The Role and Mechanism of Essential Selenoproteins for Homeostasis
Abstract
:1. Introduction
2. Synthesis and Metabolism of Selenoproteins
2.1. Diet Sources and Metabolism of Se in the Body
2.2. Se-Synthesis of Selenoproteins
3. Selenoproteins Regulate Related Diseases
3.1. Selenoproteins and Cardiovascular Diseases
3.2. Selenoproteins and Liver Disease
3.3. Selenoproteins and Brain Diseases
3.4. Selenoproteins and Intestinal Diseases
3.5. Selenoproteins and Cancer
3.6. Selenoproteins and Reproduction
4. Mechanisms That Show That Selenoproteins Regulate Disease
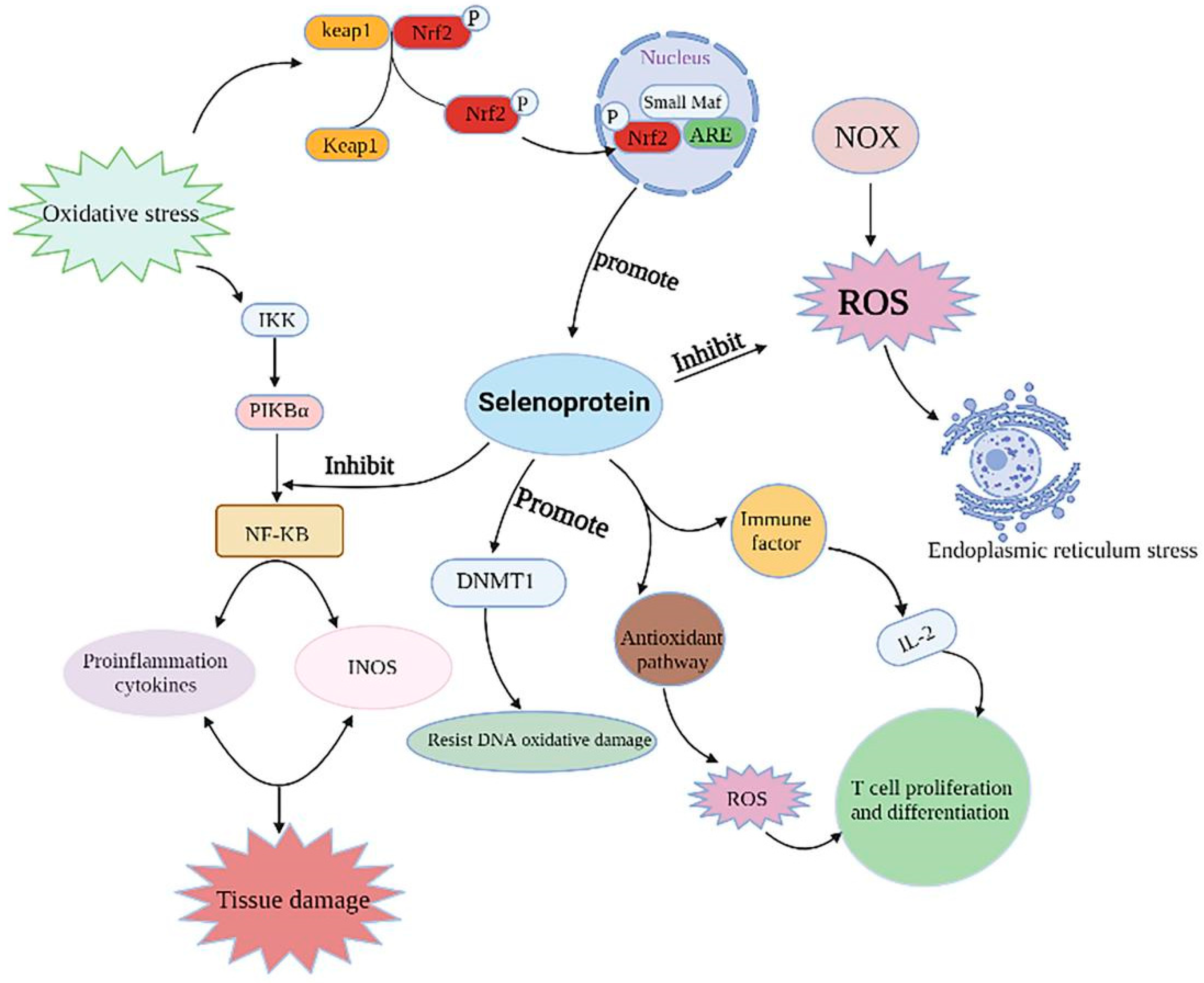
4.1. Selenoproteins Regulate Immune Responses
4.2. Selenoproteins Reduce Inflammation
4.3. Selenoproteins Inhibit ER Stress
5. Future Development Trend of Se and Selenoproteins
5.1. Se-Enriched Food Industry Develops Vigorously
5.2. Nano-Se Has the Potential to Be a High-Quality Se Supplement
6. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M. Selenium(-)fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Dinh, Q.T.; Cui, Z.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Tan, W. Expression profile analysis of selenium-related genes in peripheral blood mononuclear cells of patients with Keshan disease. Biomed Res. Int. 2019, 2019, 4352905. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2020, 50, 366–372. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Guo, X. Serious selenium deficiency in the serum of patients with kashin-beck disease and the effect of nano-selenium on their chondrocytes. Biol. Trace Elem. Res. 2020, 194, 96–104. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 8, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, R.; Farooq, M.U.; Zhu, J. Dissecting the potential of selenoproteins extracted from selenium-enriched rice on physiological, biochemical and anti-ageing effects in vivo. Biol. Trace Elem. Res. 2020, 196, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bao, Y.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. Selenoprotein synthesis: A unique translational mechanism used by a diverse family of proteins. Thyroid 2005, 15, 769–775. [Google Scholar] [CrossRef]
- Bulteau, A.L.; Chavatte, L. Update on selenoprotein biosynthesis. Antioxid. Redox Signal. 2015, 23, 775–794. [Google Scholar] [CrossRef]
- Santesmasses, D.; Mariotti, M.; Gladyshev, V.N. Bioinformatics of selenoproteins. Antioxid. Redox Signal. 2020, 33, 525–536. [Google Scholar] [CrossRef]
- Sunde, R.A.; Raines, A.M. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv. Nutr. 2011, 2, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, U.; Dehina, N.; Schomburg, L. Disorders of selenium metabolism and selenoprotein function. Curr. Opin. Pediatr. 2011, 23, 429–435. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Schoenmakers, E.; Chatterjee, K. Human disorders affecting the selenocysteine incorporation pathway cause systemic selenoprotein deficiency. Antioxid. Redox Signal. 2020, 33, 481–497. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Watanabe, M.; Das, D.K. Transgenic mice overexpressing glutathione peroxidase are resistant to myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 1996, 28, 1759–1767. [Google Scholar] [CrossRef]
- Rose, A.H.; Hoffmann, P.R. Selenoproteins and cardiovascular stress. Thromb. Haemost. 2015, 113, 494–504. [Google Scholar] [CrossRef]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The impact of selenium deficiency on cardiovascular function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yang, G.; Sadoshima, J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Investg. 2003, 112, 1395–1406. [Google Scholar] [CrossRef]
- Yang, J.; Hamid, S.; Zhang, Z. Gene expression of selenoproteins can be regulated by thioredoxin(Txn) silence in chicken cardiomyocytes. J. Inorg. Biochem. 2017, 177, 118–126. [Google Scholar] [CrossRef]
- Rocca, C.; Boukhzar, L.; Angelone, T. A selenoprotein T-derived peptide protects the heart against ischaemia/reperfusion injury through inhibition of apoptosis and oxidative stress. Acta. Physiol. 2018, 223, e13067. [Google Scholar] [CrossRef]
- Shalihat, A.; Hasanah, A.N.; Gozali, D. The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: A literature review. Biomed. Pharmacother. 2021, 134, 111125. [Google Scholar] [CrossRef]
- Rees, K.; Hartley, L.; Stranges, S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013, CD009671. [Google Scholar] [CrossRef] [Green Version]
- Benstoem, C.; Goetzenich, A.; Stoppe, C. Selenium and its supplementation in cardiovascular disease—What do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [Green Version]
- Caviglia, G.P.; Rosso, C.; Bugianesi, E. Interplay between oxidative stress and metabolic derangements in non-alcoholic fatty liver disease: The role of Selenoprotein P. Int. J. Mol. Sci. 2020, 21, 8838. [Google Scholar] [CrossRef]
- Day, K.; Seale, L.A.; Cardoso, B.R. Selenotranscriptome network in non-alcoholic fatty liver DISEASE. Front. Nutr. 2021, 8, 744825. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Z.; Shan, A. The effects of endoplasmic-reticulum-resident selenoproteins in a nonalcoholic fatty liver disease pig model induced by a high-fat diet. Nutrients 2020, 12, 692. [Google Scholar] [CrossRef] [Green Version]
- Speckmann, B.; Schulz, S.; Kipp, A.P. Selenium increases hepatic DNA methylation and modulates one-carbon metabolism in the liver of mice. J. Nutr. Biochem. 2017, 48, 112–119. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Seliger, B. Individual effects of different selenocompounds on the hepatic proteome and energy metabolism of mice. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 3323–3334. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Li, S.; Zhang, J. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef]
- Wu, B.K.; Chen, Q.H.; Sang, L.X. A novel therapeutic strategy for hepatocellular carcinoma: Immunomodulatory mechanisms of selenium and/or selenoproteins on a shift towards anti-cancer. Int. Immunopharmacol. 2021, 96, 107790. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Roberts, B.R.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef] [Green Version]
- Pitts, M.W.; Hoffmann, P.R.; Schomburg, L. Editorial: Selenium and selenoproteins in brain development, function, and disease. Front. Neurosci. 2022, 15, 821140. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rocourt, C.; Cheng, W.H. Selenoproteins and the aging brain. Mech. Ageing Dev. 2010, 131, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Song, G.L. Roles of selenoproteins in brain function and the potential mechanism of selenium in alzheimer’s disease. Front. Neurosci. 2021, 15, 646518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, R.P.; Zhu, J.H. Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech. Ageing Dev. 2019, 180, 89–96. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and the brain: A review. Nutr. Neurosci. 2001, 4, 81–97. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Barrett, C.W.; Short, S.P.; Williams, C.S. Selenoproteins and oxidative stress-induced inflammatory tumorigenesis in the gut. Cell Mol. Life Sci. 2017, 74, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Short, S.P.; Pilat, J.M.; Williams, C.S. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic. Biol. Med. 2018, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.A.; Prabhu, K.S. The essential role of selenoproteins in the resolution of Citrobacter rodentium-induced intestinal inflammation. Front. Nutr. 2020, 7, 96. [Google Scholar]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Yin, Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid. Med. Cell Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, E.R.; Kim, B.W.; Chu, F.F. The Gdac1 locus modifies spontaneous and Salmonella-induced colitis in mice deficient in either Gpx2 or Gpx1 gene. Free Radic. Biol. Med. 2013, 65, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, P.A.; Carlson, B.A.; Davis, C.D. Knockout of the 15 kDa selenoprotein protects against chemically-induced aberrant crypt formation in mice. PLoS ONE 2012, 7, e50574. [Google Scholar] [CrossRef]
- Nettleford, S.K.; Zhao, L.; Tsuji, P.A. Selenium and the 15kDa selenoprotein impact colorectal tumorigenesis by modulating intestinal barrier integrity. Int. J. Mol. Sci. 2021, 22, 10651. [Google Scholar]
- Short, S.P.; Pilat, J.M.; Williams, C.S. Colonic epithelial-derived selenoprotein P is the source for antioxidant-mediated protection in colitis-associated cancer. Gastroenterology 2021, 160, 1694–1708. [Google Scholar] [CrossRef]
- Huang, L.J.; Mao, X.T.; Cao, Q. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 54, 1728–1744. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Naranjo-Suarez, S.; Davis, C.D. Deficiency in the 15 kDa selenoprotein inhibits human colon cancer cell growth. Nutrients 2011, 3, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Murawaki, Y.; Tsuchiya, H.; Shiota, G. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008, 259, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, E. Selenoproteins in bladder cancer. Clin. Chim. Acta. 2012, 413, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Wei, R.; Qiu, H.; Gan, Z. Expression and prognostic potential of GPX1 in human cancers based on data mining. Ann. Transl. Med. 2020, 8, 124. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Hempel, N. Extracellular glutathione peroxidase GPx3 and its role in cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Ong, T.P. Selenium and breast cancer risk: Focus on cellular and molecular mechanisms. Adv. Cancer Res. 2017, 136, 173–192. [Google Scholar]
- Diamond, A.M. Selenoproteins of the human prostate: Unusual properties and role in cancer etiology. Biol. Trace Elem. Res. 2019, 192, 51–59. [Google Scholar] [CrossRef]
- Li, M.; Cheng, W.; Li, H. Selenoprotein K mediates the proliferation, migration, and invasion of human choriocarcinoma cells by negatively regulating human chorionic gonadotropin expression via ERK, p38 MAPK, and Akt signaling pathway. Biol. Trace Elem. Res. 2018, 184, 47–59. [Google Scholar] [CrossRef]
- Marciel, M.P.; Hoffmann, P.R. Molecular mechanisms by which selenoprotein K regulates immunity and cancer. Biol. Trace Elem. Res. 2019, 192, 60–68. [Google Scholar] [CrossRef]
- Ben, S.B.; Peng, B.; Chen, C.L. Overexpression of selenoprotein SelK in BGC-823 cells inhibits cell adhesion and migration. Biochemistry 2015, 80, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Gralla, O.; Schomburg, L. Selenoprotein P in seminal fluid is a novel biomarker of sperm quality. Biochem. Biophys. Res. Commun. 2014, 443, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Mojadadi, A.; Au, A.; Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L. Selenium in mammalian spermiogenesis. Biol. Chem. 2007, 388, 987–995. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Zhou, G. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Qazi, I.H.; Angel, C.; Zhou, G.B. Selenium, selenoproteins, and female reproduction: A review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.R.; Neves, C.; Soares, P. Selenium and selenoproteins in immune mediated thyroid disorders. Diagnostics 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Li, C.W.; Tomer, Y. Immunogenetics of autoimmune thyroid diseases: A comprehensive review. J. Autoimmun. 2015, 64, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.C.; Lee, S.G.; Gladyshev, V.N. Selenoprotein MsrB1 promotes anti-inflammatory cytokine gene expression in macrophages and controls immune response in vivo. Sci. Rep. 2017, 7, 5119. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Ding, D.; Mou, D.; Feng, B. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct. 2021, 12, 11214–11228. [Google Scholar] [CrossRef]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: Selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, C.; Li, S. Selenium deficiency mainly influences antioxidant selenoproteins expression in broiler immune organs. Biol. Trace Elem. Res. 2016, 172, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Krehl, S.; Loewinger, M.; Brigelius-Flohé, R. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis 2012, 33, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Zhang, Q.; Li, S. Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis. Redox Biol. 2021, 44, 102003. [Google Scholar] [CrossRef]
- Shchedrina, V.A.; Zhang, Y.; Gladyshev, V.N. Structure-function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid. Redox Signal. 2010, 12, 839–849. [Google Scholar] [CrossRef]
- Varlamova, E.G. Participation of selenoproteins localized in the ER in the processes occurring in this organelle and in the regulation of carcinogenesis-associated processes. J. Trace Elem. Med. Biol. 2018, 48, 172–180. [Google Scholar] [CrossRef]
- Addinsall, A.B.; Wright, C.R.; Stupka, N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem. J. 2018, 475, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Hoffmann, P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018, 70, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S. Effects of selenium application on plant growth and some quality parameters in peanut (Arachis hypogaea). Pak. J. Biol. Sci. 2017, 20, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Soltani, F.; Baldermann, S. Selenium fortification alters the growth, antioxidant characteristics and secondary metabolite profiles of cauliflower (Brassica oleracea var. botrytis) cultivars in hydroponic culture. Plants 2021, 10, 1537. [Google Scholar]
- Jia, L.; Wang, T.; Cui, J. Protective effect of selenium-enriched red radish sprouts on carbon tetrachloride-induced liver injury in mice. J. Food Sci. 2019, 84, 3027–3036. [Google Scholar] [CrossRef]
- Smits, J.E.; Krohn, R.M.; Raqib, R. Food as medicine: Selenium enriched lentils offer relief against chronic arsenic poisoning in Bangladesh. Environ. Res. 2019, 176, 108561. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, H.; Huang, B. Effect of selenium-enriched kiwifruit on body fat reduction and liver protection in hyperlipidaemic mice. Food Funct. 2021, 12, 2044–2057. [Google Scholar] [CrossRef]
- Fisinin, V.I.; Papazyan, T.T.; Surai, P.F. Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit. Rev. Biotechnol. 2009, 29, 18–28. [Google Scholar] [CrossRef]
- Lu, J.; Qu, L.; Wang, K.H. Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult. Sci. 2018, 97, 3102–3108. [Google Scholar] [CrossRef]
- Lu, J.; Qu, L.; Wang, K.H. Efficacy evaluation of selenium-enriched yeast in laying hens: Effects on performance, egg quality, organ development, and selenium deposition. Poult. Sci. 2020, 99, 6267–6277. [Google Scholar] [CrossRef]
- Hou, L.; Qiu, H.; Qin, S. Selenium-enriched saccharomyces cerevisiae improves the meat quality of broiler chickens via activation of the glutathione and thioredoxin systems. Poult. Sci. 2020, 99, 6045–6054. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dal, B.A.; Proietti, P. Use of Selenium-enriched olive leaves in the feed of growing rabbits: Effect on oxidative status, mineral profile and selenium speciation of longissimus dorsi meat. J. Trace Elem. Med. Biol. 2019, 51, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mrázová, J.; Gažarová, M.; Bobček, B. The effect of consumption of pork enriched by organic selenium on selenium status and lipid profile in blood serum of consumers. J. Environ. Sci. Health B 2020, 55, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Dinev, I.; Stanley, D. Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ. Sci Pollut. Res. Int. 2020, 27, 16159–16166. [Google Scholar] [CrossRef]
- Hu, S.; Hu, W.; Wang, J. Construction and structure-activity mechanism of polysaccharide nano-selenium carrier. Carbohydr. Polym. 2020, 236, 116052. [Google Scholar] [CrossRef]
- Ullah, A.; Yin, X.; Naveed, M. Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef]
- Sun, J.; Wei, C.; Liu, J. Progressive release of mesoporous nano-selenium delivery system for the multi-channel synergistic treatment of Alzheimer’s disease. Biomaterials 2019, 197, 417–431. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. The Role and Mechanism of Essential Selenoproteins for Homeostasis. Antioxidants 2022, 11, 973. https://doi.org/10.3390/antiox11050973
Ye R, Huang J, Wang Z, Chen Y, Dong Y. The Role and Mechanism of Essential Selenoproteins for Homeostasis. Antioxidants. 2022; 11(5):973. https://doi.org/10.3390/antiox11050973
Chicago/Turabian StyleYe, Ruihua, Jiaqiang Huang, Zixu Wang, Yaoxing Chen, and Yulan Dong. 2022. "The Role and Mechanism of Essential Selenoproteins for Homeostasis" Antioxidants 11, no. 5: 973. https://doi.org/10.3390/antiox11050973





