Conventional Soybean Meal as Fishmeal Alternative in Diets of Japanese Seabass (Lateolabrax japonicus): Effects of Functional Additives on Growth, Immunity, Antioxidant Capacity and Disease Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
2.2. Diets
2.3. Sample Collection
2.4. Proximate Composition of Diets and the Whole Body of Fish
2.5. Respiratory Burst Activity (RB) Assay
2.6. Serum Alternative Complement Pathway (ACP) Activities
2.7. Serum Antioxidant Enzyme Assay
2.8. Liver Malondialdehyde (MDA) Levels
2.9. Liver Vitamin E Concentrations
2.10. Liver Scavenging Rate of Hydroxyl Radical (SROH)
2.11. Challenge Test
2.12. Calculations and Statistical Methods
3. Results
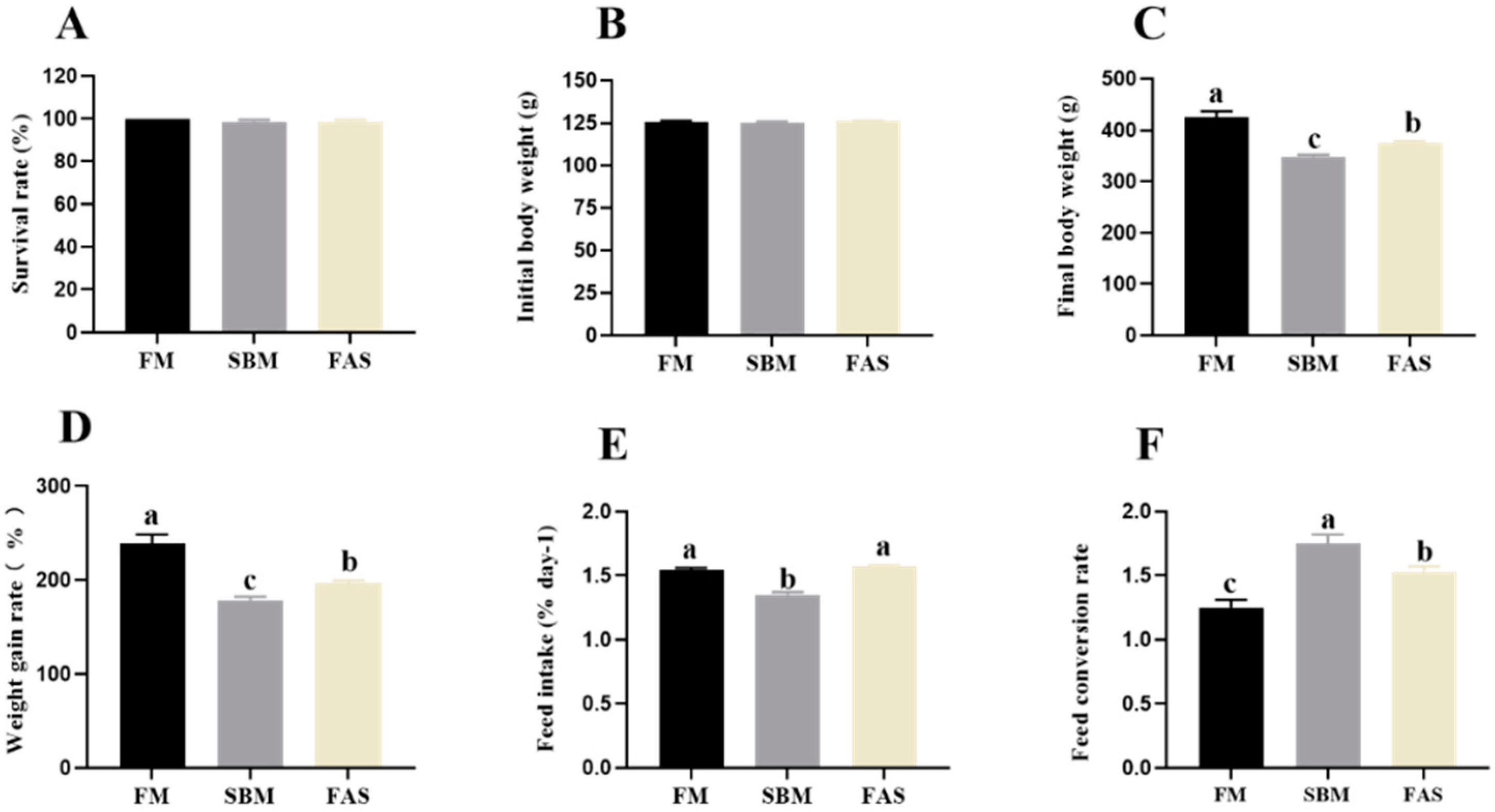
3.1. Growth Performance
3.2. Body Indexes
3.3. Proximate Composition of the Whole Body of Fish
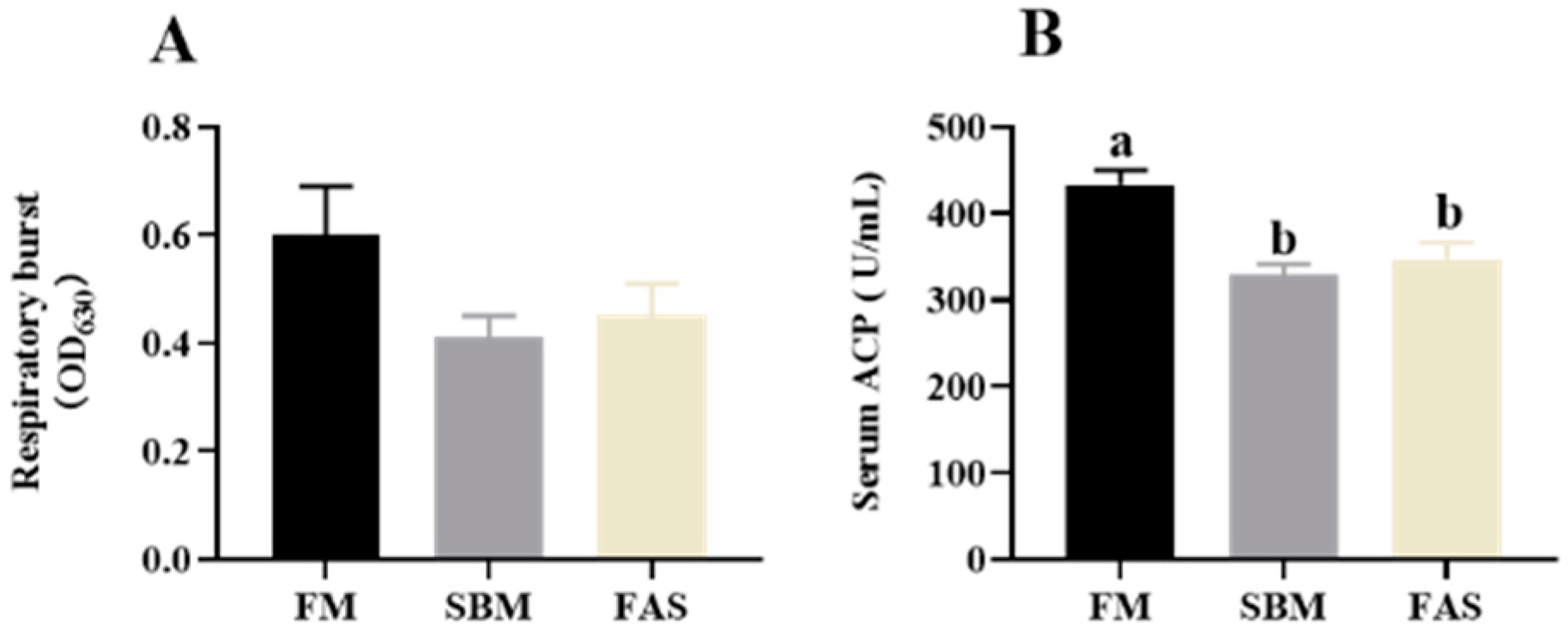
3.4. Immune Parameters
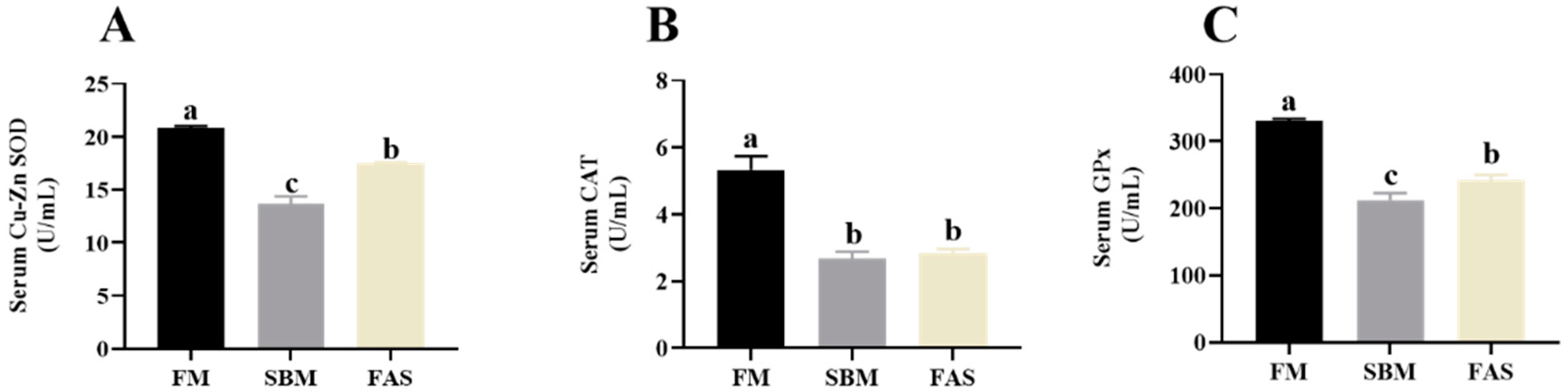
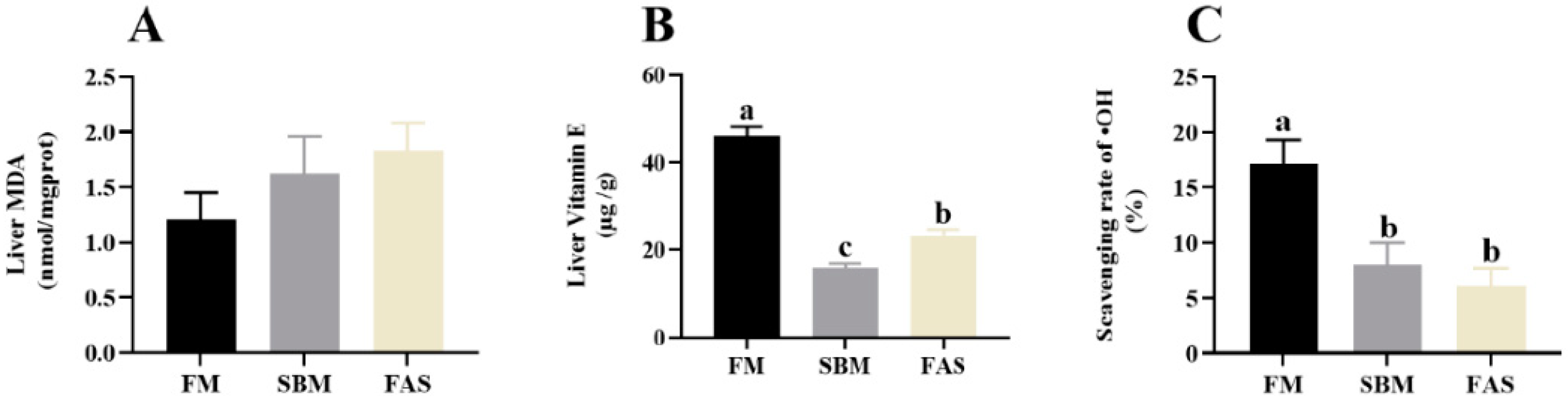
3.5. Antioxidant Capacities
3.6. Challenge Test
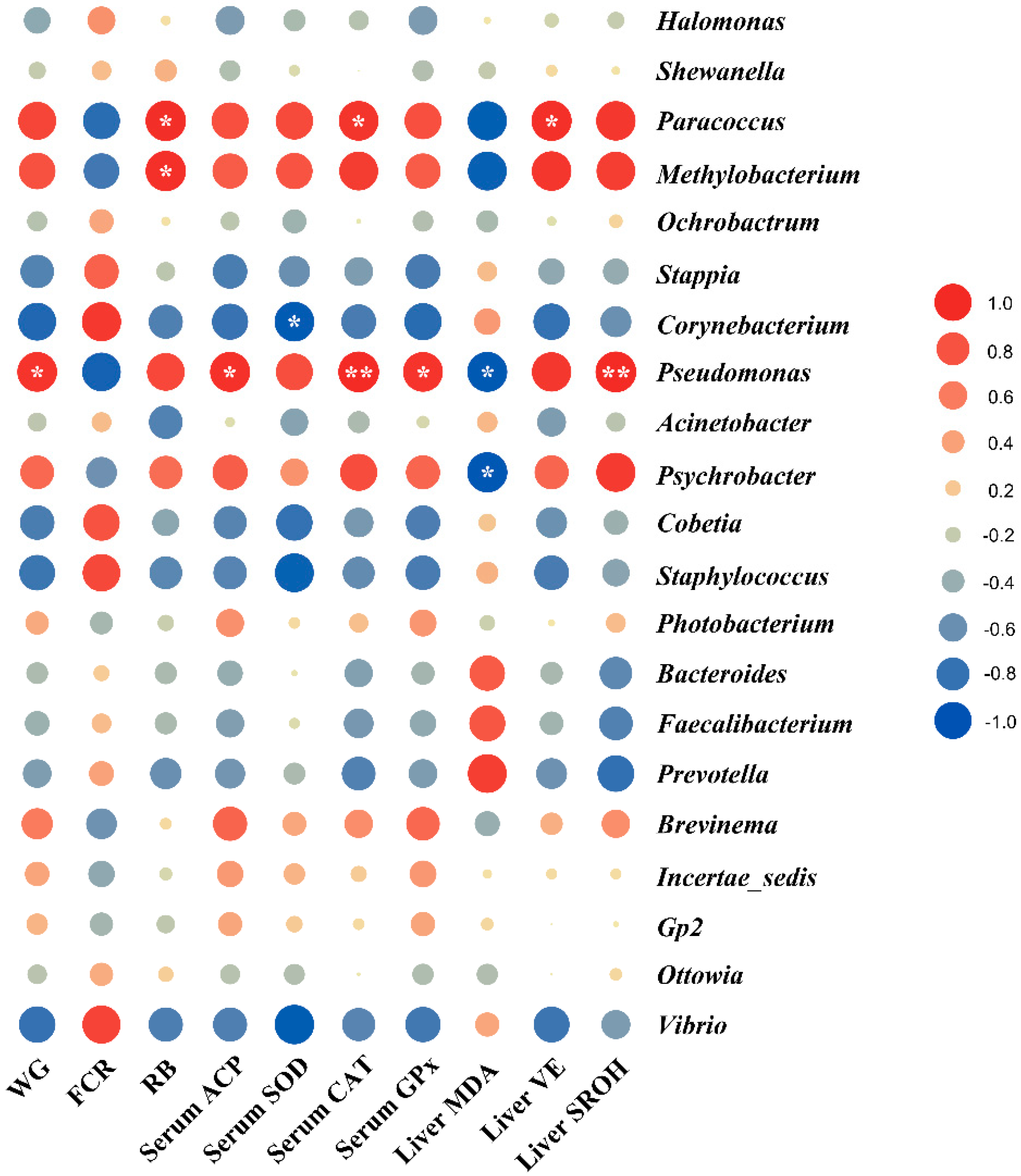
3.7. Pearson Correlation between Gut Microbiota and Host Responses
4. Discussion
4.1. Growth Performance and Body Composition
4.2. Immunity
4.3. Antioxidant Capacity
4.4. Associations between Gut Microbiota and Host Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Wang, J.; Yun, B.; Xue, M.; Wu, X.; Zheng, Y.; Li, P. Apparent digestibility coefficients of several protein sources, and replacement of fishmeal by porcine meal in diets of Japanese seabass, Lateolabrax japonicus, are affected by dietary protein levels. Aquac. Res. 2012, 43, 117–127. [Google Scholar] [CrossRef]
- Hu, L.; Yun, B.; Xue, M.; Wang, J.; Wu, X.; Zheng, Y.; Han, F. Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus). Aquaculture 2013, 372, 52–61. [Google Scholar] [CrossRef]
- Cheng, Z.; Ai, Q.; Mai, K.; Xu, W.; Ma, H.; Li, Y.; Zhang, J. Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass, Lateolabrax japonicus. Aquaculture 2010, 305, 102–108. [Google Scholar] [CrossRef]
- Men, K.; Ai, Q.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H. Effects of dietary corn gluten meal on growth, digestion and protein metabolism in relation to IGF-I gene expression of Japanese seabass, Lateolabrax japonicus. Aquaculture 2014, 428, 303–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liang, X.F.; Han, J.; Wu, X.F.; Yang, Y.H.; Xue, M. Metabolic disorder induces fatty liver in Japanese seabass, Lateolabrax japonicas fed a full plant protein diet and regulated by cAMP-JNK/NF-kB-caspase signal pathway. Fish Shellfish Immunol. 2019, 90, 223–234. [Google Scholar] [CrossRef]
- Wang, J.; Tao, Q.; Wang, Z.; Mai, K.; Xu, W.; Zhang, Y.; Ai, Q. Effects of fish meal replacement by soybean meal with supplementation of functional compound additives on intestinal morphology and microbiome of Japanese seabass (Lateolabrax japonicus). Aquac. Res. 2016, 48, 2186–2197. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mai, K.; Xu, W.; Cheng, Z. Effects of the partial substitution of dietary fish meal by two types of soybean meals on the growth performance of juvenile Japanese seabass, Lateolabrax japonicus (Cuvier 1828). Aquac. Res. 2012, 43, 458–466. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mai, K.; Xu, W.; Deng, J.; Cheng, Z. Comparison of high-protein soybean meal and commercial soybean meal partly replacing fish meal on the activities of digestive enzymes and aminotransferases in juvenile Japanese seabass, Lateolabrax japonicus (Cuvier, 1828). Aquac. Res. 2014, 45, 1051–1060. [Google Scholar] [CrossRef]
- Liang, X.F.; Hu, L.; Dong, Y.C.; Wu, X.F.; Qin, Y.C.; Zheng, Y.H.; Shi, D.D.; Xue, M.; Liang, X.F. Substitution of fish meal by fermented soybean meal affects the growth performance and flesh quality of Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2017, 229, 1–12. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.-r.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Wu, Y.; Han, H.; Qin, J.; Wang, Y. Replacement of dietary fish meal by soybean meal supplemented with crystalline methionine for Japanese seabass (Lateolabrax japonicus). Aquac. Res. 2016, 47, 243–252. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wu, Y.B.; Jiang, D.L.; Qin, J.G.; Wang, Y. Gamma-irradiated soybean meal replaced more fish meal in the diets of Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2014, 197, 155–163. [Google Scholar] [CrossRef]
- Refstie, S.; Sahlstrom, S.; Brathen, E.; Baeverfjord, G.; Krogedal, P. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 2005, 246, 331–345. [Google Scholar] [CrossRef]
- Krogdahl, A.; Gajardo, K.; Kortner, T.M.; Penn, M.; Gu, M.; Berge, G.M.; Bakke, A.M. Soya Saponins Induce Enteritis in Atlantic Salmon (Salmo salar L.). J. Agric. Food Chem. 2015, 63, 3887–3902. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; Lu, K.; Wang, L.; Song, K.; Mai, K.; Davis, D.A.; Zhang, C. Replacement of fish meal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2019, 84, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Papatryphon, E.; Soares, J.H. The effect of dietary feeding stimulants on growth performance of striped bass, Morone saxatilis, fed-a-plant feedstuff-based diet. Aquaculture 2000, 185, 329–338. [Google Scholar] [CrossRef]
- Hai, N.V. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar]
- Wang, J.; Kortner, T.M.; Chikwati, E.M.; Li, Y.; Jaramillo-Torres, A.; Jakobsen, J.V.; Ravndal, J.; Brevik, O.J.; Einen, O.; Krogdahl, A. Gut immune functions and health in Atlantic salmon (Salmo salar) from late freshwater stage until one year in seawater and effects of functional ingredients: A case study from a commercial sized research site in the Arctic region. Fish Shellfish Immunol. 2020, 106, 1106–1119. [Google Scholar] [CrossRef]
- Beth, K.; Hellwig, D.; Gill, D.; Owens, F.; Murapa, P.; Gandhapudi, S.; Skaggs, H.S.; Sarge, K.D. Effect of Agrado® on performance and health of calves new to the feedlot environment. Res. Ser.-Ark. Agric. Exp. Stn. 2000, 84–87. [Google Scholar]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2020, 13, 632–641. [Google Scholar] [CrossRef]
- Apines-Amar, M.J.S.; Satoh, S.; Caipang, C.M.A.; Kiron, V.; Watanabe, T.; Aoki, T. Amino acid-chelate: A better source of Zn, Mn and Cu for rainbow trout, Oncorhynchus mykiss. Aquaculture 2004, 240, 345–358. [Google Scholar] [CrossRef]
- Katya, K.; Lee, S.; Bharadwaj, A.S.; Browdy, C.L.; Vazquez-Anon, M.; Bai, S.C. Effects of inorganic and chelated trace mineral (Cu, Zn, Mn and Fe) premixes in marine rockfish, Sebastes schlegeli (Hilgendorf), fed diets containing phytic acid. Aquac. Res. 2017, 48, 4165–4173. [Google Scholar] [CrossRef]
- Katya, K.; Lee, S.; Yun, H.; Dagoberto, S.; Browdy, C.L.; Vazquez-Anon, M.; Bai, S.C. Efficacy of inorganic and chelated trace minerals (Cu, Zn and Mn) premix sources in Pacific white shrimp, Litopenaeus vannamei (Boone) fed plant protein based diets. Aquaculture 2016, 459, 117–123. [Google Scholar] [CrossRef]
- Secombes, C.J. Isolation of salmonid macrophages and analysis of their killing activity. Tech. Fish Immunol. 1990, 1, 137–154. [Google Scholar]
- Nilsson, U.R.; Nilsson, B. Simplified assays of hemolytic activity of the classical and alternative complement pathways. J. Immunol. Methods 1984, 72, 49–59. [Google Scholar] [CrossRef]
- Abei, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zhang, X.D.; Zhu, Y.F.; Cai, L.S.; Wu, T.X. Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 2008, 280, 136–139. [Google Scholar] [CrossRef]
- Tangney, C.C.; Driskell, J.A. Effects of vitamin E deficiency on the relative incorporation of 14C-arachidonate into platelet lipids of rabbits. J. Nutr. 1981, 111, 1839–1847. [Google Scholar] [CrossRef]
- Wang, F.; Le, G.; Shi, Y.; Chen, Z. Effect of mannose-oligosaccharides on scavenging free radicals and the activities of antioxidant enzymes in liver of rats. Acta Nutr. 2007, 5, 15. [Google Scholar]
- Ai, Q.H.; Xu, H.G.; Mai, K.S.; Xu, W.; Wang, J.; Zhang, W.B. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Van den Ingh, T.S.; Krogdahl, Å.; Olli, J.J.; Hendriks, H.G.; Koninkx, J.G.J.F. Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): A morphological study. Aquaculture 1991, 94, 297–305. [Google Scholar] [CrossRef]
- Yu, D.H.; Gong, S.Y.; Yuan, Y.C.; Luo, Z.; Lin, Y.C.; Li, Q. Effect of partial replacement of fish meal with soybean meal and feeding frequency on growth, feed utilization and body composition of juvenile Chinese sucker, Myxocyprinus asiaticus (Bleeker). Aquac. Res. 2013, 44, 388–394. [Google Scholar] [CrossRef]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; de Francesco, M.; Luzzana, U.; Harpaz, S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- Ye, J.D.; Liu, X.H.; Wang, Z.J.; Wang, K. Effect of partial fish meal replacement by soybean meal on the growth performance and biochemical indices of juvenile Japanese flounder Paralichthys olivaceus. Aquac. Int. 2011, 19, 143–153. [Google Scholar] [CrossRef]
- Silva-Carrillo, Y.; Hernandez, C.; Hardy, R.W.; Gonzalez-Rodriguez, B.; Castillo-Vargasmachuca, S. The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture 2012, 364, 180–185. [Google Scholar] [CrossRef]
- Peng, M.; Xu, W.; Ai, Q.H.; Mai, K.S.; Liufu, Z.G.; Zhang, K.K. Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybean meal (Scophthalmus maximus L.). Aquaculture 2013, 392, 51–58. [Google Scholar] [CrossRef]
- Rossi, W.; Tomasso, J.R.; Gatlin, D.M. Performance of Cage-Raised, Overwintered Hybrid Striped Bass Fed Fish Meal- or Soybean-Based Diets. N. Am. J. Aquac. 2015, 77, 178–185. [Google Scholar] [CrossRef]
- Rossi, W.; Allen, K.M.; Habte-Tsion, H.-M.; Meesala, K.-M. Supplementation of glycine, prebiotic, and nucleotides in soybean meal-based diets for largemouth bass (Micropterus salmoides): Effects on production performance, whole-body nutrient composition and retention, and intestinal histopathology. Aquaculture 2021, 532, 736031. [Google Scholar] [CrossRef]
- Mai, K.S.; Zhang, L.; Ai, Q.H.; Duan, Q.Y.; Zhang, C.X.; Li, H.T.; Wan, J.L.; Liufu, Z.G. Dietary lysine requirement of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 2006, 258, 535–542. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.H.; He, R.J.; Xu, W.; Mai, K.S.; He, G. Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2016, 464, 87–94. [Google Scholar] [CrossRef]
- Khosravi, S.; Rahimnejad, S.; Herault, M.; Fournier, V.; Lee, C.R.; Bui, H.T.D.; Jeong, J.B.; Lee, K.J. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immun. 2015, 45, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Maita, M.; Maekawa, J.; Satoh, K.; Futami, K.; Satoh, S. Disease resistance and hypocholesterolemia in yellowtail Seriola quinqueradiata fed a non-fishmeal diet. Fish. Sci. 2006, 72, 513–519. [Google Scholar] [CrossRef]
- Martinez-Alvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Torstensen, B.E.; Hemre, G.I.; Sanden, M.; Waagbo, R. Hepatic oxidative stress in Atlantic salmon (Salmo salar L.) transferred from a diet based on marine feed ingredients to a diet based on plant ingredients. Aquac. Nutr. 2011, 17, E424–E436. [Google Scholar] [CrossRef]
- Wang, J.; Jaramillo-Torres, A.; Li, Y.; Brevik, Ø.J.; Jakobsen, J.V.; Kortner, T.M.; Krogdahl, Å. Gut Health and Microbiota in Out-of-Season Atlantic Salmon (Salmo salar L.) Smolts Before and After Seawater Transfer Under Commercial Arctic Conditions: Modulation by Functional Feed Ingredients. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Wang, J.; Jaramillo-Torres, A.; Li, Y.; Kortner, T.M.; Gajardo, K.; Brevik, O.J.; Jakobsen, J.V.; Krogdahl, A. Microbiota in intestinal digesta of Atlantic salmon (Salmo salar), observed from late freshwater stage until one year in seawater, and effects of functional ingredients: A case study from a commercial sized research site in the Arctic region. Anim. Microbiome 2021, 3, 14. [Google Scholar] [CrossRef]
- Li, Y.; Bruni, L.; Jaramillo-Torres, A.; Gajardo, K.; Kortner, T.M.; Krogdahl, A. Differential response of digesta- and mucosa-associated intestinal microbiota to dietary insect meal during the seawater phase of Atlantic salmon. Anim. Microbiome 2021, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Hatje, E.; Neuman, C.; Stevenson, H.; Bowman, J.P.; Katouli, M. Population dynamics of Vibrio and Pseudomonas species isolated from farmed Tasmanian Atlantic salmon (Salmo salar L.): A seasonal study. Microb. Ecol. 2014, 68, 679–687. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (% Dry Matter) | FM | SBM | FAS |
|---|---|---|---|
| Fishmeal 1 | 42 | 21 | 21 |
| Conventional soybean meal 1 | 12 | 30 | 30 |
| Wheat 1 | 25.8 | 22.1 | 22.1 |
| Peanut meal 1 | 10 | 10 | 10 |
| Squid meal 1 | 2.5 | 3.5 | 3.5 |
| Spray blood meal 1 | 2.8 | 2.8 | |
| Soy protein concentrate 1 | 2 | 2 | |
| Fish oil | 4 | 5.5 | 5.5 |
| Vitamin and mineral premix | 1 | 1 | 1 |
| Lecithin | 1 | 1 | 1 |
| Vitamin C | 0.1 | 0.1 | 0.1 |
| Calcium propionic acid | 0.1 | 0.1 | 0.1 |
| Choline | 0.5 | 0.5 | 0.5 |
| Monocalcium phosphate | 1 | 1 | 1 |
| MeraMet 2 | 0.26 | 0.26 | |
| Threonine 2 | 0.1 | 0.1 | |
| Ethoxyquin | 0.02 | 0.02 | 0.02 |
| Functional additives 3 | 0.0665 | ||
| Proximate analysis | |||
| Crude protein | 45.2 | 45.1 | 44.9 |
| Crude lipid | 11.6 | 11.4 | 11.2 |
| Lysine | 2.74 | 2.66 | 2.68 |
| Methionine | 0.96 | 0.99 | 1.02 |
| (% Wet Weight) | FM | SBM | FAS |
|---|---|---|---|
| Moisture | 67.0 ± 0.9 b | 71.6 ± 0.4 a | 71.1 ± 0.6 a |
| Crude lipid | 11.2 ± 0.7 a | 7.0 ± 0.4 b | 8.0 ± 0.3 b |
| Crude protein | 16.9 ± 0.7 | 16.8 ± 0.1 | 16.5 ± 0.4 |
| Ash | 4.4 ± 0.2 | 5.2 ± 0.1 | 5.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Mai, K.; Ai, Q. Conventional Soybean Meal as Fishmeal Alternative in Diets of Japanese Seabass (Lateolabrax japonicus): Effects of Functional Additives on Growth, Immunity, Antioxidant Capacity and Disease Resistance. Antioxidants 2022, 11, 951. https://doi.org/10.3390/antiox11050951
Wang J, Mai K, Ai Q. Conventional Soybean Meal as Fishmeal Alternative in Diets of Japanese Seabass (Lateolabrax japonicus): Effects of Functional Additives on Growth, Immunity, Antioxidant Capacity and Disease Resistance. Antioxidants. 2022; 11(5):951. https://doi.org/10.3390/antiox11050951
Chicago/Turabian StyleWang, Jie, Kangsen Mai, and Qinghui Ai. 2022. "Conventional Soybean Meal as Fishmeal Alternative in Diets of Japanese Seabass (Lateolabrax japonicus): Effects of Functional Additives on Growth, Immunity, Antioxidant Capacity and Disease Resistance" Antioxidants 11, no. 5: 951. https://doi.org/10.3390/antiox11050951





