Phytochemical Screening, Antioxidant and Antifungal Activities of Aconitum chasmanthum Stapf ex Holmes Wild Rhizome Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Chemicals
2.3. Cold Extraction of Wild Rhizomes of A. chasmanthum
2.4. Qualitative Phytochemical Analysis
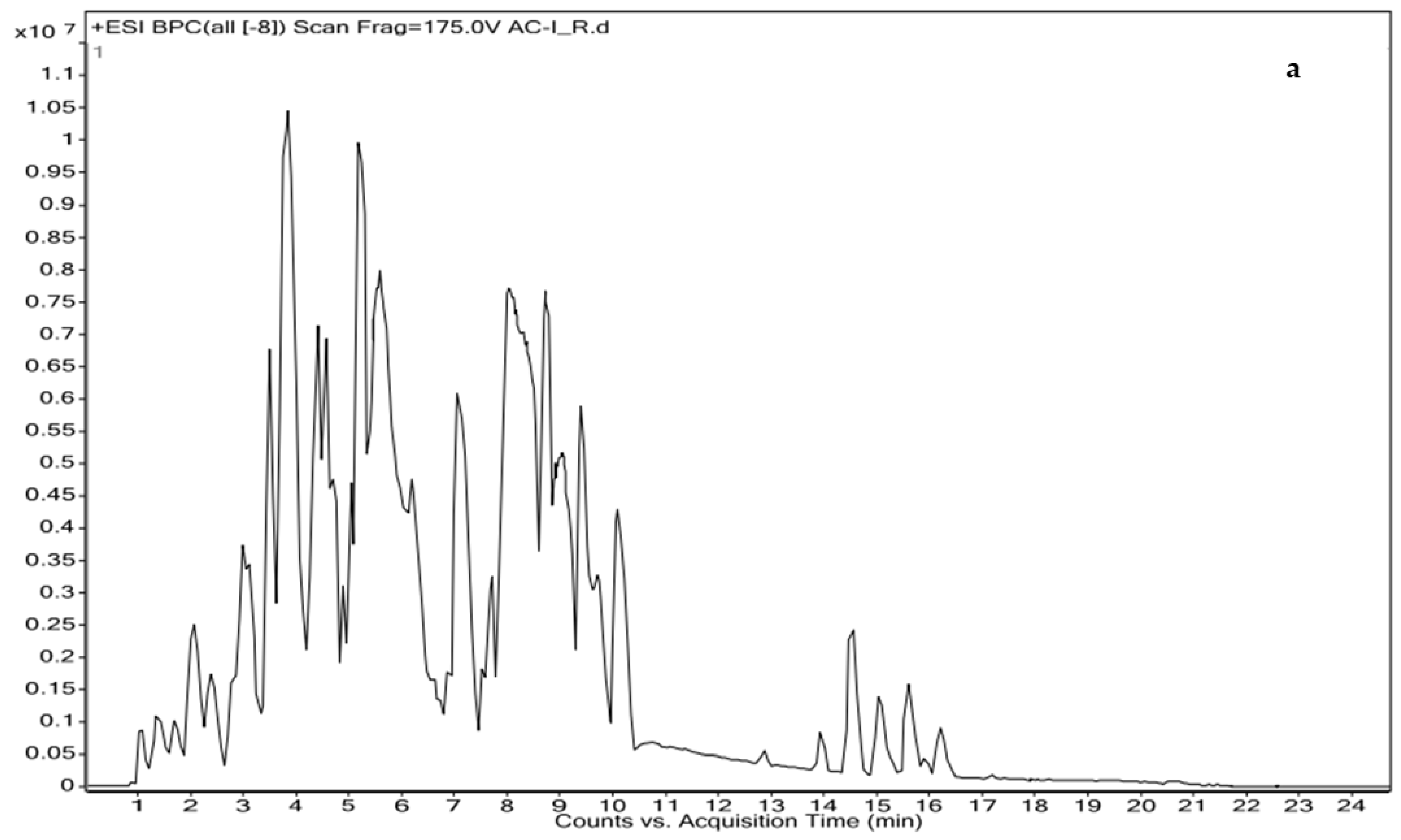
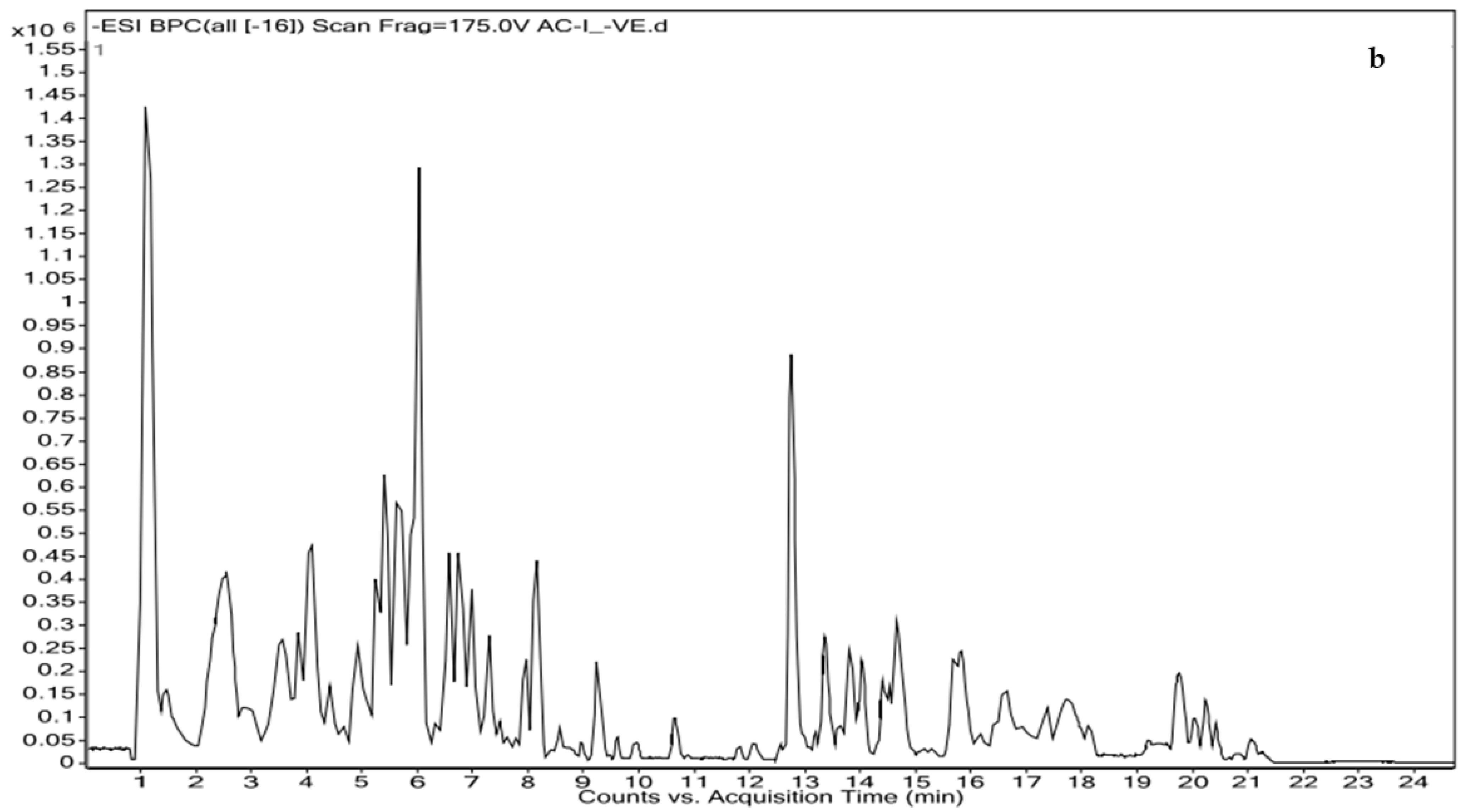
2.5. Identification of Bioactive Molecules by Liquid Chromatography Coupled with High-Resolution Mass Spectrometry
2.6. Total Phenolic Content (TPC)
2.7. Total Flavonoid Content (TFC)
2.8. Biological Activity
2.8.1. Antioxidant Assays
DPPH Radical Scavenging Assay
Ferric-Reducing Antioxidant Power (FRAP)
Superoxide Anion Radical Scavenging Activity (SOR)
Hydroxyl Radical Scavenging (OH−)
2.8.2. Antifungal Activity
Test Microorganisms
Poisoned Food Method
MIC (Minimum Inhibitory Concentrations) of the Plant Extracts
2.9. Statistical Analysis
3. Results
3.1. Qualitative Phytochemical Analysis
3.2. Phytochemical Composition
3.3. Total Phenolic Content (TPC)
3.4. Total Flavonoid Content (TFC)
3.5. Antioxidant Assays
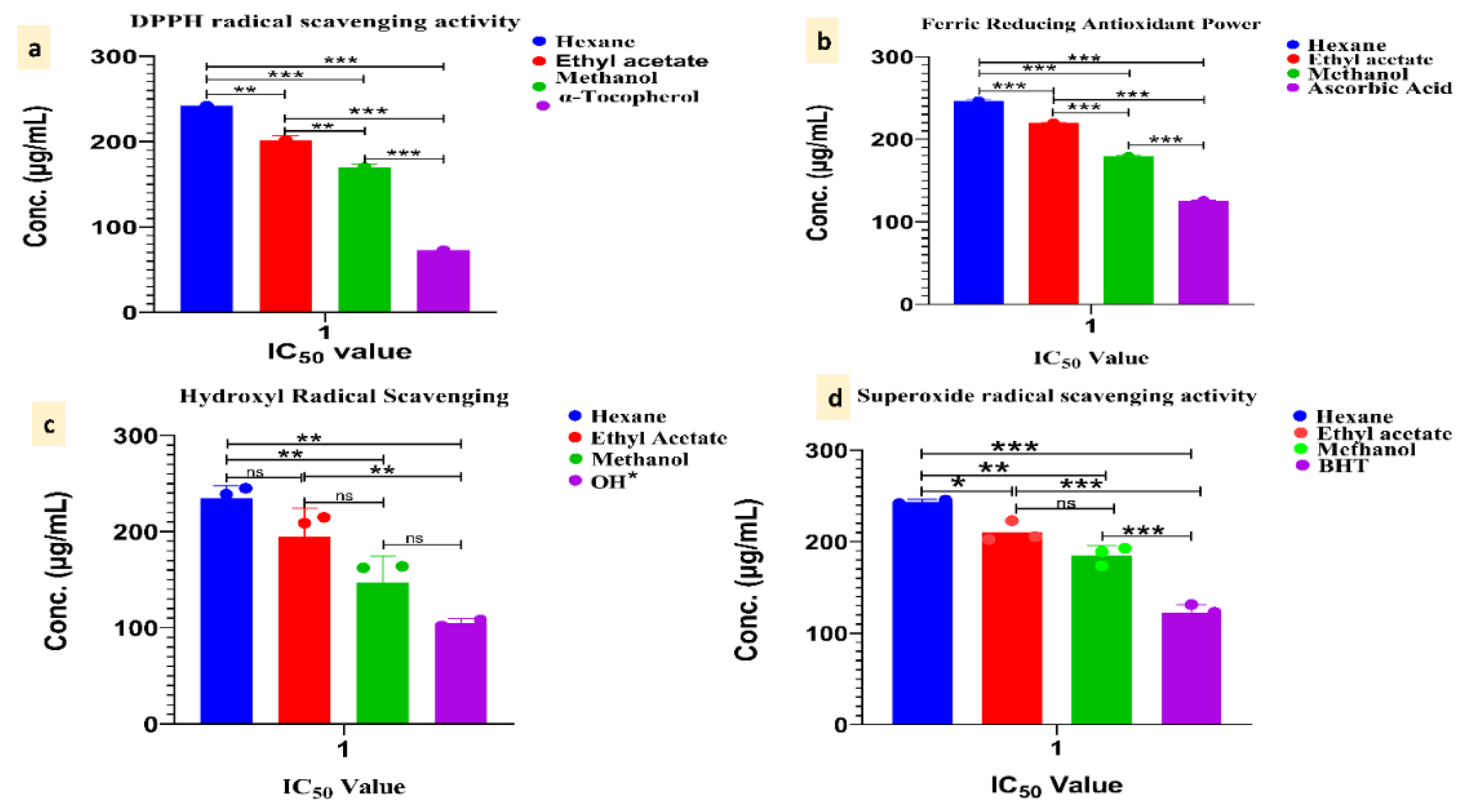
3.5.1. DPPH Radical Scavenging Activity
3.5.2. Ferric-Reducing Antioxidant Power (FRAP)
3.5.3. Superoxide Anion Radical Scavenging Activity (SOR)
3.5.4. Hydroxyl Radical Scavenging (OH−) Activity
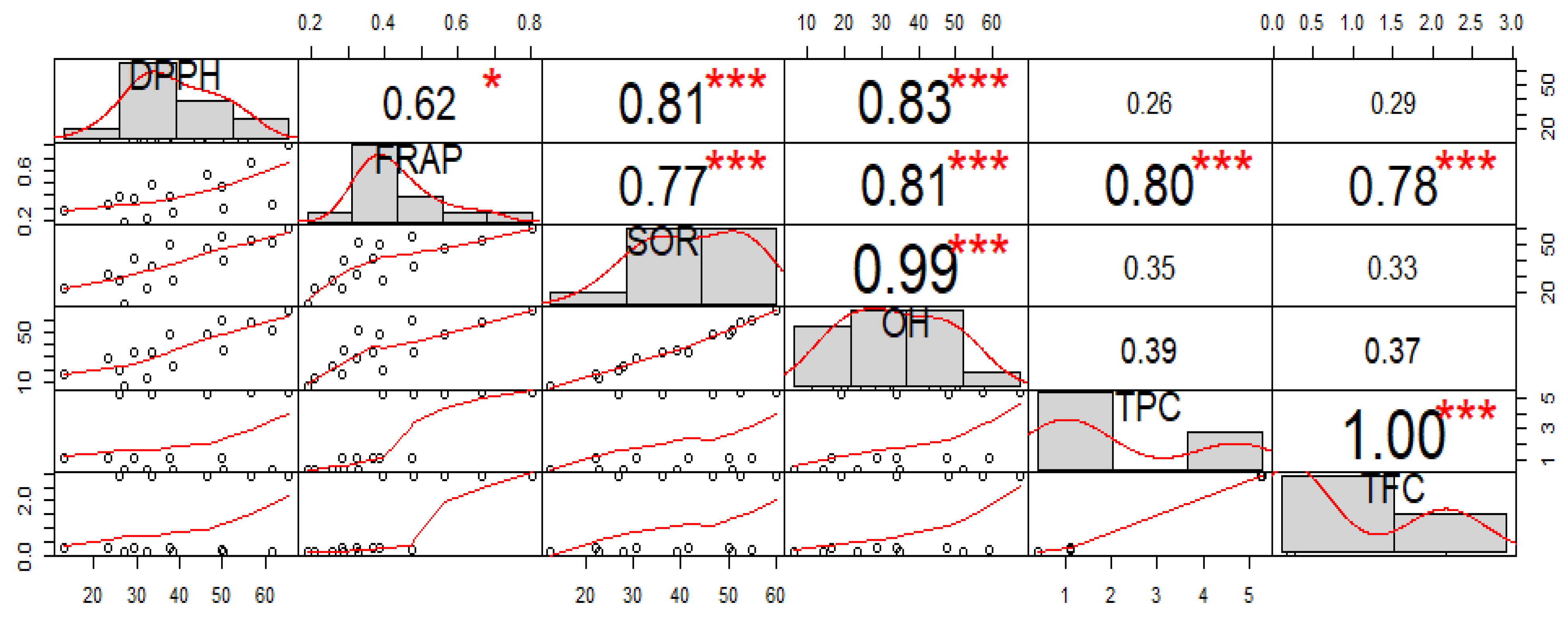
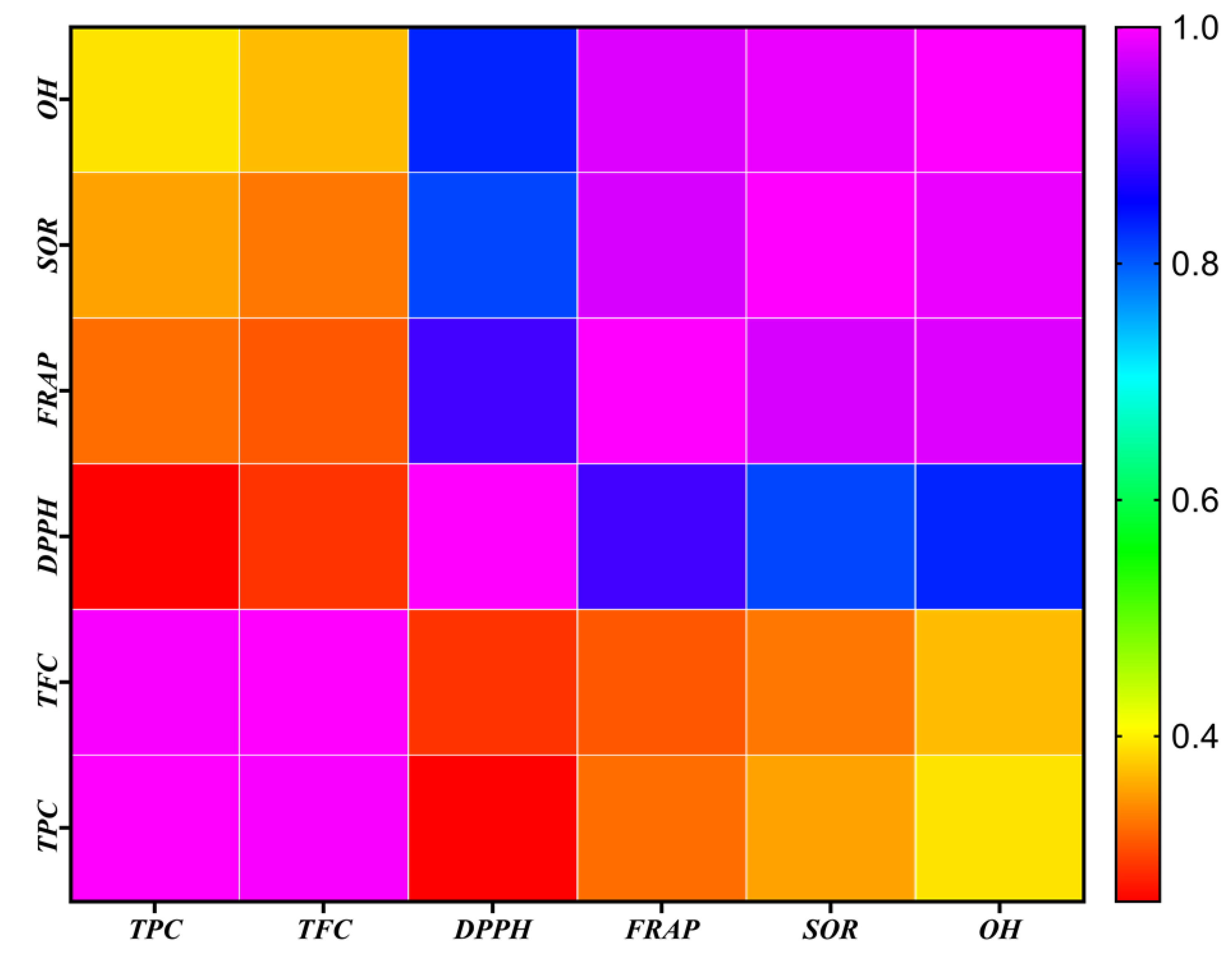
3.5.5. Relationship between Different Solvent Systems in Total Phenolic, Flavonoid and Antioxidant Activity
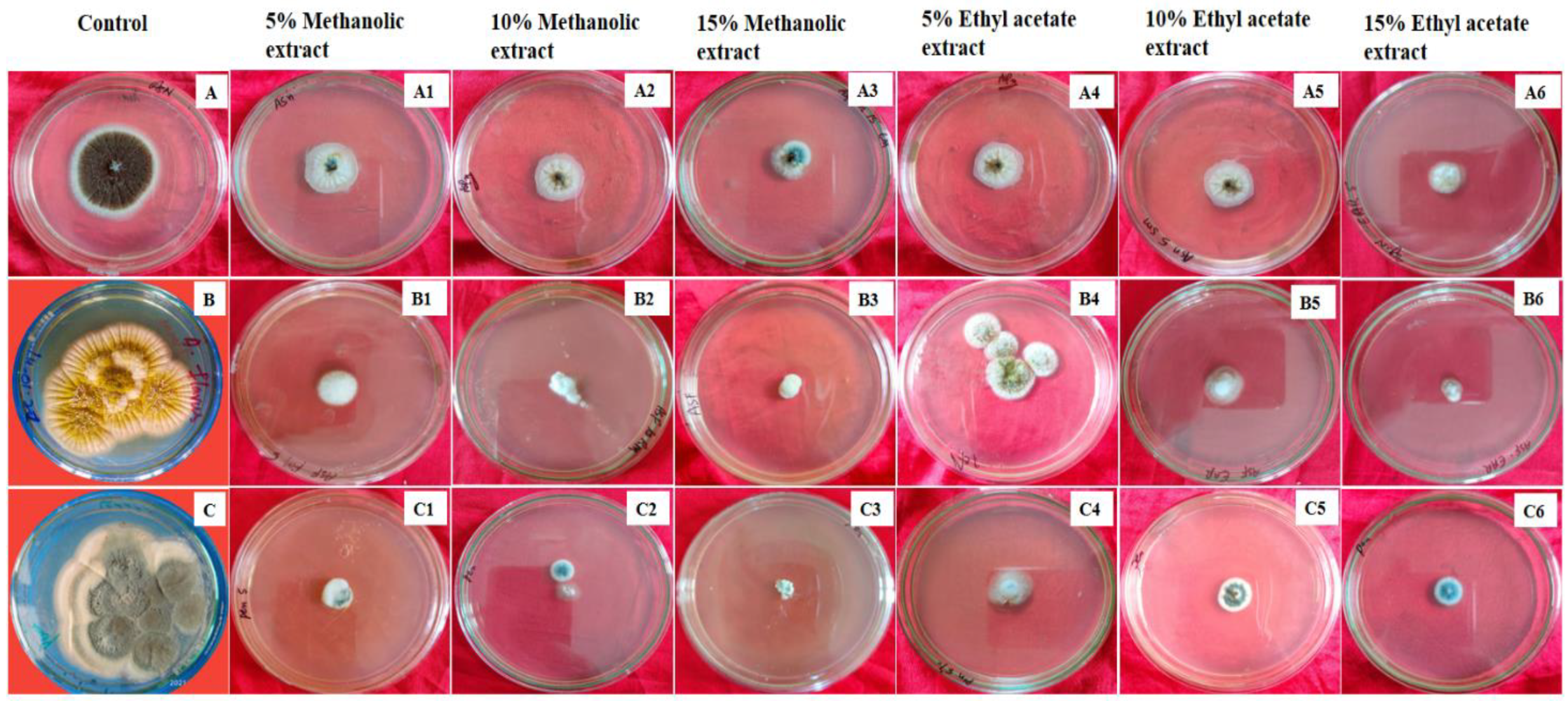
3.6. Antifungal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, B.; Shukla, V.J. Indian systems of medicine: A brief profile. Afr. J. Tradit. Complementary Altern. Med. 2007, 4, 319–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tag, H.; Kalita, P.; Dwivedi, P.; Das, A.K.; Namsa, N.D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. J. Ethnopharmacol. 2012, 141, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Wachtel-Galor, S. (Eds.) Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- George, M.; Joseph, L.; Ramaswamy. Anti-allergic, anti-pruritic, and anti-inflammatory activities of Centella asiatica extracts. Afr. J. Tradit. Complementary Altern. Med. 2009, 6, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.M.; Hanna, A.N.; Kauffman, E.M.; Newman, H.A. Inhibition of humanlow-density lipoprotein oxidation in vitro by Maharishi Ayur-Veda herbal mixtures. Pharmacol. Biochem. Behav. 1992, 43, 1175–1182. [Google Scholar] [CrossRef]
- Saranya, B.; Sulfikarali, T.; Chindhu, S.; Muneeb, A.M.; Leela, N.K.; Zachariah, T.J. Turmeric and cinnamon dominate in antioxidant potential among four major spices. J. Spices Aromat. Crops 2017, 26, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health and Family Planning, Government of India. The Ayurvedic Pharmacopoeia of India—Part I, 1st ed.; Ministry of Health and Family Planning, Government of India: New Delhi, India, 2000; pp. 180–182.
- Anwar, S.; Ahmad, B.; Subhan, M.; Gul, W.; Nazar-ul-Islam. Biological and pharmacological properties of Aconitum chasmanthum. J. Biol. Sci. 2003, 3, 989–993. [Google Scholar]
- Shyaula, S.L.; Phytochemicals, T.U. Processing of Aconitum Species in Nepal. Nepal J. Sci. Technol. 2011, 12, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Mir, A.H.; Tyub, S.; Kamili, A.N. Ecology, distribution mapping and conservation implications of four critically endangeredendemic plants of Kashmir Himalaya. Saudi J. Biol. Sci. 2020, 27, 2380–2389. [Google Scholar] [CrossRef]
- Shrikumar, S.; Ravi, T.K. Approaches towards development and promotion of herbal drugs. Pharmacog. Rev. 2007, 1, 180–184. [Google Scholar]
- Gujree, G.M.; Nawchoo, I.A.; Wafai, B.A. Meiotic System and Pollination Mechanisms of the Critically Endangered Aconitum chasmanthum Stapf ex Holmes—A Novel Species Endemic to Kashmir Himalayan Region. Int. J. Plant Rep. Biol. 2009, 1, 153–162. [Google Scholar]
- Michailides, T.; Thomidis, T. First report of Aspergillus flavus causing fruit rots of peaches in greece. Plant Pathol. 2007, 56, 352. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [Green Version]
- Marr, K.A.; Carter, R.A.; Boeckh, M.; Martin, P.; Corey, L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood 2002, 100, 4358–4366. [Google Scholar] [CrossRef] [Green Version]
- Raper, K.B.; Fennell, D.I. Aspergillus fumigatus group. In The Genus Aspergillus; Raper, K.B., Fennell, D.I., Eds.; The William & Wilkins Co.: Baltimore, MD, USA, 1965; pp. 238–268. [Google Scholar]
- Person, A.K.; Chudgar, S.M.; Norton, B.L.; Tong, B.C.; Stout, J.E. Aspergillus niger: An unusual cause of invasive pulmonary aspergillosis. J. Med. Microbiol. 2010, 59 Pt 7, 834–838. [Google Scholar] [CrossRef] [Green Version]
- Araiza, J.; Canseco, P.; Bonifaz, A. Otomycosis: Clinical and mycological study of 97 cases. Rev. Laryngol.-Otol.-Rhinol. 2006, 127, 251–254. [Google Scholar]
- Loudon, K.W.; Coke, A.P.; Burnie, J.P.; Shaw, A.J.; Oppenheim, B.A.; Morris, C.Q. Kitchens as a source of Aspergillus niger infection. J. Hosp. Infect. 1996, 32, 191–198. [Google Scholar] [CrossRef]
- Oliveri, S.; Capello, G.; Napolitano, M.G.; Triolo, C.; Grillo, C. Otomycosis: Etiology and analysis of predisposing factors. Boll. Ist. Sieroter. Milan. 1984, 63, 537–542. [Google Scholar] [PubMed]
- Tisner, J.; Millan, J.; Rivas, P.; Adiego, I.; Castellote, A.; Valles, H. Otomycosis and topical application of thimerosal: Study of 152 cases. Acta Otorrinolaringol. 1995, 46, 85–89. [Google Scholar]
- Rajasingham, R.; Rachel, M.S.; Benjamin, J.P.; Joseph, N.J.; Nelesh, P.G.; Tom, M.C.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W. The ambitious “95-95 by 2025” roadmap for the diagnosis and management of fungal diseases. Thorax 2015, 70, 613–614. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.K. Forms of Aspergillosis. In The Genus Aspergillus; Powell, K.A., Renwick, A., Peberdy, J.F., Eds.; Federation of European Microbiological Societies Symposium Series; Springer: Boston, MA, USA, 1994; Volume 69, pp. 313–319. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3, 4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Gende, L.B.; Floris, I.; Fritz, R.; Eguaras, M.J. Antimicrobial activity of cinnamon (Cinnamomum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentine. Bull. Insectol. 2008, 61, 1. [Google Scholar]
- Abramson, D.; Lombaert, G.; Clear, R.M.; Sholberg, P.; Trelka, R.; Rosin, E. Production of patulin and citrinin by Penicillium expansum from British Columbia (Canada) apples. Mycotoxin Res. 2009, 25, 85–88. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Abarca, M.L.; Cabanes, F.J. Low occurrence of patulin-and citrinin-producing species isolated from grapes. Lett. Appl. Microbiol. 2008, 47, 286–289. [Google Scholar] [CrossRef]
- Stevic, T.; Beric, T.; Šavikin, K.; Sokovic, M.; Godevac, D.; Dimkic, I.; Stankovic, S. Antifungal activity of selected essential oils againstfungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Abdel-Kader, M.; El-Mougy, N.; Lashin, S. Essential oils and Trichoderma harzianum as an integrated control measure against faba bean root pathogens. J. Plant Prot. Res. 2011, 51, 306–313. [Google Scholar] [CrossRef]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Pharmacognosy; Nirali Prakashan: Pune, India, 2005. [Google Scholar]
- Harborne, A.J. Phytochemical Methods A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Springer: Berlin/Heidelberg, Germany; Amsterdam, The Netherlands, 1998; ISBN 978-0-412-57260-9. [Google Scholar]
- Sadashivan, S.; Manickam, A. Biochemical Methods, 2nd ed.; New Age International (P) Ltd. Publisher: New Delhi, India, 2005. [Google Scholar]
- Wallis, T.E. Textbook of Pharmacognosy, 5th ed.; CBS Publishers and Distributors: New Delhi, India, 1990. [Google Scholar]
- Singleton, V.L. Lamuela-Raventos: Analysis of Total Phenoles and Other Oxidation Substartes and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, H.; Li, Y.F.; Xu, J.L.; Yu, M.Q. In vitro studies on the radical Scavenging activity of hulless barley pigment. Adv. Mater. Res. 2011, 183–185, 145–150. [Google Scholar] [CrossRef]
- Mohana, D.C.; Raveesha, K.A. Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. J. Agric. Technol. 2007, 4, 119–137. [Google Scholar]
- Sridhar, S.R.; Rajagopal, R.V.; Rajavel, R.; Masilamani, S.; Narasimhan, S. Antifungal activity of some essential oils. J. Agric. Food Chem. 2003, 51, 7596–7599. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Approved Standard M2-A9; Performance Standards for Antimicrobial Disk Susceptibility Tests; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2006. [Google Scholar]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Therapeutic uses of Amaranthus caudatus L. Trop. Biomed. 2019, 36, 1038–1053. [Google Scholar]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 341–361. [Google Scholar] [CrossRef]
- Thakur, M. Wild Mushrooms as Natural Untapped Treasures. Natural Products: Recent Advances; Write & Print Publications: New Delhi, India, 2015; pp. 214–226. [Google Scholar]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alia, M.; Horcajo, C.; Bravo, L.; Goya, L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr. Res. 2003, 23, 1251–1267. [Google Scholar] [CrossRef] [Green Version]
- Amic, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Kiliçgün, H.; Altiner, D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn. Mag. 2010, 6, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Qin, W. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cere. Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Yang, C.H.; Liu, J.H. Recent advance of diterpenoid alkaloids in genus Aconitum. Strait Pharmaceut. J. 2009, 21, 1–10. [Google Scholar]
- Wang, F.P.; Chen, Q.H. The C19-diterpenoid alkaloids. Alkaloids Chem. Biol. 2010, 69, 1–577. [Google Scholar] [CrossRef]
- Yin, T.; Zhou, H.; Cai, L.; Ding, Z. Non-alkaloidal constituents from the genus Aconitum: A review. RSC Adv. 2019, 9, 10184–10194. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.A.; Khan, S.; Khan, N.M.; Khan, H.; Khan, S.; Ahmad, S.; Rehman, N.; Aziz, R. Antimicrobial and Antioxidant Role of the Aerial Parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021, 65, 84–93. [Google Scholar] [CrossRef]
- Konda, V.G.R.; Eerike, M.; Raghuraman, L.P.; Rajamanickam, M.K. Antioxidant and nephroprotective activities of Aconitum heterophyllum root in glycerol induced acute renal failure in rats. J. Clin. Diagn. Res. JCDR 2016, 10, FF01. [Google Scholar] [CrossRef]
- BKim, J.H.; Lee, S.Y.; Kwon, O.J. Anti-aging and anti-diabetes effects of Aconitum pesudolaeve var. erectum extracts. J. Life Sci. 2013, 23, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, C.; Pan, H.H.; Kang, J.; Yu, X.T.; Wang, H.Q.; Li, B.M.; Xie, Y.Z.; Chen, R.Y. Chemical constituents from Inonotus obliquus and their biological activities. J. Nat. Prod. 2014, 77, 35–41. [Google Scholar] [CrossRef]
- Balakumar, S.; Rajan, S.; Thirunalasundari, T.; Jeeva, S. Antifungal activity of Aegle marmelos (L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pac. J. Trop. Biomed. 2011, 1, 309–312. [Google Scholar] [CrossRef] [Green Version]
- Al-Rahmah, A.N.; Mostafa, A.A.; Abdel-Megeed, A.; Yakout, S.M.; Hussein, S.A. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr. J. Microbiol. Res. 2013, 7, 517–524. [Google Scholar]
- Al-Samarrai, G.F.; Singh, H.; Syarhabil, M. Extracts some plants on controlling green mold of orange and on postharvest quality parameters. World Appl. Sci. J. 2013, 22, 564–570. [Google Scholar] [CrossRef]
- El-Samawaty, A.M.A.; Yassin, M.A.; Moslem, M.A.; Omar, M.R. Effectiveness of some Plant Extracts against Fusarium spp. Causing Cotton Seedlings Damping off. Life Sci. J. 2013, 10, 510–515. [Google Scholar]
- Kumar, S.; Javed, M.S.; Kumar, P. In-vitro antifungal and anti-bacterial activity of chloroform extract from tubers of Aconitum laeve Royle: Endangered species, India. Mater. Today Proc. 2021, 34, 563–568. [Google Scholar] [CrossRef]
- Munir, N.; Ijaz, W.; Altaf, I.; Naz, S. Evaluation of antifungal and antioxidant potential of two medicinal plants: Aconitum heterophyllum and Polygonum bistorta. Asian Pac. J. Trop. Biomed. 2014, 4, S639–S643. [Google Scholar] [CrossRef] [Green Version]
| Constituents | Chemical Tests | Rhizome | Stem | Leaves | Flower | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | EA | M | H | EA | M | H | EA | M | H | EA | M | ||
| Alkaloids | Sodium hydroxide test Wagner’s test | − | + | ++ | − | + | ++ | − | + | ++ | − | + | ++ |
| Lead acetate test Mayer’s test | − | + | ++ | − | + | ++ | − | + | ++ | − | + | ++ | |
| Flavonoids | Keller–Kiliani test Sodium hydroxide test | + | + | ++ | + | − | ++ | − | + | ++ | + | + | ++ |
| Fehling’s test Lead acetate test | + | + | ++ | + | − | ++ | − | + | + | + | + | ++ | |
| Glycosides | Phenol’s test Keller–Kiliani test | + | + | + | + | + | ++ | + | + | + | + | + | ++ |
| Frothing/foam test Fehling’s test | − | + | + | + | + | ++ | + | + | + | + | + | ++ | |
| Phenols | Salkowski test Phenols test | + | + | ++ | − | − | − | − | − | − | + | + | ++ |
| Saponins | LB test Frothing/foam test | + | + | ++ | − | − | − | − | − | − | + | + | ++ |
| Steroids | Ferric chloride test Liebermann–Burchard test | + | + | ++ | + | + | + | + | + | + | + | + | ++ |
| Tannin | Salkowski test FeCl3 test | − | + | + | + | + | + | − | − | − | + | + | + |
| Terpenoids | Sodium hydroxide test Salkowski test | − | + | + | + | + | ++ | − | + | + | + | + | ++ |
| Terpenes | Salkowski’s test | − | − | − | − | − | − | − | − | − | + | + | + |
| S. No. | Ret. Time | m/z | Adduct | Compound Name | Comp. Formula | Category/Class/Subclass | Exact Mass |
|---|---|---|---|---|---|---|---|
| 1 | 1.215 | 180.1013 | (M+H)+ | 2(N)-Methyl-norsalsolinol | C10H13NO2 | Alkaloid | 179.0941 |
| 2 | 1.332 | 377.0898 | (M+HCOO)− | 3,3′,5-Trihydroxy-4′,7-dimethoxyflavanone | C17H16O7 | Flavonoid derivative | 332.0896 |
| 3 | 1.76 | 376.2468 | (M+H)+ | Icaceine | C22H33NO4 | Diterpene alkaloid | 375.2395 |
| 4 | 1.939 | 470.273 | (M+H)+ | Dimethylaminoethylreserpilinate | C26H35N3O5 | Reserpilinate derivative alkaloid | 469.2659 |
| 5 | 2.16 | 197.0481 | (M+H)− | Syringic acid | C9H10O5 | Phenolic compound | 198.0554 |
| 6 | 2.261– 2.635 | 454.2782 | (M+H)+ | Delcosine | C24H39NO7 | Diterpene alkaloid | 453.271 |
| 7 | 2.319 | 503.1451 | (M−H)− | 6-Caffeoylsucrose | C21H28O14 | Glycoside | 504.1523 |
| 8 | 2.536 | 353.0873 | (M+H)− | Chlorogenic acid | C16H18O9 | phenolic derivative | 354.0945 |
| 9 | 3.434 | 378.2628 | (M+H)+ | Karakoline | C22H35NO4 | Diterpene alkaloid | 377.2554 |
| 10 | 3.619 | 408.273 | (M+H)+ | Cammaconine | C23H37NO5 | Diterpene alkaloid | 407.2656 |
| 11 | 4.658– 4.986 | 468.2945 | (M+H)+ | Browniine | C25H41NO7 | Diterpene alkaloid | 467.2871 |
| 12 | 4.886 | 422.2891 | (M+H)+ | Talatizamine | C24H39NO5 | Diterpene | 421.2819 |
| 13 | 4.988 | 480.2943 | (M+H)+ | Delcorine | C26H41NO7 | Diterpene alkaloid | 479.2871 |
| 14 | 5.107 | 659.1804 | (M+HCOO)− | Catechin 3′,5-diglucoside | C27H34 O | Flavonoid O-glycosides | 614.18 |
| 15 | 5.13–6.038 | 510.3051 | (M+H)+ | Germine | C27H43NO8 | Alkaloid | 509.2977 |
| 16 | 5.189 | 355.1577 | (M−H)− | Gingerenone A | C21H24 O5 | Polyphenol | 355.1577 |
| 17 | 5.27 | 491.1621 | (M+HCOO)− | Osmanthuside A | C23H26O9 | Coumaric acid esters | 446.1638 |
| 18 | 5.407– 7.383 | 494.3118 | (M+H)+ | Zygadenine | C27H43NO7 | Alkaloid | 493.3043 |
| 19 | 5.805 | 693.2109 | (M−H)− | Sucrose 1′,4′-(4,4′-dihydroxy-3,3′-20dimethoxy-b-truxinate) | C32H38O17 | Stilbene glycoside | 694.2182 |
| 20 | 5.815 | 579.1804 | (M−H)− | (S)-Naringenin 8-C-(2″-rhamnosylglucoside) | C32H38 O17 | Flavonoid glycoside | 580.1874 |
| 21 | 5.97 | 545.1942 | (M+CH3COO)− | Haplodimerine | C28H26N2O6 | Quinoline alkaloid | 486.1804 |
| 22 | 6.032 | 521.2094 | (M−H)− | Isolariciresinol 9′-O-β-D-glucoside | C26H34O11 | Lignan glycoside | 522.2163 |
| 23 | 6.352 | 555.1701 | (M−H)− | 7-Dehydrologanin tetraacetate | C25H32O14 | Terpene | 556.1776 |
| 24 | 7.077 | 521.1375 | (M−H)− | Sudachiin A | C24H26O13 | Flavonoid glycoside | 522.1445 |
| 25 | 7.451 | 616.3103 | (M+H)+ | Hypaconitine | C33H45NO10 | Diterpene alkaloid | 615.3031 |
| 26 | 7.807 | 533.2579 | (M−H)− | 7,8-Dihydrovomifoliol 9-[rhamnosyl-(1->6)-glucoside] | C25H42O12 | Flavonoid glycoside | 534.2651 |
| 27 | 8.13 | 630.3276 | (M+H)+ | Indaconitine | C34H47NO10 | Diterpene alkaloid | 629.32 |
| 28 | 8.521– 11.332 | 630.3276 | (M+H)+ | Falaconitine | C34H47NO10 | Diterpene alkaloid | 629.32 |
| 29 | 9.897– 11.561 | 630.3276 | (M+H)+ | Finaconitine | C33H46N2O10 | Diterpene alkaloid | 629.32 |
| 30 | 12.749 | 535.1612 | (M+HCOO)− | Edulisin I | C28H26O8 | Furanocoumarins | 490.1603 |
| 31 | 13.263– 14.128 | 639.3123 | (M−H)− | N1,N5,N10-Tris-trans-p-coumaroylspermine | C37H44N4O6 | Coumaric acid derivative | 640.3186 |
| 32 | 13.913 | 563.4011 | (M+HCOO)− | Ganoderiol C | C32H54O5 | Triterpenoid | 518.4029 |
| 33 | 16.648 | 593.2809 | (M+CH3COO)− | 3-Hydroxy-beta-ionol 3-[glucosyl-(1->6)-glucoside] | C25H42O12 | Terpene glycoside | 534.2668 |
| 34 | 16.764 | 473.3327 | (M−H)− | (3-β,17-α,23S)-17,23-Epoxy-3,28,29-trihydroxy-27-norlanost-8-en-24-one | C29H46O5 | Triterpenoid | 474.3399 |
| 35 | 19.711 | 597.4014 | (M−H)− | Idoxanthin | C40H54O4 | Carotenoid (xanthophyll) | 598.4063 |
| Conc. (μg/mL) | Hexane | Ethyl Acetate | Methanol | α-tocopherol |
|---|---|---|---|---|
| 50 | 12.92 ± 0.73 | 22.38 ± 0.42 | 27.99 ± 0.55 | 50.24 ± 1.10 |
| 100 | 23.42 ± 0.52 | 31.48 ± 0.54 | 39.69 ±0.44 | 55.67 ± 0.87 |
| 150 | 29.46 ± 0.54 | 38.28 ± 1.05 | 45.50 ± 0.47 | 61.24 ± 0.27 |
| 200 | 37.67 ± 0.75 | 50.23 ±1.42 | 57.48 ± 0.63 | 75.37 ± 0.32 |
| 250 | 49.99 ± 1.10 | 59.59 ± 0.77 | 65.21 ± 0.95 | 81.10 ± 1.16 |
| IC50 Value | 263.01 ± 1.70 μg/mL | 199.5 ± 1.99 μg/mL | 163.71 ± 2.69 μg/mL | 118.79 ± 1.27 μg/mL |
| Conc. (μg/mL) | Hexane Extract | Ethyl Acetate | Methanol | Ascorbic Acid |
|---|---|---|---|---|
| 50 | 7.72 ± 0.41 | 11.67 ± 0.88 | 16.31 ± 0.24 | 32.56 ± 0.10 |
| 100 | 16.68 ± 0.37 | 25.50 ± 0.96 | 31.41 ± 0.11 | 43.55 ± 0.43 |
| 150 | 28.65 ± 1.55 | 35.33 ± 0.13 | 42.67 ± 0.14 | 59.34 ± 0.96 |
| 200 | 41.43 ± 0.15 | 46.36 ± 0.62 | 55.54 ± 0.15 | 70.46 ± 0.51 |
| 250 | 50.26 ± 0.48 | 54.30 ± 0.75 | 68.64 ± 0.37 | 80.25 ± 1.27 |
| IC50 value | 246.37 ± 0.56 μg/mL | 219.71 ± 0.32 μg/mL | 179.11 ± 0.73 μg/mL | 125.80 ± 0.63 μg/mL |
| Conc. (μg/mL) | Hexane | Ethyl Acetate | Methanol | BHT |
|---|---|---|---|---|
| 50 | 12.59 ± 0.38 | 22.18 ± 0.46 | 26.79 ± 0.91 | 34.44 ± 1.31 |
| 100 | 22.83 ± 0.61 | 30.49 ± 0.89 | 36.11 ± 1.03 | 42.35 ± 0.32 |
| 150 | 27.96 ± 0.28 | 41.54 ± 0.41 | 46.54 ± 1.01 | 52.9 ± 0.92 |
| 200 | 39.13 ± 0.34 | 50.18 ± 1.03 | 52.46 ± 0.54 | 60.61 ± 0.53 |
| 250 | 50.86 ± 0.75 | 54.75 ± 1.07 | 60.0 ± 0.21 | 68.39 ± 1.72 |
| IC50 Value | 243.54 ± 0.34 μg/mL | 192.23 ± 0.39 μg/mL | 178.33 ± 0.91 μg/mL | 122.61 ± 0.57 μg/mL |
| Conc. (μg/mL) | Hexane | Ethyl Acetate | Methanol | OH− |
|---|---|---|---|---|
| 50 | 6.32 ± 0.29 | 16.61 ± 0.70 | 19.21 ± 0.68 | 37.62 ± 0.39 |
| 100 | 14.01 ± 0.74 | 28.92 ± 0.40 | 34.01 ± 0.29 | 48.81 ± 0.85 |
| 150 | 23.48 ± 1.81 | 34.01 ± 0.29 | 48.01 ± 0.59 | 57.62 ± 0.58 |
| 200 | 35.02 ± 0.88 | 47.79 ± 1.41 | 57.17 ± 0.29 | 68.02 ± 0.49 |
| 250 | 51.86 ± 1.95 | 58.98 ± 0.70 | 67.24 ± 0.49 | 74.68 ± 0.59 |
| IC50 value | 238.85 ± 0.23 μg/mL | 208.85 ± 0.45 μg/mL | 159.64 ± 0.42 μg/mL | 101.99 ± 0.66 μg/mL |
| Pathogenic Fungi | % Mean Mycelial Inhibition | Negative Control | Hexaconazole | MIC (μg/mL) | ||
|---|---|---|---|---|---|---|
| Concentrations (%) of Culture Filtrate | ||||||
| 5% | 10% | 15% | ||||
| Aspergillus niger ME | 41.16 ± 3.32 a | 52.94 ± 1.46 a | 66.18 ± 1.03 b | 25.23 ± 1.13 a | 83.76 ± 0.26 a | 230 |
| Aspergillus flavus ME | 53.81 ± 1.11 b | 69.36 ± 1.05 b | 78.91 ± 1.19 c | 15.47 ± 2.23 b | 90.53 ± 1.32 b | 200 |
| Penicillium notatum ME | 58.81 ± 0.76 b | 71.46 ± 1.06 b | 83.14 ± 0.97 bc | 12.35 ± 0.73 b | 95.65 ± 2.23 b | 190 |
| Aspergillus niger EAE | 49.42 ± 0.99 b | 49.98 ± 3.78 a | 59.56 ± 3.14 a | 25.23 ± 1.13 a | 83.76 ± 0.26 a | 250 |
| Aspergillus flavus EAE | 36.75 ± 3.72 a | 65.88 ± 1.74 b | 74.33 ± 0.71 bc | 15.47 ± 2.23 b | 90.53 ± 1.32 b | 210 |
| Penicillium notatum EAE | 55.34 ± 1.06 b | 69.75 ± 0.99 b | 78.21 ± 1.31 bc | 12.35 ± 0.73 b | 95.65 ± 2.23 b | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, S.; Wagay, N.A.; Elansary, H.O.; Malik, M.A.; Bhat, I.A.; Kaloo, Z.A.; Hadi, A.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Phytochemical Screening, Antioxidant and Antifungal Activities of Aconitum chasmanthum Stapf ex Holmes Wild Rhizome Extracts. Antioxidants 2022, 11, 1052. https://doi.org/10.3390/antiox11061052
Rafiq S, Wagay NA, Elansary HO, Malik MA, Bhat IA, Kaloo ZA, Hadi A, Alataway A, Dewidar AZ, El-Sabrout AM, et al. Phytochemical Screening, Antioxidant and Antifungal Activities of Aconitum chasmanthum Stapf ex Holmes Wild Rhizome Extracts. Antioxidants. 2022; 11(6):1052. https://doi.org/10.3390/antiox11061052
Chicago/Turabian StyleRafiq, Shah, Nasir Aziz Wagay, Hosam O. Elansary, Mansoor Ahmad Malik, Irshad Ahmad Bhat, Zahoor Ahmad Kaloo, Abdul Hadi, Abed Alataway, Ahmed Z. Dewidar, Ahmed M. El-Sabrout, and et al. 2022. "Phytochemical Screening, Antioxidant and Antifungal Activities of Aconitum chasmanthum Stapf ex Holmes Wild Rhizome Extracts" Antioxidants 11, no. 6: 1052. https://doi.org/10.3390/antiox11061052








