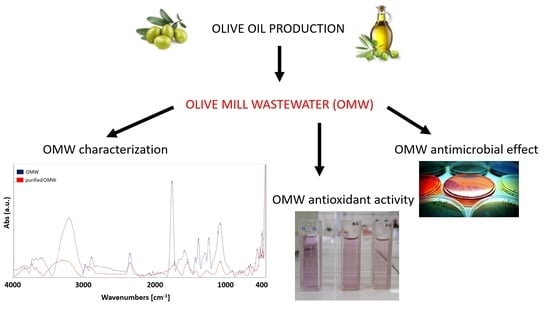
Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
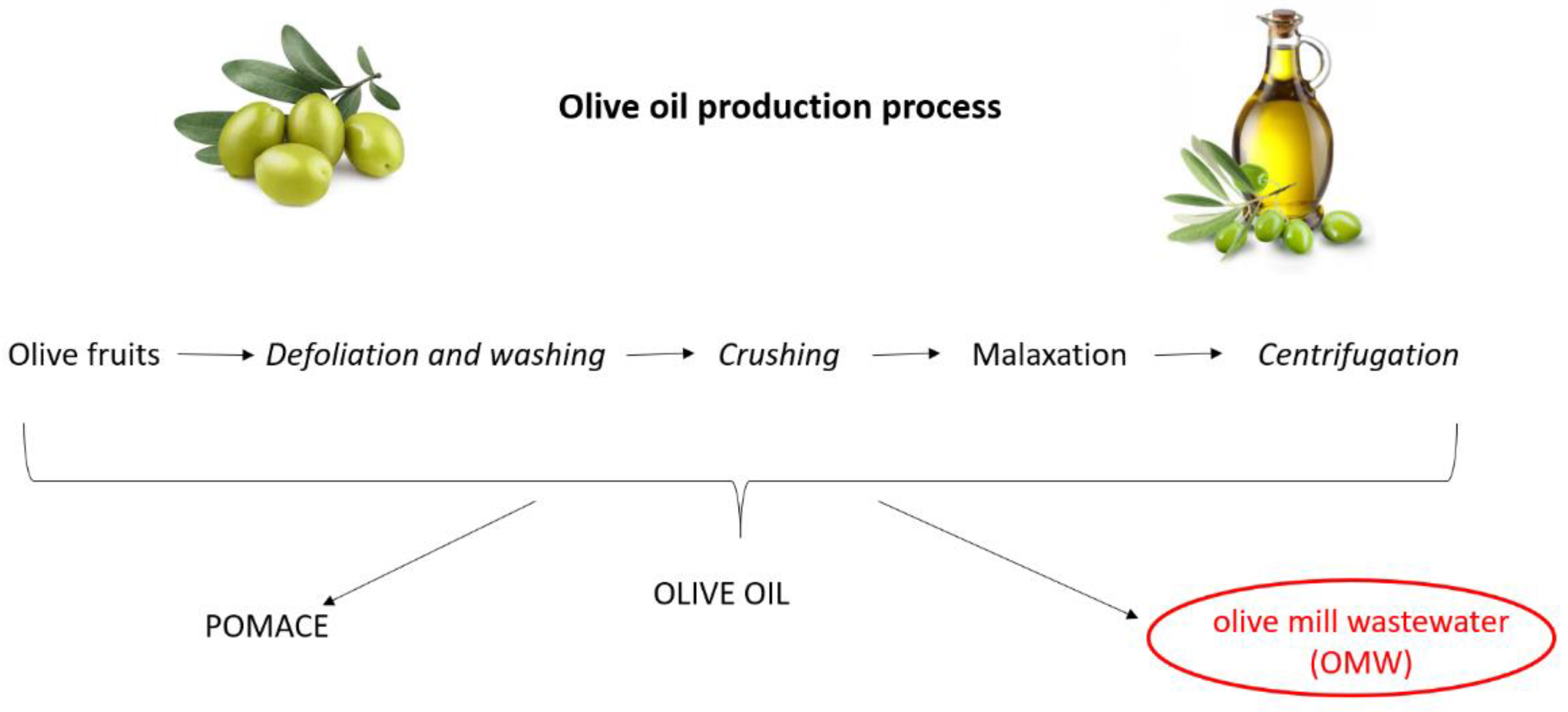
2.2. Plant Material and OMW Used
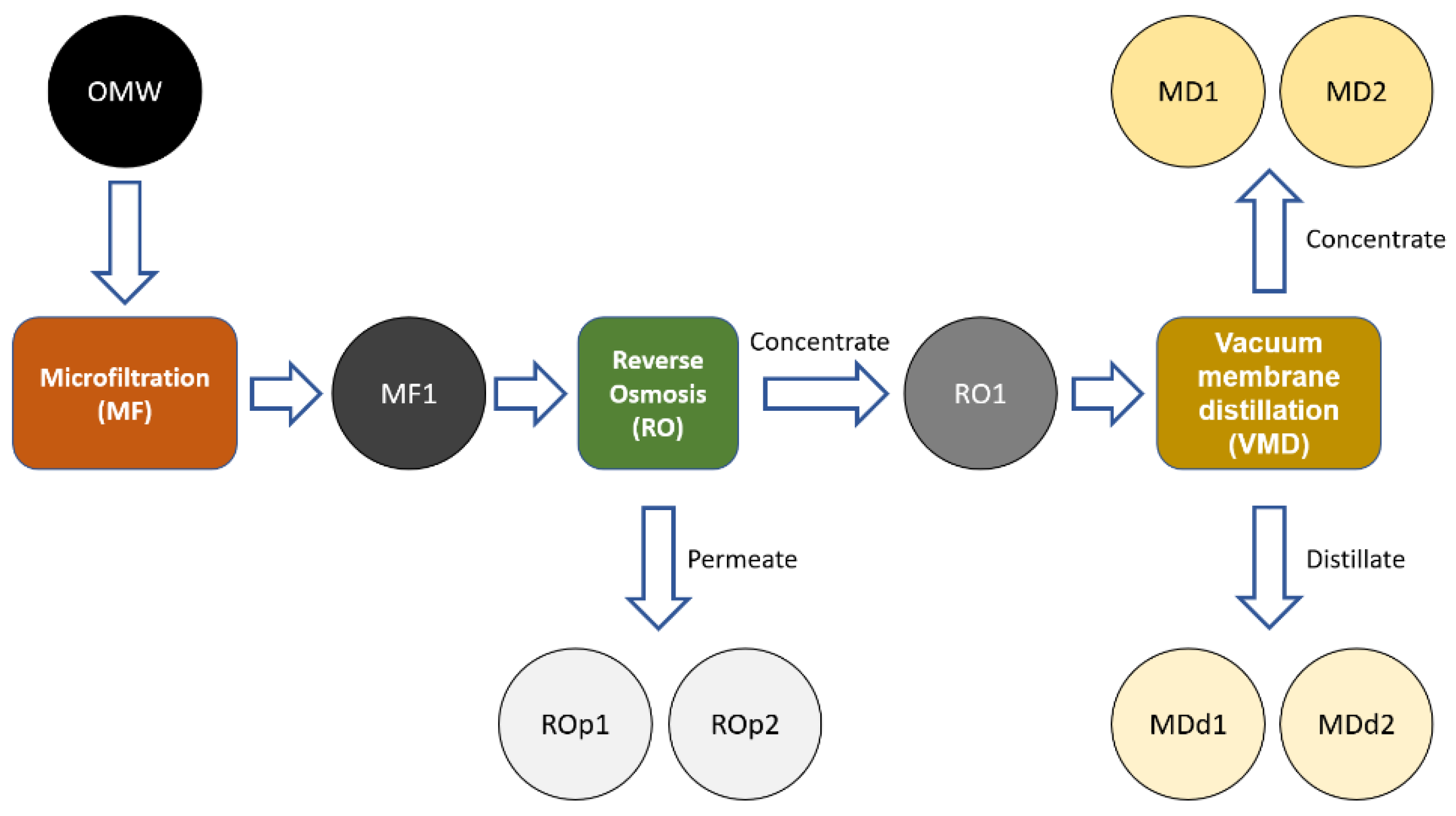
2.3. Filtration Procedure
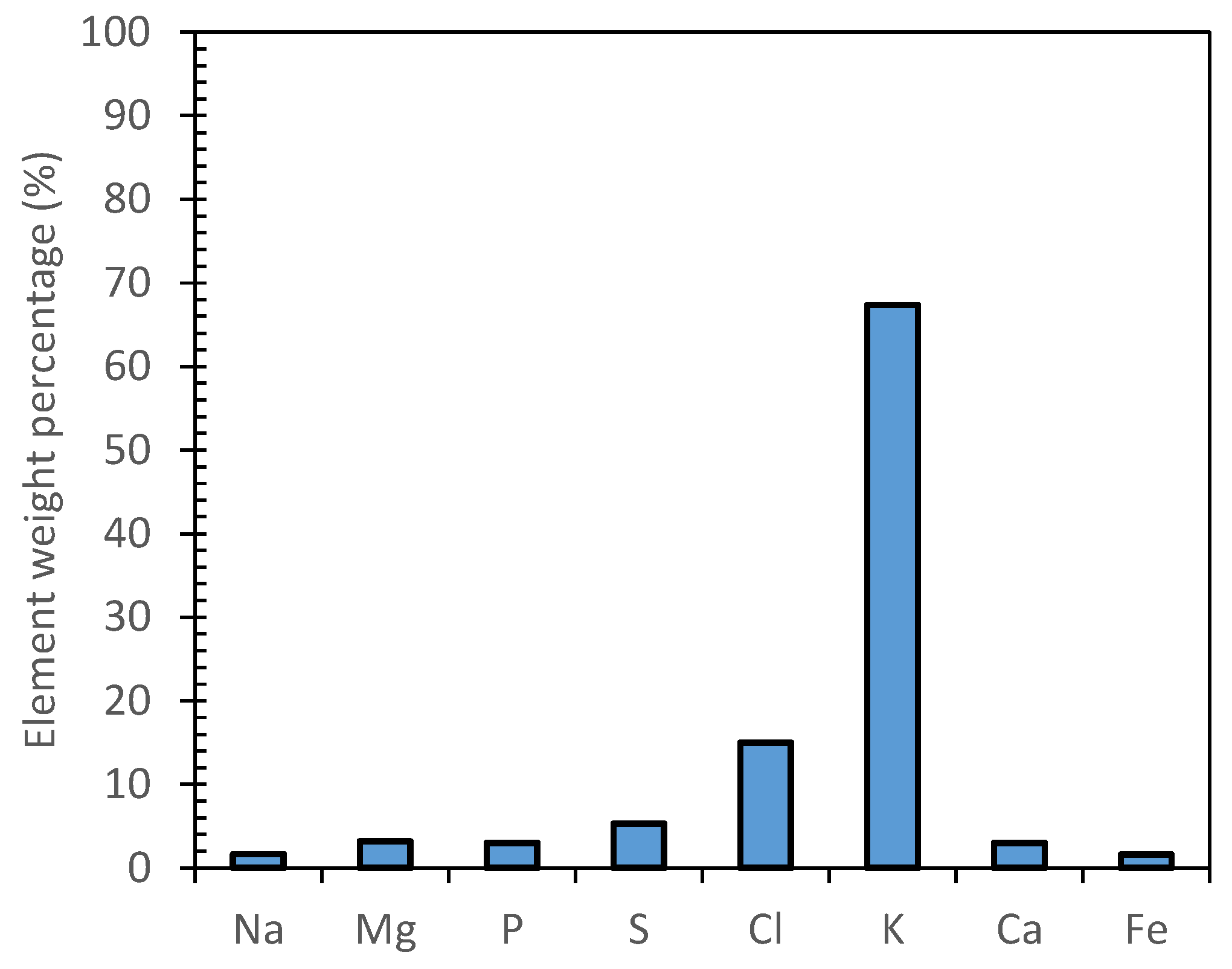
2.4. Determination of Electric Conductivity and Elemental Composition of Isolated Fractions
2.5. Fourier Transform Infrared Spectroscopy
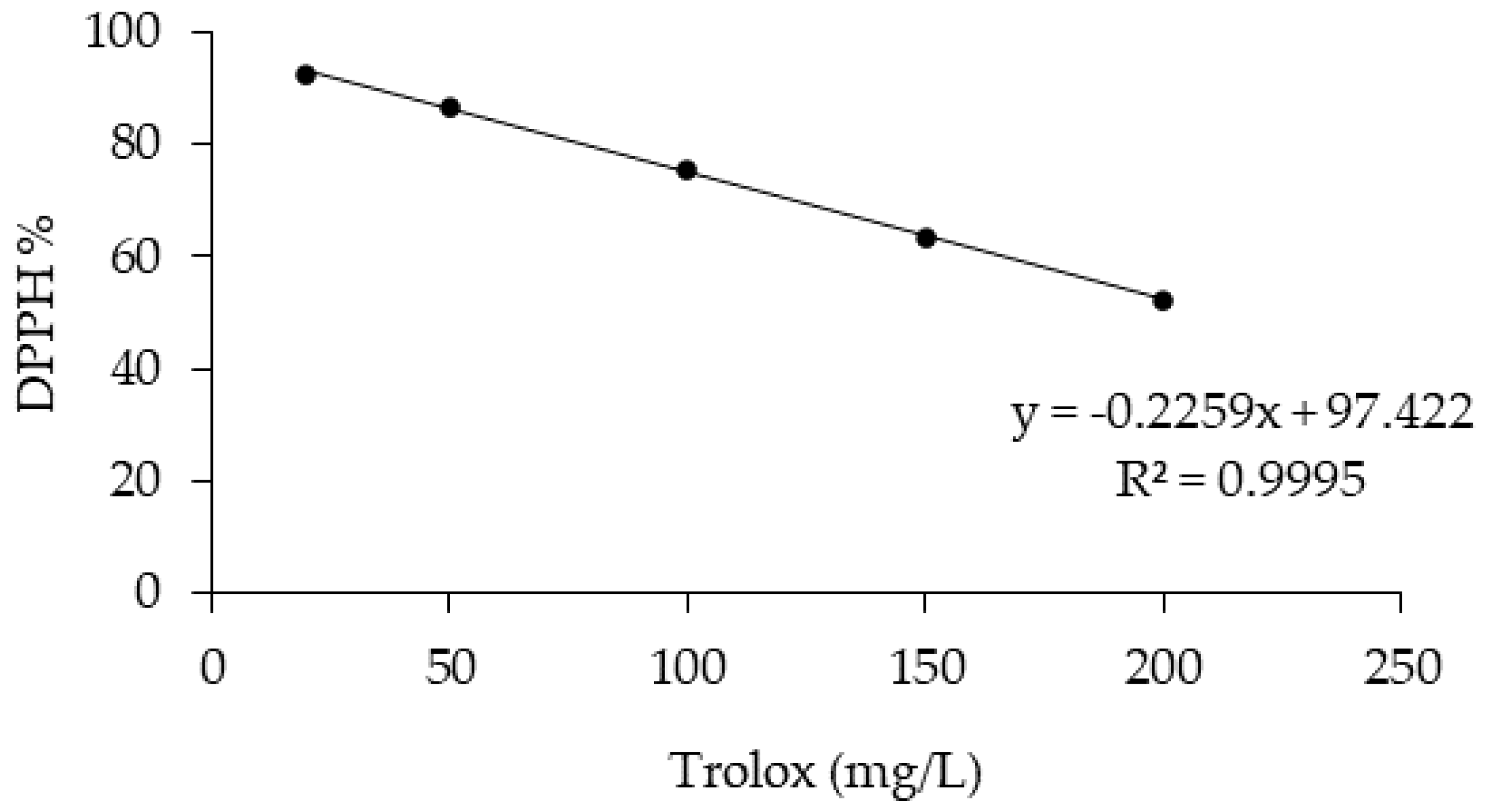
2.6. DPPH Radical-Scavenging Activity
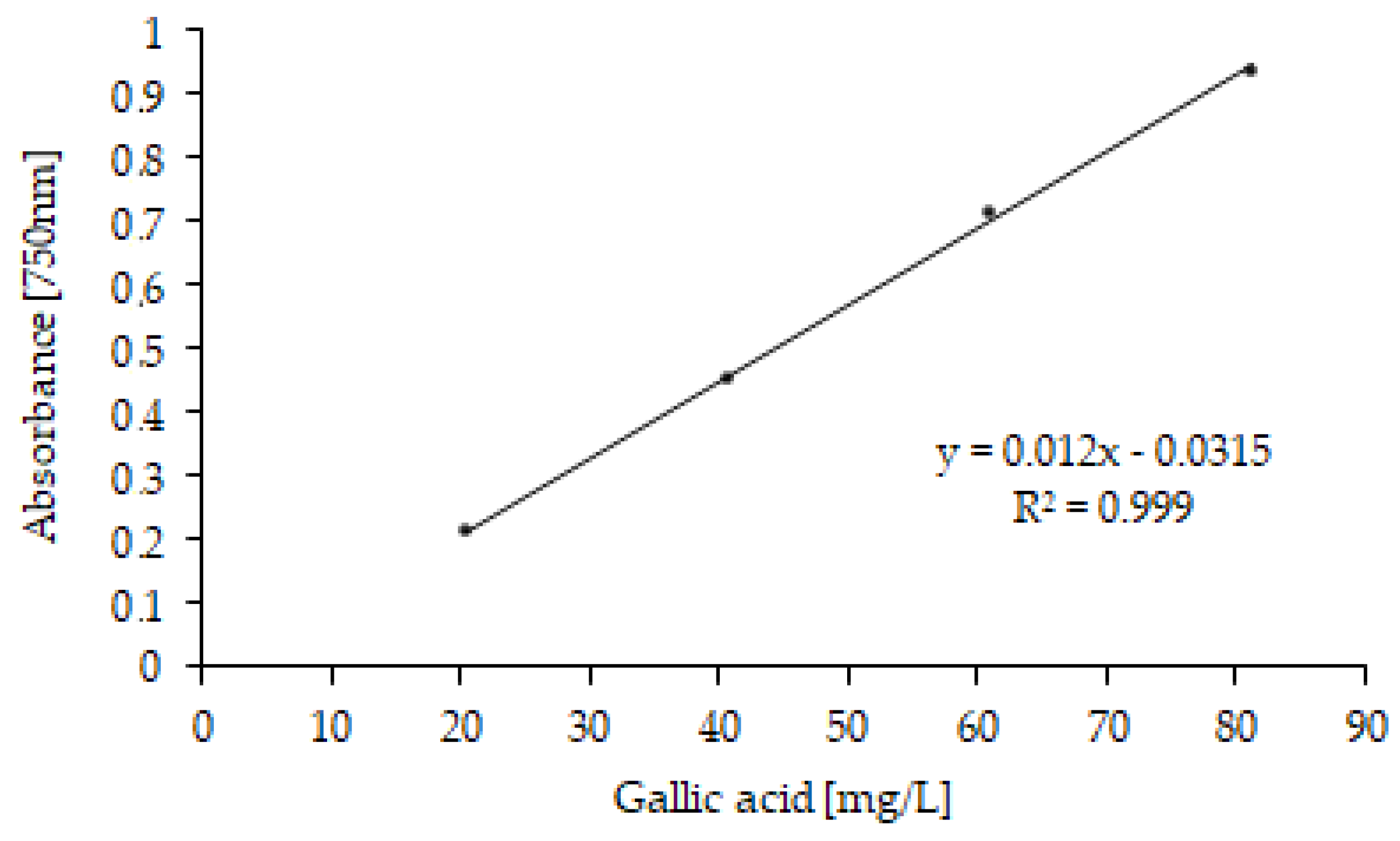
2.7. Folin–Ciocalteu Spectrophotometric Determination
2.8. Antibacterial Activity of OMW
2.8.1. Bacterial Strains
2.8.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of OMW
2.9. Statistical Analysis
3. Results and Discussion
3.1. OMW Concentration from Membrane Processes
3.2. OMW Characterization
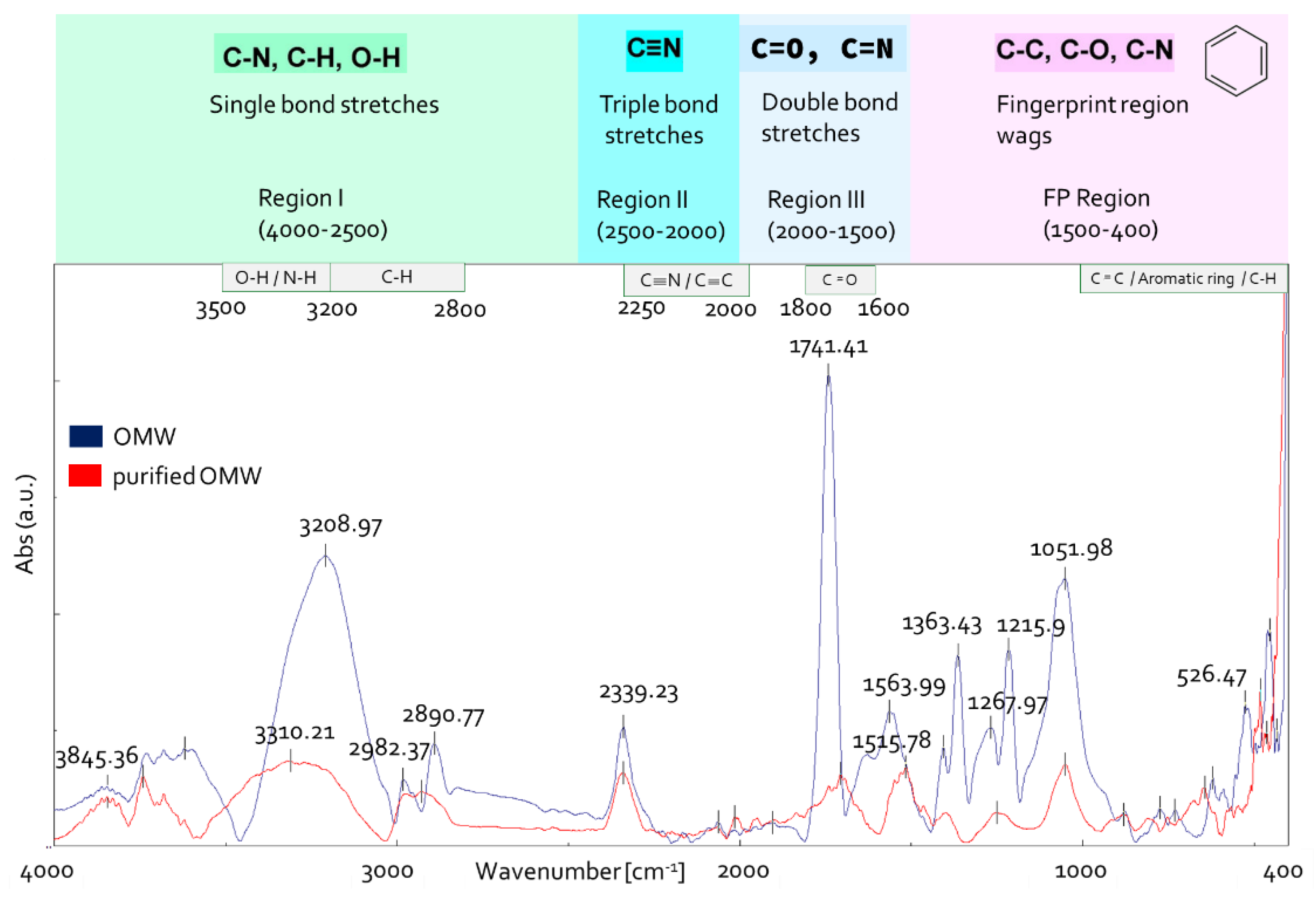
3.2.1. FTIR Analysis
3.2.2. TPs and DPPH Radical-Scavenging Activity in OMW
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional Recommendations for Cardiovascular Disease Prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuffrè, A.M. Influence of cultivar and harvest year on triglyceride composition of olive oils produced in Calabria (S outhern Italy). Eur. J. Lipid Sci. Technol. 2013, 115, 928–934. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; Janik, J., Ed.; Johnwiley & Sons, Ltsd.: Chichester, UK, 2011; Volume 38, pp. 83–148. [Google Scholar]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Lama Muñoz, A.; Ruiz-Méndez, M.V.; Rubio-Senent, F.; Fernàndez-Bolaños, J. New Olive-Pomace Oil Improved By Hydrothermal Pre-Treatments; INTECH Open Access Publisher: London, UK, 2012; pp. 249–266. [Google Scholar]
- Leouifoudi, I.; Zyad, A.; Amechrou, Q.A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Sci. Technol Camp. 2014, 34, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.; Mailer, R.; Prenzler, P.D.; Robards, K. Impact of Cultivar, Harvesting Time, and Seasonal Variation on the Content of Biophenols in Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 8851–8858. [Google Scholar] [CrossRef]
- Giuffrè, A.M. Biometric evaluation of twelve olive cultivars under rainfed conditions in the region of Calabria, South Italy. Emir. J. Food Agric. 2017, 29, 696. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental Effects Caused by Olive Mill Wastewaters: Toxicity Comparison of Low-Molecular-Weight Phenol Components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef]
- Hu, Z.; Grasso, D. Chemical Oxygen Demand. In Water Analysis; Encyclopedia of Analytical Science, Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 325–330. [Google Scholar]
- Italian Legislative Decree, 06/07/2005, Official Gazette (G.U. Serie Generale n. 166, 19/07/2005). Available online: https://www.gazzettaufficiale.it/eli/id/2005/07/19/05A07191/sg (accessed on 30 March 2022).
- Rupani, P.F.; Singh, R.P.; Ibrahim, M.H.; Esa, N. Review of current palm oil mill effluent (POME) treatment methods: Vermicomposting as a sustainable practice. World Appl. Sci. J. 2010, 11, 70–81. [Google Scholar]
- Council Directive of 21 May 1991 concerning urban wastewater treatment (91/271/EEC). Off. J. Eur. Communities 1991, 50, 1.
- Komnitsas, K.; Zaharaki, D. Pre-treatment of olive mill wastewaters at laboratory and mill scale and subsequent use in agri-culture: Legislative framework and proposed soil quality indicators. Resour. Conserv. Recycl. 2012, 69, 82–89. [Google Scholar] [CrossRef]
- Babić, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kužić, A.; Čož-Rakovac, R.; Trebše, P. Toxicity evaluation of olive oil mill wastewater and its polar fraction using multiple whole-organism bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Italian Law, L 574, 1996, Official Gazette (G.U. Serie Generale n. 265, 12/11/1996). Available online: https://web.camera.it/parlam/leggi/96574l.htm (accessed on 22 March 2022).
- Italian Legislative Decree, 152, 1999, Official Gazette (G.U. Serie Generale n. 246, 20/10/ 2000-Ordinary Supplement n. 172). Available online: https://web.camera.it/parlam/leggi/deleghe/99152dl.htm (accessed on 22 March 2022).
- Italian Legislative Decree, 258, 2000, Official Gazette (G.U. Serie Generale n. 218, 18/09/2000). Available online: https://web.camera.it/parlam/leggi/deleghe/testi/00258dl.htm (accessed on 30 March 2022).
- El-Abbassi, A.; Kiai, H.; Hafidi, A.; García-Payo, M.; Khayet, M. Treatment of olive mill wastewater by membrane distillation using polytetrafluoroethylene membranes. Sep. Purif. Technol. 2012, 98, 55–61. [Google Scholar] [CrossRef]
- Carnevale, M.; Gnisci, E.; Hilal, J.; Criscuoli, A. Direct Contact and Vacuum Membrane Distillation application for the olive mill wastewater treatment. Sep. Purif. Technol. 2016, 169, 121–127. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Costa, C.; Firpo, R.; Jezowska, A.; Pagliero, M. Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes. Membranes 2020, 10, 334. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of different ultrafiltration membranes as first step for the recovery of phenolic compounds from olive-oil washing wastewater. Process Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef] [PubMed]
- Neffa, M.; Taourirte, M.; Ouazzani, N.; Hanine, H. Eco-friendly approach for elimination of olive mill wastewaters (OMW) toxicity using cactus prickly pears juice as a coagulant. Water Pract. Technol. 2020, 15, 1050–1067. [Google Scholar] [CrossRef]
- Tagliabue, M.; Tonziello, J.; Bottino, A.; Capannelli, G.; Comite, A.; Pagliero, M.; Boero, F.; Cattaneo, C. Laboratory Scale Evaluation of Fertiliser Factory Wastewater Treatment through Membrane Distillation and Reverse Osmosis. Membranes 2021, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation. J. Membr. Sci. 2020, 596, 117575. [Google Scholar] [CrossRef]
- Bottino, A.; Comite, A.; Costa, C.; Di Felice, R.; Varosio, E. Wetting of Polypropylene Membranes by Aqueous Solutions in CO2Absorbing Devices. Sep. Sci. Technol. 2015, 50, 1860–1869. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Structure–antioxidant activity relationship of β-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press Ltd; Elsevier Science Ltd: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Herbello-Hermelo, P.; Lamas, J.P.; Lores, M.; González, M.R.D.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Polyphenol bioavailability in nuts and seeds by an in vitro dialyzability approach. Food Chem. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_bacteria/ (accessed on 30 March 2022).
- Zghari, B.; Doumenq, P.; Romane, A.; Boukir, A. GC-MS, FTIR and 1H,13C NMR Structural Analysis and Identification of Phenolic Compounds in Olive Mill Wastewater Extracted from Oued Oussefrou Effluent (Beni Mellal-Morocco). J. Mater. Environ. Sci. 2017, 8, 4496–4509. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Method Enzymol. 1986, 130, 290–311. [Google Scholar] [CrossRef]
- Francioso, O.; Ferrari, E.; Saladini, M.; Montecchio, D.; Gioacchini, P.; Ciavatta, C. TG–DTA, DRIFT and NMR characterisation of humic-like fractions from olive wastes and amended soil. J. Hazard. Mater. 2007, 149, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ait Baddi, G.; Hafidi, M.; Gilard, V.; Revel, J.C. Characterization of humic acids produced during composting of olive mill wastes: Elemental and spectroscopic analyses (FTIR and 13C NMR). Agronomie 2003, 23, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Senent, F.; Martos, S.; Lama-Muñoz, A.; Fernandez-Bolanos, J. Isolation and identification of minor secoiridoids and phenolic components from thermally treated olive oil byproducts. Food. Chem. 2015, 187, 166–173. [Google Scholar] [CrossRef]
- Sienkiewicz-Gromiuk, J.; Tarasiuk, B.; Mazur, L. New organic single crystal of (benzylthio)acetic acid: Synthesis, crystal structure, spectroscopic (ATR-FTIR, 1H and 13C NMR) and thermal characterization. J. Mol. Struct. 2016, 1110, 65–71. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV—Vis, FTIR and 1 H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Elhajjouji, H.; Fakharedine, N.; Aitbaddi, G.; Winterton, P.; Bailly, J.; Revel, J.; Hafidi, M. Treatment of olive mill waste-water by aerobic biodegradation: An analytical study using gel permeation chromatography, ultraviolet–visible and Fourier transform infrared spectroscopy. Bioresour. Technol. 2007, 98, 3513–3520. [Google Scholar] [CrossRef]
- Boukir, A.; Guiliano, M.; Asia, L.; El Hallaoui, A.; Mille, G. A fraction to fraction study of photo-oxidation of BAL 150 crude oil asphaltenes. Analusis 1998, 26, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Ayers, S.; Zink, D.L.; Mohn, K.; Powell, J.S.; Brown, C.M.; Murphy, T.; Brand, R.; Pretorius, S.; Stevenson, D.; Thompson, D.; et al. Flavones from Struthiola argentea with anthelmintic activity in vitro. Phytochemistry 2008, 69, 541–545. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Pessanha, S.; Figueirinhas, J.; Carvalho, M.L. Artificial aging paper to assess long-term effects of conservative treatment. Monitoring by infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), and energy dispersive X-ray fluorescence (EDXRF). Microchem. J. 2016, 124, 646–656. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Weiss, D.S. Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria. Antibiotics 2015, 4, 18–41. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, A.; Samadi Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–772. [Google Scholar]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.W.; Grogan, R.G. Bacterial speck of tomato: Sources of in-oculum and establishment of a resident population. Phytopathology 1977, 67, 388–394. [Google Scholar] [CrossRef]
- Belaqziz, M.; Tan, S.P.; EL Abbassi, A.; Kiai, H.; Hafidi, A.; Donovan, O.O.; McLoughlin, P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE 2017, 12, e0182622. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef]
- Schlupp, P.; Schmidts, T.M.; Pössl, A.; Wildenhain, S.; Franco, G.L.; Franco, A.L.; Franco, B.L. Effects of a Phenol-Enriched Purified Extract from Olive Mill Wastewater on Skin Cells. Cosmetics 2019, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Roila, R.; Branciari, R.; Ranucci, D.; Ortenzi, R.; Urbani, S.; Servili, M.; Valiani, A. Antimicrobial Activity of Olive Mill Wastewater Extract Against Pseudomonas Fluorescens Isolated from Mozzarella Cheese. Ital. J. Food Saf. 2016, 5, 5760. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Conductivity (mS/cm) |
|---|---|
| ROp1 | 0.14 |
| ROp2 | 1.00 |
| MDd1 | 0.42 |
| MDd2 | 0.63 |
| Sample Name | A (λ = 750 nm) | g GAE/L |
|---|---|---|
| MF1 | 0.188 | 1.304 ± 0.150 d |
| MF2 | 0.333 | 0.251 ± 0.056 e |
| ROP1 | 0.042 | 0.001 ± 0.001 e |
| ROP2 | 0.097 | 0.055 ± 0.006 e |
| RO1 | 0.131 | 8.292 ± 0.251 b |
| MD1 | 0.110 | 6.542 ± 0.227 c |
| MD2 | 0.216 | 15.375 ± 0.015 a |
| MDd1 | 0.180 | 0.012 ± 0.001 e |
| MDd2 | 0.150 | 0.010 ± 0.001 e |
| Sample Name | A (517 nm) | DPPH% | AA% |
|---|---|---|---|
| MF1 | 0.861 | 88.95 ± 0.3 | 11.05 ± 0.3 e |
| MF2 | 0.893 | 92.25 ± 0.2 | 7.75 ± 0.2 f |
| ROP1 | 0.898 | 92.77 ± 0.4 | 7.23 ± 0.4 f |
| ROP2 | 0.708 | 73.14 ± 0.1 | 26.86 ± 0.1 d |
| RO1 | 0.633 | 65.39 ± 0.2 | 34.61 ± 0.2 c |
| MD1 | 0.514 | 53.10 ± 0.2 | 46.90 ± 0.2 b |
| MD2 | 0.17 | 17.56 ± 0.3 | 82.44 ± 0.3 a |
| MDd1 | 0.858 | 88.64 ± 0.5 | 11.36 ± 0.5 e |
| MDd2 | 0.864 | 89.26 ± 0.1 | 10.74 ± 0.1 e |
| MF1 | RO1 | ROP2 | MD1 | MD2 | MDd1 | MDd2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus | ||||||||||||||
| 17 MRSA | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 8 | 16 | 125 | >125 | 62 | >125 |
| 18 MRSA | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 16 | 8 | 16 | 125 | >125 | 62 | >125 |
| 187 MRSA | 125 | >125 | 31 | 31 | 125 | >125 | 16 | 31 | 8 | 16 | 125 | >125 | 62 | >125 |
| 188 MRSA | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 16 | 8 | 16 | 125 | >125 | 62 | >125 |
| S. epidermidis | ||||||||||||||
| 22 MRSE | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 16 | 8 | 16 | 125 | >125 | 62 | 125 |
| 180 MRSE | 62 | >125 | 16 | 16 | 125 | >125 | 16 | 16 | 8 | 16 | 62 | 125 | 62 | 62 |
| 181 MRSE | 62 | >125 | 16 | 16 | 125 | >125 | 16 | 16 | 8 | 8 | 125 | >125 | 62 | 125 |
| 222 MRSE | 125 | >125 | 16 | 16 | 125 | >125 | 16 | 16 | 16 | 16 | 125 | >125 | 62 | 125 |
| E. faecalis | ||||||||||||||
| 1 VRE | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| 4 | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 31 | 62 | >125 | 31 | >125 |
| 50 VRE | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| 365 VRE | 125 | >125 | 16 | 125 | 125 | >125 | 16 | 125 | 16 | 31 | 62 | >125 | 31 | >125 |
| E. faecium | ||||||||||||||
| 21 | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 16 | 62 | >125 | 62 | >125 |
| 40 | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 16 | 62 | >125 | 62 | >125 |
| 300 VRE | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 16 | 62 | >125 | 62 | >125 |
| 362 VRE | 125 | >125 | 16 | 62 | 125 | >125 | 16 | 62 | 16 | 16 | 62 | >125 | 31 | >125 |
| MF1 | RO1 | ROP2 | MD1 | MD2 | MDd1 | MDd2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| P. aeruginosa | ||||||||||||||
| 403 | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 432 | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 265c | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 16 | 62 | >125 | 31 | 125 |
| 1 | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 16 | 62 | >125 | 31 | 125 |
| 2v | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 19v | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 16b | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 16 | 62 | >125 | 31 | 125 |
| 12b | 62 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 8g | 125 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| A. baumannii | ||||||||||||||
| 245 | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 16 | 62 | 125 | 31 | 125 |
| M. morganii | ||||||||||||||
| 372 | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 31 | 62 | >125 | 31 | 125 |
| P. stuarti | ||||||||||||||
| 374 | 125 | >125 | 16 | 31 | 125 | >125 | 16 | 31 | 16 | 16 | 62 | >125 | 62 | 125 |
| K. pneumoniae | ||||||||||||||
| 375 * | 125 | >125 | 31 | 62 | 125 | >125 | 31 | 62 | 16 | 31 | 62 | >125 | 62 | 125 |
| 376 * | 62 | >125 | 16 | 62 | 62 | >125 | 16 | 62 | 8 | 31 | 62 | >125 | 62 | >125 |
| 377 * | 125 | >125 | 31 | 31 | 125 | >125 | 31 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| S. marcescens | ||||||||||||||
| 400 | 125 | >125 | 31 | 31 | 125 | >125 | 16 | 31 | 16 | 31 | 62 | >125 | 31 | 125 |
| S. maltophilia | ||||||||||||||
| 391 | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 16 | 62 | >125 | 31 | 125 |
| 392 | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 16 | 16 | 62 | >125 | 31 | 125 |
| E. coli | ||||||||||||||
| 224 | 125 | >125 | 31 | 62 | 125 | >125 | 31 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| 238 * | 125 | >125 | 31 | 62 | 125 | >125 | 31 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| 4 | 125 | >125 | 31 | 62 | 125 | >125 | 31 | 62 | 16 | 31 | 62 | >125 | 62 | >125 |
| P. syringae | ||||||||||||||
| 266 | 62 | >125 | 16 | 31 | 62 | >125 | 16 | 31 | 8 | 31 | 62 | >125 | 31 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. https://doi.org/10.3390/antiox11050903
Russo E, Spallarossa A, Comite A, Pagliero M, Guida P, Belotti V, Caviglia D, Schito AM. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants. 2022; 11(5):903. https://doi.org/10.3390/antiox11050903
Chicago/Turabian StyleRusso, Eleonora, Andrea Spallarossa, Antonio Comite, Marcello Pagliero, Patrizia Guida, Vittorio Belotti, Debora Caviglia, and Anna Maria Schito. 2022. "Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production" Antioxidants 11, no. 5: 903. https://doi.org/10.3390/antiox11050903
APA StyleRusso, E., Spallarossa, A., Comite, A., Pagliero, M., Guida, P., Belotti, V., Caviglia, D., & Schito, A. M. (2022). Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants, 11(5), 903. https://doi.org/10.3390/antiox11050903