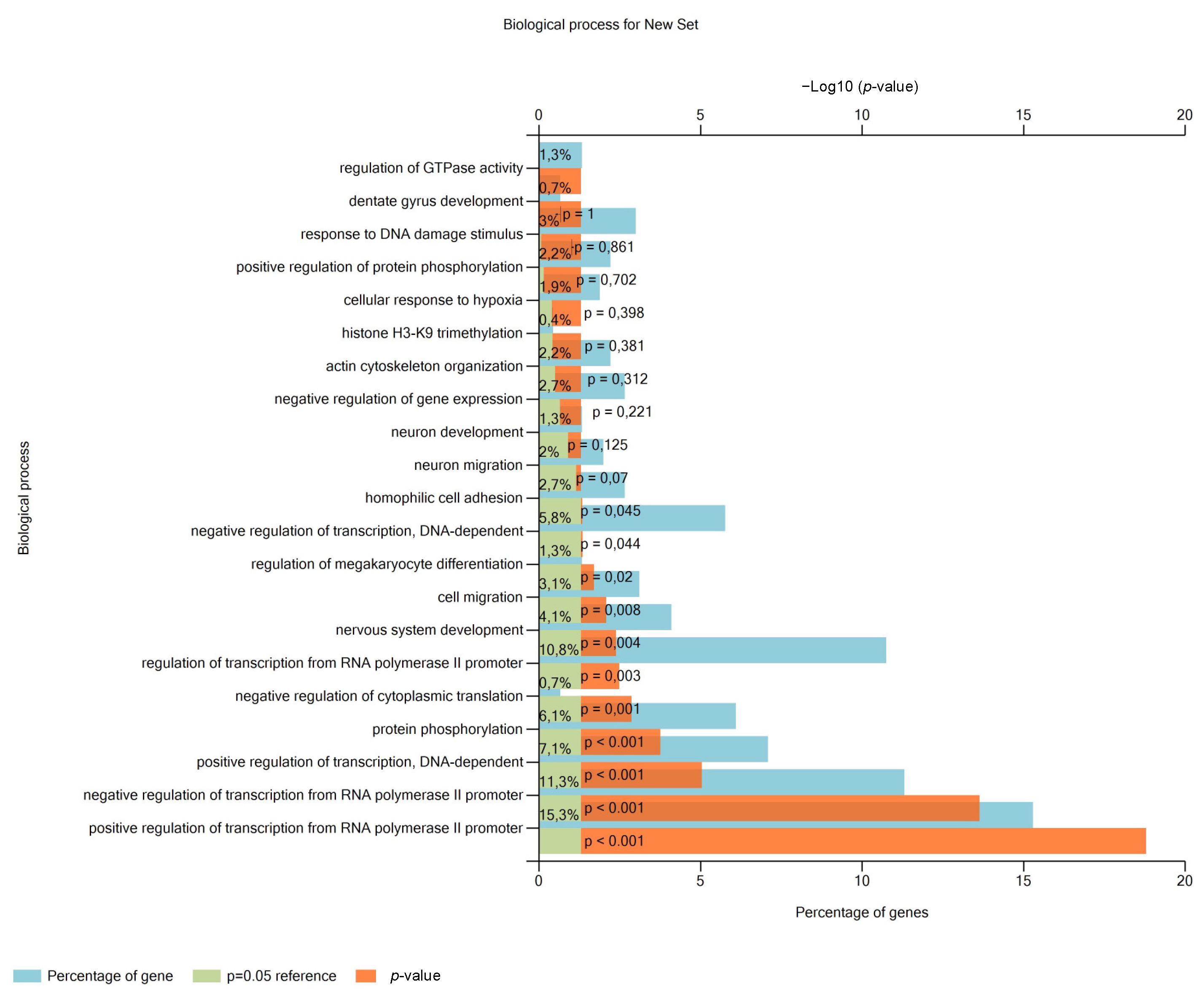
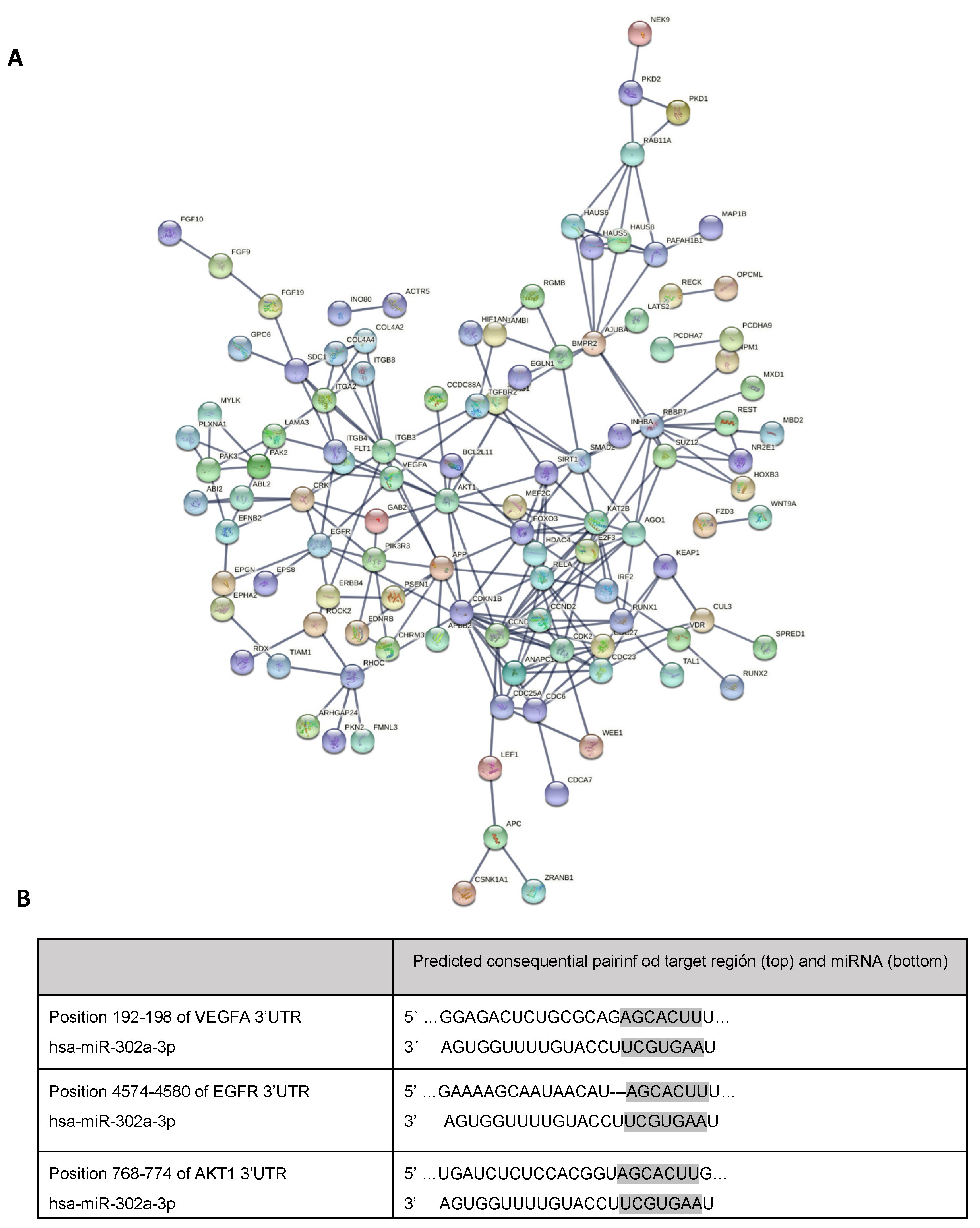
2.6. Analysis of Pathways and miRNA Target Genes
2.7. Transfection of miR-302a-3p Mimic
2.8. RNA Isolation from ARPE-19 Cells
2.9. RNA Isolation from sEVs
2.10. mRNA Expression Analysis
2.11. miRNA Expression Analysis
2.12. Statistical Analysis
3. Results
3.1. H2O2-Induced SEVs Promoted Oxidative Stress, Decreasing Cell Viability in ARPE-19 Cells
3.2. miR-302a-3p Targets and Related Pathways
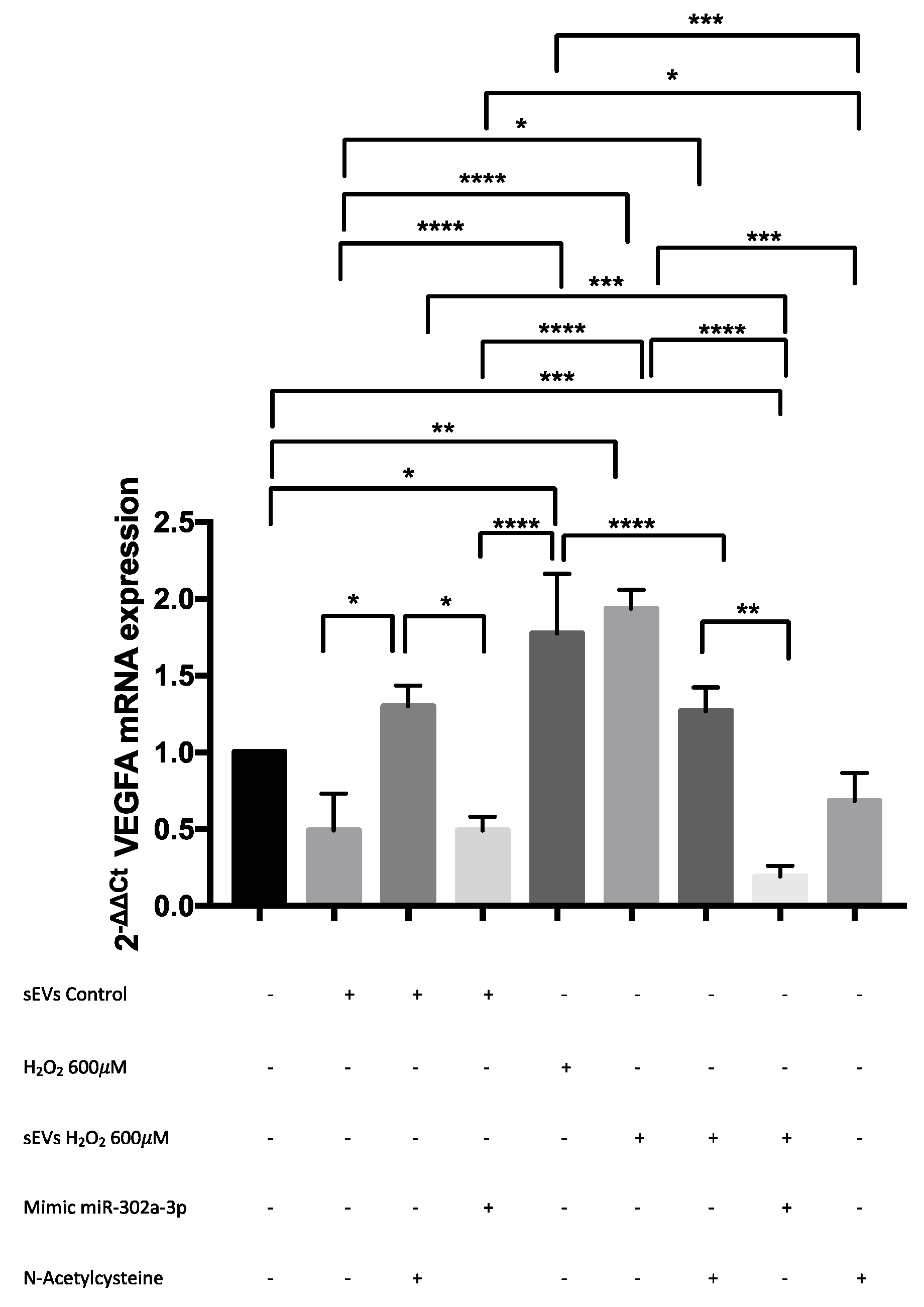
3.3. VEGFA mRNA and Angiogenesis Were Regulated by H2O2 and SEVs
3.4. miR-302a-3p sEV Cargo Regulated Oxidative-Induced Angiogenesis
3.5. VEGFA mRNA Was Overexpressed by H2O2 and Modulated by miR-302a-3p
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative Stress in Retinal Pigment Epithelium Cells Increases Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. J. Cell Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Biasutto, L.; Chiechi, A.; Couch, R.; Liotta, L.A.; Espina, V. Retinal Pigment Epithelium (RPE) Exosomes Contain Signaling Phosphoproteins Affected by Oxidative Stress. Exp. Cell Res. 2013, 319, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Oltra, S.S.; Romero, F.J.; Sancho-Pelluz, J.; Barcia, J.M. MiR302a and 122 Are Deregulated in Small Extracellular Vesicles from ARPE-19 Cells Cultured with H2O2. Sci. Rep. 2019, 9, 17954. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Simpson, R.J. Understanding Extracellular Vesicle Diversity–Current Status. Expert Rev. Proteomics 2018, 15, 887–910. [Google Scholar] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular Vesicles: Exosomes, Microparticles, Their Parts, and Their Targets to Enable Their Biomanufacturing and Clinical Applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Zhong, H.; Yuan, H.; Liang, F.; Liu, J.; Tang, W. Extracellular Vesicles in Inter-Kingdom Communication in Gastrointestinal Cancer-PubMed. Am. J. Cancer Res. 2021, 11, 1087–1103. [Google Scholar]
- Chen, W.; Li, Z.; Deng, P.; Li, Z.; Xu, Y.; Li, H.; Su, W.; Qin, J. Advances of Exosomal MiRNAs in Breast Cancer Progression and Diagnosis. Diagnostics 2021, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Faict, S.; Muller, J.; De Veirman, K.; De Bruyne, E.; Maes, K.; Vrancken, L.; Heusschen, R.; De Raeve, H.; Schots, R.; Vanderkerken, K.; et al. Exosomes Play a Role in Multiple Myeloma Bone Disease and Tumor Development by Targeting Osteoclasts and Osteoblasts. Blood Cancer J. 2018, 8, 4484. [Google Scholar] [CrossRef]
- Waldenmaier, M.; Seibold, T.; Seufferlein, T.; Eiseler, T. Pancreatic Cancer Small Extracellular Vesicles (Exosomes): A Tale of Short- and Long-Distance Communication. Cancers 2021, 13, 4844. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhang, Y.; Ge, M.; Liu, S.; Jiang, X.; Shang, Z.; Liu, H.; Cao, C.; Xiao, H. Cancer Cell Derived Small Extracellular Vesicles Contribute to Recipient Cell Metastasis Through Promoting HGF/c-Met Pathway. Mol. Cell Proteom. 2019, 18, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, X.; Zhou, J.; Meng, M.; Gao, Y.; Yi, G.; Fu, M. Exosomes in the Pathogenesis and Treatment of Ocular Diseases. Exp. Eye Res. 2021, 209, 108626. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Ishii, M.; Brandon, C.; Ablonczy, Z.; Cai, J.; Liu, Y.; Chou, C.J.; Rohrer, B. Extracellular Vesicle-Mediated Long-Range Communication in Stressed Retinal Pigment Epithelial Cell Monolayers. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2610–2622. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. Microrna-1281 as a Novel Circulating Biomarker in Patients with Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.W.; Wang, Y.; Fu, Y.H.; Gao, X.Y. MicroRNAs: Potential Targets in Diabetic Retinopathy. Horm. Metab. Res. 2020, 52, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Liu, Q.; Wei, Q.; Cai, W.; He, M.; Du, Y.; Xu, D.; Wu, Y.; Yu, J. Circulating MiRNAs as Potential Biomarkers of Age-Related Macular Degeneration. Cell Physiol. Biochem. 2017, 41, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Ingenito, F.; Roscigno, G.; Affnito, A.; Nuzzo, S.; Scognamiglio, I.; Quintavalle, C.; Condorelli, G. The Role of Exo-MiRNAs in Cancer: A Focus on Therapeutic and Diagnostic Applications. Int. J. Mol. Sci. 2019, 20, 4687. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Xu, M.; Wang, Z.; Yang, M. Engineered Exosomes Loaded with MiR-449a Selectively Inhibit the Growth of Homologous Non-Small Cell Lung Cancer. Cancer Cell Int. 2021, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bellver, M.; Mighty, J.; Aparicio-Domingo, S.; Li, K.V.; Shi, C.; Zhou, J.; Cobb, H.; McGrath, P.; Michelis, G.; Lenhart, P.; et al. Extracellular Vesicles Released by Human Retinal Pigment Epithelium Mediate Increased Polarised Secretion of Drusen Proteins in Response to AMD Stressors. J. Extracell. Vesicles 2021, 10, e12165. [Google Scholar] [CrossRef] [PubMed]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative Stress-Induced Angiogenesis Is Mediated by MiR-205-5p. J. Cell Mol. Med. 2020, 24, 1428–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Kim, D.; Kim, S.; Park, G.B.; Hur, D.Y.; Yang, J.W.; Park, S.G.; Kim, Y.S. MiR-9 Regulates the Post-Transcriptional Level of VEGF165a by Targeting SRPK-1 in ARPE-19 Cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1369–1376. [Google Scholar]
- Smith, G.A.; Fearnley, G.W.; Harrison, M.A.; Tomlinson, D.C.; Wheatcroft, S.B.; Ponnambalam, S. Vascular Endothelial Growth Factors: Multitasking Functionality in Metabolism, Health and Disease. J. Inherit. Metab. Dis. 2015, 38, 753–763. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Y.; Chen, Z.; Xiong, Y.; Zhou, T.; Tao, W.; Xu, F.; Yang, H.; Ylä-Herttuala, S.; et al. MicroRNA-15b Targets VEGF and Inhibits Angiogenesis in Proliferative Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2020, 105, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef] [PubMed]
- Farnoodian, M.; Wang, S.; Dietz, J.; Nickells, R.W.; Sorenson, C.M.; Sheibani, N. Negative Regulators of Angiogenesis: Important Targets for Treatment of Exudative AMD. Clin. Sci. 2017, 131, 1763–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, F.; Osswald, M.; Wick, W. Anti-Angiogenics: Their Role in the Treatment of Glioblastoma. Oncol. Res. Treat. 2018, 41, 181–186. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-Angiogenic Agents in Combination with Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Barroso-del Jesus, A.; Lucena-Aguilar, G.; Menendez, P. The MiR-302-367 Cluster as a Potential Stemness Regulator in ESCs. Cell Cycle 2009, 8, 394–398. [Google Scholar] [CrossRef]
- Cao, J.; Li, L.; Han, X.; Cheng, H.; Chen, W.; Qi, K.; Chen, C.; Wu, Q.; Niu, M.; Zeng, L.; et al. MiR-302 Cluster Inhibits Angiogenesis and Growth of K562 Leukemia Cells by Targeting VEGFA. Onco. Targets Ther. 2019, 12, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Mi, C.; Wang, K.S.; Lee, J.J.; Jin, X. Zinc Finger Protein 91 (ZFP91) Activates HIF-1α via NF-ΚB/P65 to Promote Proliferation and Tumorigenesis of Colon Cancer. Oncotarget 2016, 7, 36551–36562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.N.; Hong, Y.; Ma, Z.L.; Pang, R.P.; Lei, Q.Q.; Lv, X.F.; Zhou, J.G.; Huang, H.; Zhang, T.T. MiR-302a Limits Vascular Inflammation by Suppressing Nuclear Factor-κ B Pathway in Endothelial Cells. Front. Cell Dev. Biol. 2021, 9, 682574. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Betí, C.; Novella, S.; Lázaro-Franco, M.; Pérez-Cremades, D.; Heras, M.; Sanchís, J.; Hermenegildo, C. An Affordable Method to Obtain Cultured Endothelial Cells from Peripheral Blood. J. Cell Mol. Med. 2013, 17, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-MiRPath v3.0: Deciphering MicroRNA Function with Experimental Support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic Tumor Cell Modulates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Exosomes. Mol. Cell Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small Extracellular Vesicles Secreted from Senescent Cells Promote Cancer Cell Proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef]
- Qi, H.; Wang, Y.; Fa, S.; Yuan, C.; Yang, L. Extracellular Vesicles as Natural Delivery Carriers Regulate Oxidative Stress Under Pathological Conditions. Front. Bioeng. Biotechnol. 2021, 9, 752019. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, Y.; Wang, L.; Huang, X. Circ0001429 Regulates Progression of Bladder Cancer through Binding MiR-205-5p and Promoting VEGFA Expression. Cancer Biomark. 2019, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.K.A.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-Directed Regulation of VEGF and Other Angiogenic Factors under Hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Wang, P.; Xu, J.; Zhu, Y.; Sun, G.; Pang, Q.; Tao, R. MicroRNA-205 Functions as a Tumor Suppressor in Human Glioblastoma Cells by Targeting VEGF-A. Oncol. Rep. 2012, 27, 1200–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, L.; Li, X. MiR-526b-3p Mediates Doxorubicin-Induced Cardiotoxicity by Targeting STAT3 to Inactivate VEGFA. Biomed. Pharmacother. 2020, 123, 109751. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, B.; Ge, B.J. MicroRNA-150-5p Inhibits Proliferation and Invasion of Osteosarcoma Cells by down-Regulating VEGFA. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9265–9273. [Google Scholar]
- Chen, N.; Wang, J.; Hu, Y.; Cui, B.; Li, W.; Xu, G.; Liu, L.; Liu, S. MicroRNA-410 Reduces the Expression of Vascular Endothelial Growth Factor and Inhibits Oxygen-Induced Retinal Neovascularization. PLoS ONE 2014, 9, e95665. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Byzova, T.V. Oxidative Stress in Angiogenesis and Vascular Disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and Oxidative Stress in Angiogenesis and Vascular Disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ji, L.Y.; Li, L.; Li, J.M. Oxidative Stress, Autophagy and Pyroptosis in the Neovascularization of Oxygen-induced Retinopathy in Mice. Mol. Med. Rep. 2019, 19, 927–934. [Google Scholar]
- Kosmidou, I.; Xagorari, A.; Roussos, C.; Papapetropoulos, A. Reactive Oxygen Species Stimulate VEGF Production from C(2)C(12) Skeletal Myotubes through a PI3K/Akt Pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L585–L592. [Google Scholar] [CrossRef] [PubMed]


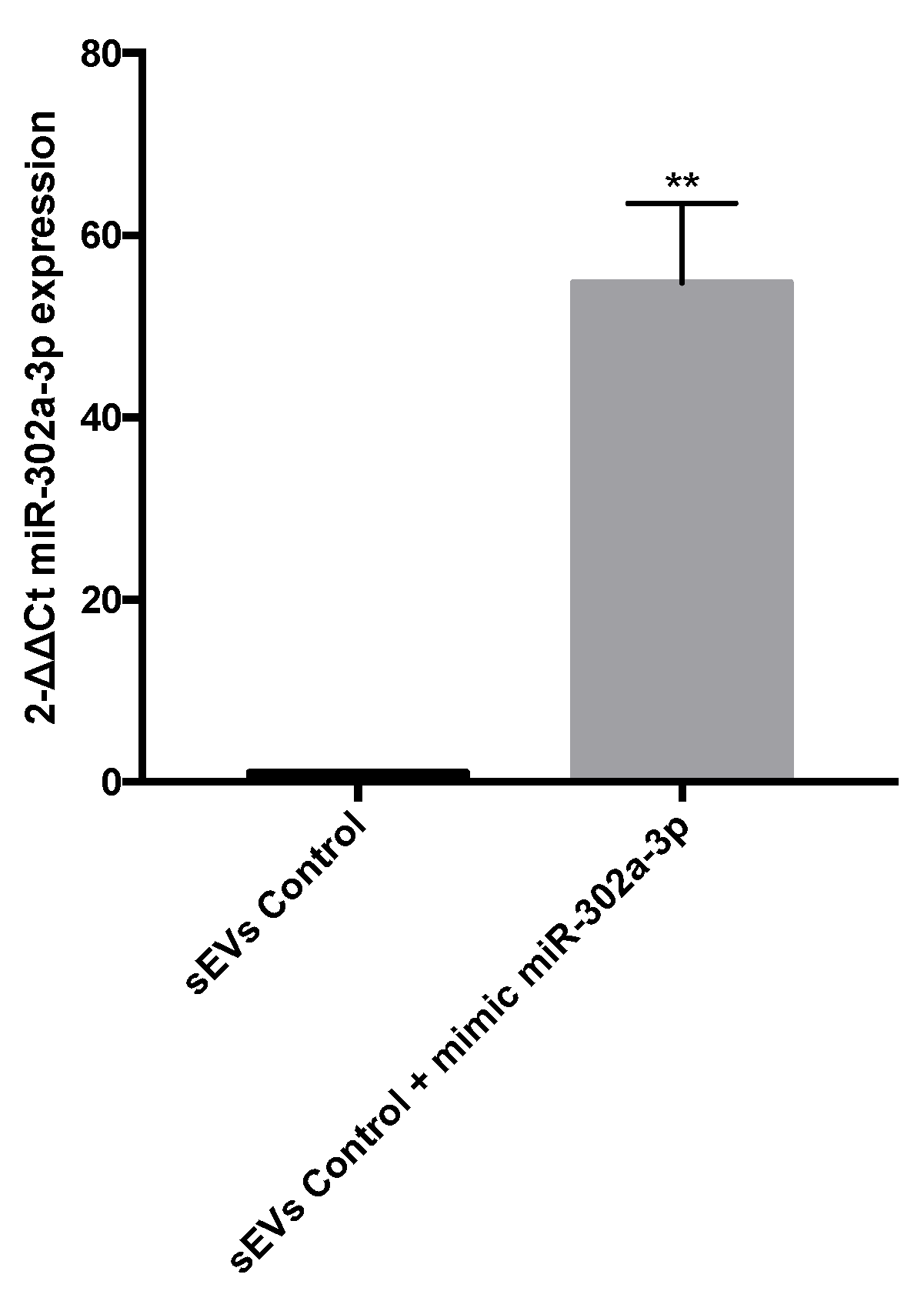
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oltra, M.; Martínez-Santos, M.; Ybarra, M.; Rowland, H.; Muriach, M.; Romero, J.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells. Antioxidants 2022, 11, 818. https://doi.org/10.3390/antiox11050818
Oltra M, Martínez-Santos M, Ybarra M, Rowland H, Muriach M, Romero J, Sancho-Pelluz J, Barcia JM. Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells. Antioxidants. 2022; 11(5):818. https://doi.org/10.3390/antiox11050818
Chicago/Turabian StyleOltra, Maria, Miriam Martínez-Santos, María Ybarra, Hugo Rowland, María Muriach, Javier Romero, Javier Sancho-Pelluz, and Jorge M. Barcia. 2022. "Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells" Antioxidants 11, no. 5: 818. https://doi.org/10.3390/antiox11050818
APA StyleOltra, M., Martínez-Santos, M., Ybarra, M., Rowland, H., Muriach, M., Romero, J., Sancho-Pelluz, J., & Barcia, J. M. (2022). Oxidative-Induced Angiogenesis Is Modulated by Small Extracellular Vesicle miR-302a-3p Cargo in Retinal Pigment Epithelium Cells. Antioxidants, 11(5), 818. https://doi.org/10.3390/antiox11050818







