Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
2.3. Bee Pollen Extract (BPE) Preparation
2.4. Chemical Composition of BPE
2.4.1. Determination of Moisture
2.4.2. Determination of Total Phenol Content (TPC)
2.4.3. Determination of Total Flavonoid Content (TFC)
2.4.4. Phenolic Compound Analysis by UHPLC-DAD-ESI/MS
2.4.5. GC-MS Analysis
2.5. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. Methods Applied in the Biological Activity of BPE
2.6.1. Qualitative Evaluation of Antimicrobial Activity
2.6.2. Quantitative Evaluation of Antimicrobial Activity
2.6.3. Semiquantitative Assessment of Microbial Adherence to the Inert Substratum
2.6.4. Evaluation of the Inhibitory Effect of the BPE Samples on the Ability of the Tested Microbial Strains to Attach to the Cellular Substrate
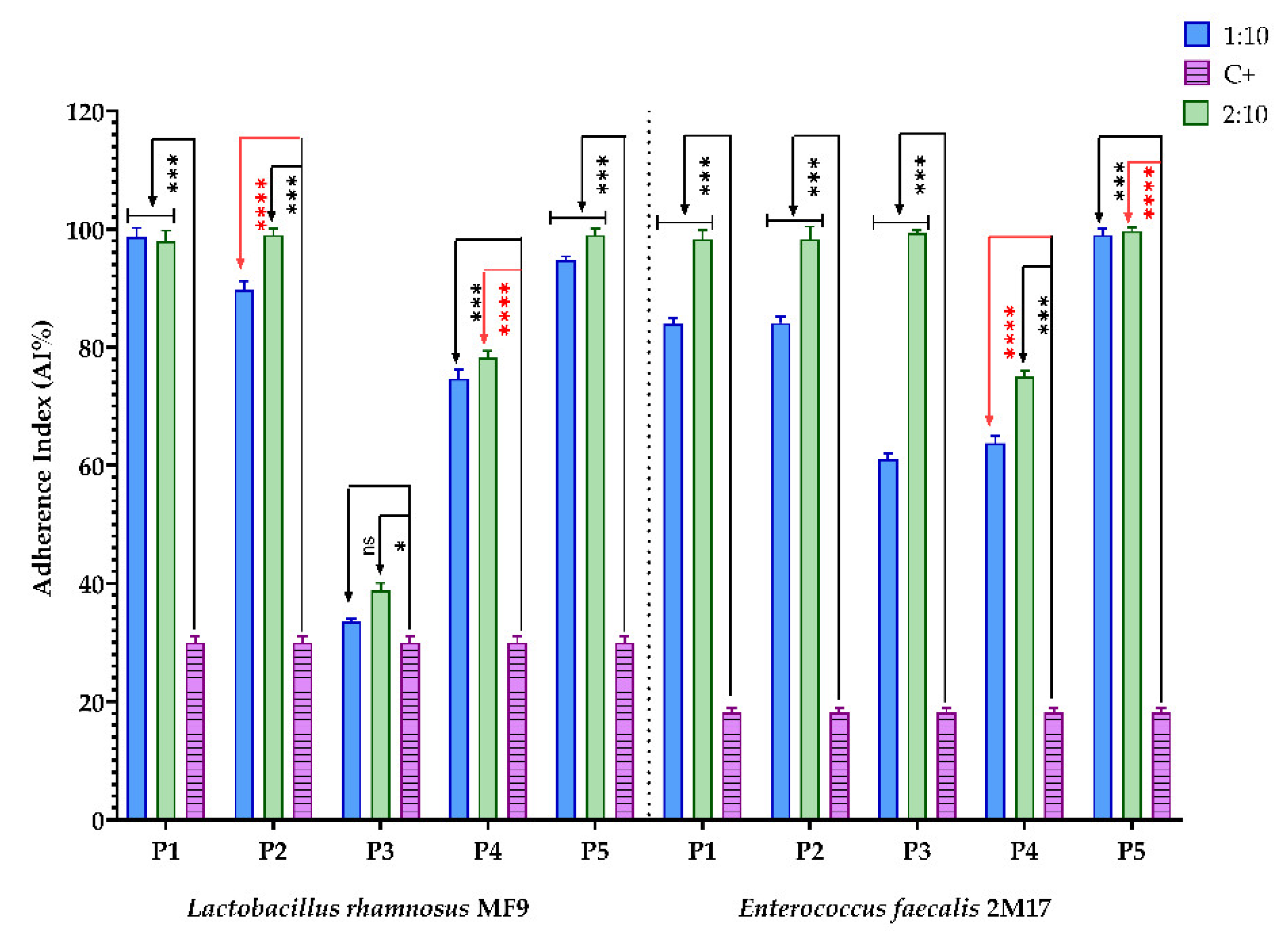
2.6.5. Evaluation of the Prebiotic Effect of the BPE Samples on the Ability of Two Microbial Strains with Probiotic Potential to Adhere to a Cellular Substrate
2.6.6. Assessment of the Prebiotic Effect of the BPE on the Growth Curve for Two Microbial Strains with Probiotic Potential
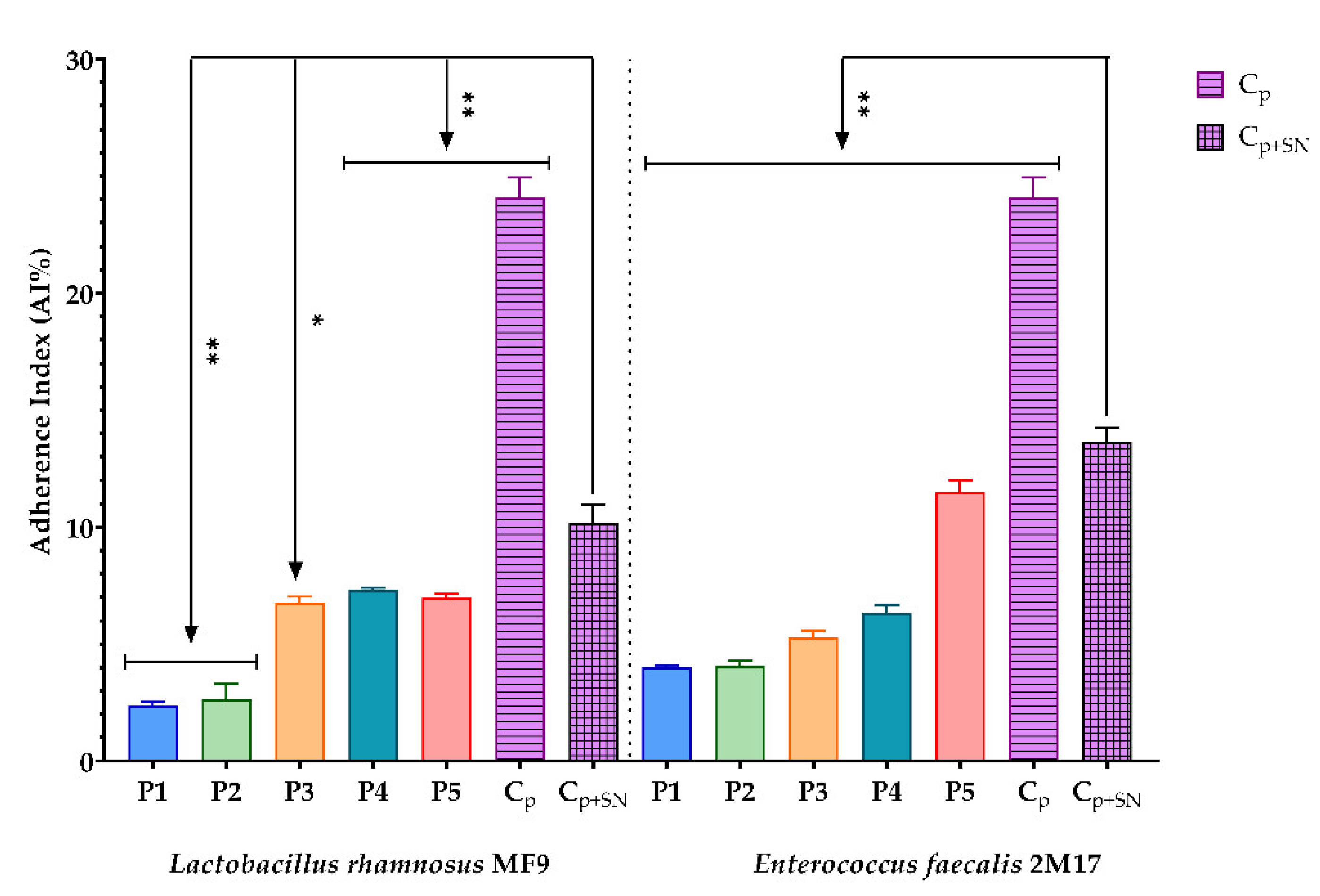
2.6.7. Assessment of the Synergic Influence of BPE and Probiotic Soluble Compounds on the Capacity of Some Pathogenic Strains to Adhere to the Cellular Substratum
2.7. Cytototoxic Activity of BPEs
2.8. Statistical Analysis
3. Results and Discussions
3.1. Chemical Composition of BPE
3.1.1. Determination of Moisture
3.1.2. Determination of TPC, TFC, and TEAC
3.1.3. Phenolic Compound Profile by UHPLC-DAD-ESI/MS
3.1.4. BPE Analysis by GC-MS
3.2. Biological Activity of BPE
3.2.1. Qualitative Evaluation of Antimicrobial Activity
3.2.2. Quantitative Evaluation of Antimicrobial Activity
3.2.3. Semiquantitative Assessment of Microbial Adherence to the Inert Substratum
3.2.4. Evaluation of the Inhibitory Effect of the BPE Samples on the Ability of the Tested Microbial Strains to Attach to the Cellular Substrate
3.2.5. Evaluation of the Prebiotic Effect of the BPE Samples on the Ability of Two Microbial Strains with Probiotic Potential to Adhere to a Cellular Substrate
3.2.6. Assessment of the Prebiotic Effect of the BPE on the Growth for Two Bacterial Strains with Probiotic Potential
3.2.7. Assessment of the Synergic Influence of BPE and Probiotic Soluble Compounds on the Capacity of Some Pathogenic Strains to Adhere to the Cellular Substratum
3.3. Cytototoxic Activity of BPEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komosinska-Vassev, K.; Olczyk, P.; Kazmierczak, J.; Mecner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complementary Altern. Med. Ecam. 2015, 2015, 297425–297431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayacan, S.; Sagdic, O.; Doymaz, I. Effects of hot-air and vacuum drying on drying kinetics, bioactive compounds and color of bee pollen. J. Food Meas. Charact. 2018, 12, 1274–1283. [Google Scholar] [CrossRef]
- De-Melo, A.; Almeida-Muradian, L. Chemical Composition of Bee Pollen. In Bee Products—Chemical and Biological Properties; Springer International Publishing: Cham, Switzerland, 2017; pp. 221–259. [Google Scholar]
- Kieliszek, M.; Piwowarek, K.; Błażejak, S.; Chlebowska-Śmigiel, A. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Spulber, R.; Dogaroglu, M.; Badeau, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotech. Lett. 2018, 23, 13357–13365. [Google Scholar]
- De Arruda, V.A.S.; Vieria dos Santos, A.; Sampaio, D.F.; da Silva Araújo, E.; Luís de Castro Peixoto, A.; Estevinho, M.L.F.; de Almeida-Muradian, L.B. Microbiological quality and physicochemical characterization of Brazilian bee pollen. J. Apic. Res. 2017, 56, 231–238. [Google Scholar] [CrossRef]
- Parvanov, P.; Dinkov, D. Proposals for desiccated bee pollen production and labeling. J. Nutr. Hum. Health 2017, 1, 13–16. [Google Scholar] [CrossRef]
- Jannesar, M.; Shoushtari, M.S.; Majd, A.; Pourpak, Z. Bee Pollen Flavonoids as a Therapeutic Agent in Allergic and Immunological Disorders. Iran. J. Allergy Asthma Immunol. 2017, 16, 171–182. [Google Scholar]
- Li, Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Maragou, N.C.; Pavlidis, G.; Hatjina, F. Major and minor element levels in Greek apicultural products. Glob. Nest J. 2017, 19, 423–429. [Google Scholar]
- Schulte, F.; Mader, J.; Kroh, L.W.; Panne, U.; Kneipp, J. Characterization of Pollen Carotenoids with in situ and High-Performance Thin-Layer Chromatography Supported Resonant Raman Spectroscopy. Anal. Chem. 2009, 81, 8426–8433. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Omar, W.A.W.; Azhar, N.A.; Fadzilah, N.H.; Kamal, N.N.S.N.M. Bee pollen extract of Malaysian stingless bee enhances the effect of cisplatin on breast cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Kostić, A.Z.; Milincic, D.D.; Barać, M.B.; Shariati, M.A.; Tešić, L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.; Nahar, L.; Sarker, S.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef]
- Graikou, K.; Kapeta, S.; Aligiannis, N.; Sotiroudis, G.; Chondrogianni, N.; Gonos, E.; Chinou, I. Chemical analysis of Greek pollen—Antioxidant, antimicrobial and proteasome activation properties. Chem. Central J. 2011, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Abouda, Z.; Zerdani, I.; Kalalou, I.; Faid, M.; Ahami, M.T. The Antibacterial Activity of Maroccan Bee Bread and Bee-Pollen (Fresh and Dried) against Pathogenic Bacteria. Res. J. Microbiol. 2011, 6, 376–384. [Google Scholar]
- Bridi, R.; Atala, E.; Pizzaro, P.N.; Montenegro, G. Honeybee Pollen Load: Phenolic Composition and Antimicrobial Activity and Antioxidant Capacity. J. Nat. Prod. 2019, 82, 559–565. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixera, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Karadal, F.; Onmaz, N.E.; Abay, S.; Yildirim, Y.; Al, S.; Tatyuz, I.; Akcay, A. A Study of Antibacterial and Antioxidant Activities of Bee Products: Propolis, Pollen and Honey Samples. Ethiop. J. Health Dev. 2018, 32, 116–122. [Google Scholar]
- Urcan, A.; Criste, A.; Dezmirean, D.; Bobiș, O.; Mărghitaș, L.; Mărgăoan, R.; Hrinca, A. Antimicrobial Activity of Bee Bread Extracts Against Different Bacterial Strains. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričik, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health. Part. B Pestic. Food Contam. Agric. Wastes 2013, 48, 133–138. [Google Scholar] [CrossRef]
- AbdElsalam, E.; Foda, H.S.; Abdel-Aziz, M.S.; El-Hady, K.A. Antioxidant and Antimicrobial activities of Egyptian Bee Pollen. Middle East J. Appl. Sci. 2018, 8, 1248–1255. [Google Scholar]
- Šimunovič, K.; Abramovič, H.; Lilek, N.; Angelova, M.; Podrzaj, L.; Mozina, S.S. Microbiological quality, antioxidative and antimicrobial properties of Slovenian Bee Pollen. AGROFOR Int. J. 2019, 4, 82–92. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.; Montfort, G.R.-C.; Zavala, J.F.A.; González-Aguilar, G. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Khan, A.; Alghanem, B.; Shaibah, H. Simultaneous identification of phenolic and flavonoid contents in bee pollen by HPLC-ESI-MS data. Biomed. Res. 2019, 30, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-Assisted Extraction of Polyphenols from Crude Pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial Bee Pollen with Different Geographical Origins: A Comprehensive Approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Mărghitas, L.; Dezmirean, D.S.; Bobis, O.; Mihai, C.M. Physical-Chemical Composition of Fresh Bee Pollen from Transylvania. Bull. UASVM Anim. Sci. Biotechnol. 2012, 69, 351–355. [Google Scholar]
- Commission regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2020, 1–170.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Marinas, I.; Oprea, E.; Geana, E.-I.; Chifiriuc, C.; Lazar, V. Antimicrobial and antioxidant activity of the vegetative and reproductive organs of Robinia pseudoacacia. J. Serb. Chem. Soc. 2014, 79, 1363–1378. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Salleh, S.; Hanapiah, N.A.M.; Johari, W.L.W.; Ahmad, H.; Osman, N.H. Analysis of bioactive compounds and chemical composition of Malaysian stingless bee propolis water extracts. Saudi J. Biol. Sci. 2021, 28, 6705–6710. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of Chemical Composition of Bee Pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Bakchiche, B.; Temizer, İ.K.; Güder, A.; Çelemli, O.G.; Yegin, S.C.; Bardaweel, S.K.; Ghareeb, M.A. Chemical Composition and Biological Activities of Honeybee Products from Algeria. J. Appl. Biotechnol. Rep. 2020, 7, 93–103. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ronald, L.; Prior, X.W.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agr. Food Chem. 2005, 53, 4290–4302. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.-D.; Ilie, C.-I.; Oprea, O.-C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Drăghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2019, 55, 1915–1932. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Cravioto, A.; Tello, A.; Navarro, A.; Ruiz, J.; Villafán, H.; Uribe, F.; Eslava, C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 1991, 337, 262–264. [Google Scholar] [CrossRef]
- Ditu, L.-M.; Chifiriuc, M.C.; Bezirtzoglou, E.; Voltsi, C.; Bleotu, C.; Pelinescu, D.; Mihaescu, G.; Lazar, V. Modulation of virulence and antibiotic susceptibility of enteropathogenic Escherichia coli strains by Enterococcus faecium probiotic strain culture fractions. Anaerobe 2011, 17, 448–451. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Bleotu, C.; Pelinescu, D.-R.; Lazar, V.; Ditu, L.-M.; Vassu, T.; Stoica, I.; Dracea, O.; Avram, I.; Sasarman, E. Patterns of colonization and immune response elicited from interactions between enteropathogenic bacteria, epithelial cells and probiotic fractions. Int. J. Biotechnol. Mol. Biol. Res. 2010, 1, 47–57. [Google Scholar]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karalidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: Evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Krishnamurthi, V.R.; Niyonshuti, I.I.; Chen, J.; Wang, Y. A new analysis method for evaluating bacterial growth with microplate readers. PLoS ONE 2021, 16, e0245205. [Google Scholar] [CrossRef]
- Samoila, I.; Dinescu, S.; Pircalabioru, G.G.; Marutescu, L.; Fundueanu, G.; Aflori, M.; Constantin, M. Pullulan/Poly(Vinyl Alcohol) Composite Hydrogels for Adipose Tissue Engineering. Materials 2019, 12, 3220. [Google Scholar] [CrossRef] [Green Version]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2021, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Pełka, K.; Otłowska, O.; Worobo, R.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobis, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Özkök, K.Ș.; Mayda, N.; Urcan, A.C.; Cornea-Cipcigan, M.A. Bee collected pollen as a value-added product rich in bioactive compounds and unsaturated fatty acids: A comparative study from Turkey and Romania. Food Sci. Technol. 2021, 149, 111925–111936. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. Food Sci. Technol. 2021, 142, 110973–110982. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133–9146. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Prdun, S.; Svecnjak, L.; Valentic, M.; Marijanovic, Z.; Jerkovic, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Santa Bárbara, M.F.; Moreira, M.M.; Machado, C.S.; Chambo, E.D.; Pascoal, A.; de Carvalho, C.A.; da Silva Sodre, G.; Delerue-Matos, C.; Estevinho, L.M. Storage methods, phenolic composition, and bioactive properties of Apis mellifera and Trigona spinipes pollen. J Apic. Res. 2020, 60, 99–107. [Google Scholar] [CrossRef]
- Velásquez, P.; Rodríguez, K.; Retamal, R.; Giardano, A.; Valenzuela, L.M.; Montenegro, G. Relation between composition, antioxidant and antibacterial activities and botanical origin of multi-floral bee pollen. J. Appl. Bot. Food Qual. 2017, 90, 306–314. [Google Scholar]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegusmonogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110–117. [Google Scholar] [CrossRef]
- Eugenia Martínez Valenzuela, M.; Díaz Peralta, K. Taraxacum Genus: Potential Antibacterial and Antifungal Activity. In Herbal Medicine; IntechOpen: London, UK, 2019; pp. 247–270. [Google Scholar]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C.F.R.; Lyoussi, B. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A.A. Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci. Biotechnol. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Mahmoud, A.A.; Mohamed, H.H.R.; Smetanska, I.; Ramadan, M.F. Phenolic Extract from Propolis and Bee Pollen: Composition, Antioxidant and Antibacterial Activities. J. Food Biochem. 2015, 39, 538–547. [Google Scholar] [CrossRef]
- Atsalakisa, E.; Chinou, I.; Makropoulou, M.; Karabournioti, S.; Graikou, K. Evaluation of Phenolic Compounds in Cistus creticus Bee Pollen from Greece. Antioxidant and Antimicrobial Properties. Nat. Prod. Commun. 2017, 12, 1813–1816. [Google Scholar] [CrossRef] [Green Version]
- Kačániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dżugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Singh, M.; Govindarajan, R.; Rawat AK, S.; Khare, P.B. Antimicrobial Flavonoid Rutin from Pteris Vittata L. Against Pathogenic Gastrointestinal Microflora. Am. Fern. J. 2008, 98, 98–103. [Google Scholar] [CrossRef]
- Rym, K.H.; Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antimicrobial activity and acute toxicity of natural rutin. Korean J. Pharmacogn. 1996, 27, 309–315. [Google Scholar]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simoes, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [Green Version]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Belhamra, Z.; Harzallah, D.; Naili, O.; Belhadj, H. Probiotic potential of lactobacillus strains isolated from fresh bee pollen. Pharm. Lett. 2016, 8, 357–365. [Google Scholar]
- Asama, T.; Arima, T.H.; Gomi, T.; Keishi, T.; Tani, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K. Lactobacillus kunkeei YB38 from honeybee products enhances IgA production in healthy adults. J. Appl. Microbiol. 2015, 119, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, H.; Harzallah, D.; Bouamra, D.; Khennouf, S.; Dahamna, S.; Ghadbane, M. Phenotypic and Genotypic Characterization of Some Lactic Acid Bacteria Isolated from Bee Pollen: A Preliminary Study. Biosci. Microbiota Food Health 2014, 33, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu RM, V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure—Activity relationship: An update review. Phytotherapy Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, M.F.G.; Sales, J.A.; Da Rocha, M.G.; Galdino, L.M.; De Aguiar, L.; Pereira-Neto, W.D.A.; Cordeiro, R.D.A.; Castelo-Branco, D.D.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Millimouno, F.M.; Eltayb, W.A.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Settu, K.; Manju, D.V. Pharmacological Applications of Isorhamnetin. Int. J. Trend Sci. Res. Dev. 2017, 1, 672–678. [Google Scholar]
- Ouyang, J.; Sun, F.; Feng, W.; Xie, Y.; Ren, L.; Chen, Y. Antimicrobial Activity of Galangin and Its Effects on Murein Hydrolases of Vancomycin-Intermediate Staphylococcus aureus (VISA) Strain Mu50. Chemotherapy 2018, 63, 20–28. [Google Scholar] [CrossRef]
- Monisha, S.I.; Vimala, J.R. Extraction, Identification and Pharmacological Evaluation of Phyto-Active Compound in Manilkara Hexandra (Roxb.) Dubard Stem Bark. Biosci. Biotechnol. Res. Asia 2018, 15, 687–698. [Google Scholar] [CrossRef]
- Das, S.; Burman, S.; Chandra, G. In-vitro Bactericidal Activity of a Novel Plant Source Plumeria pudica against Some Human and Fish Pathogenic Bacteria. Curr. Drug Discov. Technol. 2021, 18, 503–510. [Google Scholar] [CrossRef]
- Najar, B.; Nardi, V.; Cervelli, C.; Mecacci, G.; Mancianti, F.; Ebani, V.V.; Nardoni, S.; Pistelli, L. Volatilome Analyses and In Vitro Antimicrobial Activity of the Essential Oils from Five South African Helichrysum Species. Molecules 2020, 25, 3196. [Google Scholar] [CrossRef]
- Oo, T.; Saiboonjan, B. Inhibition of Bacterial Efflux Pumps by Crude Extracts and Essential Oil from Myristica fragrans Houtt. (Nutmeg) Seeds against Methicillin-Resistant Staphylococcus aureus. Molecules 2021, 26, 4662. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef] [Green Version]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, E.L.; de Albuquerque, T.M.R.; Dos Santos, A.S.; Massa NM, L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1–15. [Google Scholar] [CrossRef]
- Parkar, S.G.; Redgate, E.L.; McGhie, T.; Hurst, R.D. In vitro studies of modulation of pathogenic and probiotic bacterial proliferation and adhesion to intestinal cells by blackcurrant juices. J. Funct. Foods 2014, 8, 35–44. [Google Scholar] [CrossRef]
- Boubakeur, B.; Tirtouil, A.; Khadem, H. The evaluation of the effect of synthetic flavonoids on growth of pathogenic and probiotic bacteria. J. Chem. Pharm. 2015, 7, 228–236. [Google Scholar]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.; Schieber, A.; Gänzle, M. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Alberto, M.R.; Farías, M.E.; de Nadra, M.C.M. Effect of Gallic Acid and Catechin on Lactobacillus hilgardii 5w Growth and Metabolism of Organic Compounds. J. Agric. Food Chem. 2001, 49, 4359–4363. [Google Scholar] [CrossRef]
- Guldas, M. Effects of Royal Jelly and Bee Pollen on the Growth of Selected Probiotic Bacteria (Bf. animalis Spp. Lactis, L. acidophilus and L. casei). J. Apic. Sci. 2016, 60, 129–140. [Google Scholar]
- Puupponen-Pimia, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kahkonen, M.; Heinonen, M.; Maatta-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; De las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of Schisandra Chinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kročko, M.; Čanigová, M. Effect of Nutrition with Propolis and Bee Pollen Supplements on Bacteria Colonization Pattern in Gastrointestinal Tract of Broiler Chickens. J. Anim. Sci. Biotechnol. 2012, 45, 63–67. [Google Scholar]
- Olczyk, P.; Koprowski, R.; Kaźmierczak, J.; Mencner, L.; Wojtyczka, R.D.; Stojko, J.; Olczyk, K.; Komosinska-Vassev, K. Bee Pollen as a Promising Agent in the Burn Wounds Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 8473937–8473949. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Forbes-Hernandez, T.Y.; Afrin, S.; Giovanetti, E.; Battino, M. Apis mellifera vs. Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. Food Sci. Technol. 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Soltan-Dallal, M.M.; Validi, M.; Douraghi, M.; Fallah-Mehrabadi, J.; Lormohammadi, L. Evaluation the cytotoxic effect of cytotoxin-producing Klebsiella oxytoca isolates on the HEp-2 cell line by MTT assay. Microb. Pathog. 2017, 113, 416–420. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Cell Viability Assays. In Assay Guidance Manual; Markossian, G.A., Brimacombe, S., Eli Lilly, K., Eds.; Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013; pp. 1–24. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Abu Shady, H.M.; Mohamed, W.F.; Sayed-Ahmed, E.F.; Amer, S.A. A Comparative Study on Propolis and Pollen Extracts: Chemical Profile Analysis, Antioxidant and Anticancer Activity. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 397–414. [Google Scholar] [CrossRef]
- Uçar, M.; Gerigelmez, A.Y.; Cengiz, S.; Barlak, Y.; Değer, O.; Ovalı, E. Effect of Turkish pollen and propolis extracts on caspase-3 activity in myeloid cancer cell lines. Trop. J. Pharm. Res. 2016, 15, 2445. [Google Scholar] [CrossRef] [Green Version]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Sukemi; Yadi; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 7182–7189. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Dizaj, S.M. Molecular mechanisms of anticancer effect of rutin. Phytotherapy Res. 2020, 35, 2500–2513. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S. Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice. Med. Sci. 2014, 2, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharm. Base 2021, 14, 1069. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Lewandowski, W. Plant-Derived and Dietary Hydroxybenzoic Acids-A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]







| Sample | Flowering Plants | Date of Harvest |
|---|---|---|
| P1 | Cornus mas, Corylus avellana, Armeniaca vulgaris, Prunus cerasifera, Salix sp., Colchicum autumnale, Taraxacum officinale, Viola odorata, Helleborus sp. etc. | 1 April 2020 |
| P2 | Cornus mas, Corylus avellana, Armeniaca vulgaris, Prunus cerasifera, Ribes sp., Salix sp., Taraxacum officinale, Viola odorata etc. | 10 April 2020 |
| P3 | Prunus domestica, Malus sp., Cerasum sp. etc. | 24 April 2020 |
| P4 | Malus sp., Pyrus sp., Cydonia oblonga, Crataegus monogyna, Fraxinus excelsior etc. | 1 May 2020 |
| P5 | Robinia pseudocacia, Rosa centifolia, Rosa canina, Cornus sanguinea, Acer campestre etc. | 1 June 2020 |
| Sample | Moisture (%) ± SD |
|---|---|
| P1 | 27.83 ± 0.01 a |
| P2 | 22.62 ± 0.02 a |
| P3 | 26.47 ± 0.01 a |
| P4 | 31.91 ± 0.02 a |
| P5 | 35.87 ± 0.02 a |
| Sample | TPC (GAE) 1 | TFC (QE) 2 | TEAC 3 |
|---|---|---|---|
| P1 | 15.51 ± 0.01 c,d | 0.27 ± 0.01 d | 0.06 ± 0.02 b |
| P2 | 16.15 ± 0.02 c,d | 0.30 ± 0.01 d | 0.07 ± 0.01 b,d |
| P3 | 13.24 ± 0.01 d | 0.20 ± 0.02 d | 0.04 ± 0.02 d |
| P4 | 14.46 ± 0.01 d | 0.26 ± 0.01 d | 0.05 ± 0.01 b |
| P5 | 10.77 ± 0.02 d | 0.19 ± 0.01 d | 0.03 ± 0.01 a,d |
| Phenolic Compound | Sample | ||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Phenolic Acids | |||||
| gallic acid | 0.015 | 0.087 | 0.157 | 0.075 | 2.310 |
| 3,4-dihydroxybenzoic acid | 0.254 | 0.401 | 0.454 | 0.209 | 0.549 |
| 4-hydroxybenzoic acid | 7.603 | 19.770 | 8.455 | 4.458 | 2.685 |
| chlorogenic acid | 0.733 | 2.441 | 6.481 | 46.939 | 0.275 |
| caffeic acid | 0.404 | 0.471 | 0.734 | 1.167 | 0.275 |
| syringic acid | 0.090 | ND | ND | ND | 0.325 |
| p-coumaric acid | 1.227 | 1.151 | 1.153 | 1.256 | 1.036 |
| ferulic acid | 2.978 | 2.894 | 0.961 | 1.271 | 1.199 |
| cinnamic acid # | 0.898 | 0.227 | 2.236 | 1.286 | 0.400 |
| Flavonoids | |||||
| epicatechin | 0.868 | 1.029 | 0.070 | 1.286 | 0.137 |
| catechin | 1.257 | 0.959 | 0.332 | 0.224 | 0.587 |
| rutin | 45.662 | 1.691 | 135.301 | 69.451 | ND |
| myricetin | 0.943 | 0.506 | 0.052 | 0.224 | 0.012 |
| quercetin | 3.577 | 1.639 | 3.214 | 7.883 | 5.981 |
| kaempferol | 2.155 | 1.308 | 7.337 | 8.676 | 26.472 |
| isorhamnetin | 3.502 | 0.558 | 12.141 | 5.086 | 40.220 |
| apigenin | 0.015 | 0.052 | 0.070 | 0.165 | 2.735 |
| pinocembrin | 0.644 | 2.197 | 0.559 | 1.227 | 0.412 |
| galangin | ND | 0.506 | 0.017 | 0.239 | 0.175 |
| chrysin | 0.464 | 1.970 | 0.384 | 0.853 | 0.387 |
| Compounds | RI a | RA b (%) | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||
| Isophorone | 1132 | ND | ND | ND | ND | 0.32 |
| Lilac aldehyde A | 1149 | 0.12 | 0.17 | ND | ND | ND |
| Lilac aldehyde B | 1159 | 0.27 | 0.20 | ND | ND | ND |
| Lilac aldehyde D | 1174 | 0.11 | 0.16 | ND | ND | ND |
| Caprylic acid | 1181 | ND | ND | 0.17 | ND | ND |
| Lilac alcohol B | 1222 | 0.15 | 0.45 | ND | ND | ND |
| Lilac alcohol D | 1237 | 0.09 | 0.19 | ND | ND | ND |
| 5-Hydroxymethylfurfural | 1245 | ND | ND | 0.44 | ND | ND |
| β-Ionone | 1499 | 0.25 | 0.42 | 0.15 | 0.07 | 0.53 |
| 2,4-Di-tert-butylphenol | 1521 | 0.36 | 0.45 | 0.57 | 0.12 | 0.75 |
| Dodecanoic acid | 1568 | ND | ND | 0.23 | ND | ND |
| Globulol | 1572 | ND | ND | 0.19 | ND | 0.36 |
| Methoxyeugenol | 1619 | ND | ND | ND | ND | 0.42 |
| Myristic acid | 1762 | ND | 0.48 | ND | 0.14 | 0.83 |
| Benzoic acid, phenylmethyl ester | 1787 | ND | ND | 0.24 | 0.11 | 0.00 |
| Myristic acid, isopropyl ester | 1824 | 0.19 | 0.38 | 0.22 | ND | 0.71 |
| Pentadecanoic acid ethyl ester | 1881 | 0.17 | 0.14 | 0.66 | ND | ND |
| 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 1940 | 0.88 | 1.34 | 0.64 | 0.42 | 2.34 |
| Palmitic acid | 1968 | 2.92 | 13.53 | 3.65 | 0.79 | 5.82 |
| Palmitic acid, ethyl ester | 1996 | 4.46 | 2.66 | 1.08 | 0.25 | 0.86 |
| 9,12,15-Octadecatrienoic acid, methyl ester | 2084 | ND | ND | 2.28 | ND | ND |
| Linoleic acid, methyl ester | 2090 | ND | ND | 2.98 | ND | ND |
| Linolenic acid, methyl ester | 2096 | 12.92 | 19.24 | 25.87 | 2.10 | 9.02 |
| Linolenic acid | 2107 | 14.52 | 24.03 | 43.23 | 6.08 | 43.42 |
| Linoleic acid | 2112 | 19.40 | 4.74 | 4.92 | 0.59 | 3.40 |
| Linolenic acid, methyl ester | 2119 | 14.27 | 3.82 | 9.84 | 1.01 | 6.12 |
| Stearic acid, methyl ester | 2126 | 27.51 | 12.58 | ND | 88.31 | 10.64 |
| Stearic acid, ethyl ester | 2222 | ND | 15.04 | ND | ND | 5.10 |
| Unidentified compounds | - | 1.41 | 0.00 | 2.36 | 0.00 | 9.34 |
| Total | - | 98.59 | 100.00 | 97.37 | 100.00 | 90.66 |
| Fatty acids and esters | - | 96.36 | 96.64 | 95.13 | 99.28 | 85.93 |
| Terpenes and terpenoides | - | 0.25 | 0.42 | 0.34 | 0.07 | 1.32 |
| Strain | GIZD (mm) | |||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | CEt | |
| Gram-Positive Bacteria | ||||||
| 1. Enterococcus faecalis ATCC 19433 | 14.00± 1.00 d | 13.50 ± 0.50 b | 11.50 ± 0.50 a | 14.40± 0.20 d | 11.50 ± 0.50 b | 7.00 ± 1.00 |
| 2. Staphylococcus aureus ATCC 25422 | 13.00 ± 0.50 b | 13.00 ± 1.00 a | 12.00 ± 0.23 | 13.00 ± 0.35 b | 14.00± 0.52 c | 9.70 ± 0.60 |
| Gram-Negative Bacteria | ||||||
| 1. Enterobacter cloacae | 15.00± 0.80 b | 14.30 ± 0.57 a | 13.30 ± 0.25 | 13.00 ± 0.06 | 14.00 ± 0.45 a | 12.00 ± 1.00 |
| 2. Escherichia coli ATCC 25923 | 14.50 ± 0.50 a | 16.00 ± 0.50b | 14.90 ± 0.90 a | 14.40 ± 0.20 a | 14.50 ± 0.50 a | 12.70 ± 0.60 |
| 3. Pseudomonas aeruginosa ATCC 25785 | 12.00 ± 0.50 a | 12.70± 0.30 a | 10.90 ± 0.15 | 10.80 ± 0.15 | 12.00± 0.25 a | 9.70 ± 0.60 |
| Yeasts | ||||||
| 1. Candida albicans ATTC 1688 | 15.10 ± 0.15 | 14.70 ± 0.07 | 15.10 ± 0.17 | 16.00± 0.06 b | 15.70± 0.05 a | 14.40 ± 0.15 |
| 2. Candida famata | 17.60± 0.70 a | 17.00± 0.40 a | 17.40± 0.40 a | 16.90 ± 0.15 | 16.20 ± 0.50 | 16.50 ± 0.50 |
| 3. Candida glabrata | 15.50± 0.50 a | 15.00± 0.30 a | 14.50 ± 0.25 | 14.50 ± 0.25 | 14.40 ± 0.17 | 14.00 ± 1.00 |
| 4. Candida guillermondii | 18.10± 0.17 c | 17.50 ± 0.05 b | 17.10 ± 0.15 a | 16.90 ± 0.11 b | 18.50± 0.10 c | 16.00 ± 0.05 |
| 5. Candida krusei | 16.20 ± 0.25 a | 16.40 ± 0.10 a | 16.90 ± 0.15 a | 18.10± 0.10 b | 16.00 ± 0.25 a | 12.50 ± 0.50 |
| 6. Candida lusitaniae | 17.80± 0.28 a | 16.50 ± 0.10 a | 16.50 ± 0.25 a | 15.50 ± 0.25 | 16.10 ± 0.10 a | 14.50 ± 0.50 |
| Strain | MIC | ||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Gram-Positive Bacteria | |||||
| 1. Enterococcus faecalis ATCC 19433 | 1250 | 2150 | 4290 | 2510 | 3000 |
| 2. Staphylococcus aureus ATCC 25422 | 1250 | 1080 | 2150 | 630 | 380 |
| Gram-Negative Bacteria | |||||
| 1. Enterobacter cloacae | 2510 | 2150 | 540 | 2510 | 750 |
| 2. Escherichia coli ATCC 25923 | 1250 | 540 | 540 | 1250 | 1500 |
| 3. Pseudomonas aeruginosa ATCC 25853 | 630 | 540 | 270 | 1250 | 1500 |
| Yeasts | |||||
| 1. Candida albicans ATTC 1688 | 5010 | 2150 | 4290 | 630 | 750 |
| 2. Candida famata | 1250 | 2150 | 1070 | 2510 | 3000 |
| 3. Candida glabrata | 630 | 540 | 1070 | 1250 | 3000 |
| 4. Candida guillermondii | 630 | 2150 | 2150 | 1250 | 750 |
| 5. Candida krusei | 2510 | 1080 | 270 | 1250 | 3000 |
| 6. Candida lusitaniae | 1250 | 2150 | 270 | 2510 | 3000 |
| Strain | MCBE | ||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |
| Gram-Positive Bacteria | |||||
| 1. Enterococcus faecalis ATCC 19433 | 1250 | 2.150 | 2150 | 2510 | 3000 |
| 2. Staphylococcus aureus ATCC 25422 | 1250 | 1080 | 2150 | 1250 | 750 |
| Gram-Negative Bacteria | |||||
| 1. Enterobacter cloacae | 1250 | 1080 | 1070 | 2510 | 1500 |
| 2. Escherichia coli ATCC 25923 | 2510 | 270 | 540 | 1250 | 1500 |
| 3. Pseudomonas aeruginosa ATCC 25853 | 630 | 540 | 270 | 1250 | 1500 |
| Yeasts | |||||
| 1. Candida albicans ATTC 1688 | 2510 | 2150 | 2150 | 630 | 750 |
| 2. Candida famata | 1250 | 2150 | 1070 | 2510 | 3000 |
| 3. Candida glabrata | 630 | 540 | 1070 | 1250 | 3000 |
| 4. Candida guillermondii | 630 | 2150 | 2150 | 1250 | 750 |
| 5. Candida krusei | 2510 | 1080 | 270 | 1250 | 1500 |
| 6. Candida lusitaniae | 630 | 2150 | 1070 | 2510 | 3000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilie, C.-I.; Oprea, E.; Geana, E.-I.; Spoiala, A.; Buleandra, M.; Gradisteanu Pircalabioru, G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants 2022, 11, 959. https://doi.org/10.3390/antiox11050959
Ilie C-I, Oprea E, Geana E-I, Spoiala A, Buleandra M, Gradisteanu Pircalabioru G, Badea IA, Ficai D, Andronescu E, Ficai A, et al. Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants. 2022; 11(5):959. https://doi.org/10.3390/antiox11050959
Chicago/Turabian StyleIlie, Cornelia-Ioana, Eliza Oprea, Elisabeta-Irina Geana, Angela Spoiala, Mihaela Buleandra, Gratiela Gradisteanu Pircalabioru, Irinel Adriana Badea, Denisa Ficai, Ecaterina Andronescu, Anton Ficai, and et al. 2022. "Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria" Antioxidants 11, no. 5: 959. https://doi.org/10.3390/antiox11050959
APA StyleIlie, C.-I., Oprea, E., Geana, E.-I., Spoiala, A., Buleandra, M., Gradisteanu Pircalabioru, G., Badea, I. A., Ficai, D., Andronescu, E., Ficai, A., & Ditu, L.-M. (2022). Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants, 11(5), 959. https://doi.org/10.3390/antiox11050959














