The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish, Experimental Conditions, and Feeding Procedures
2.3. Sample Collection and Air Exposure Stress Challenge
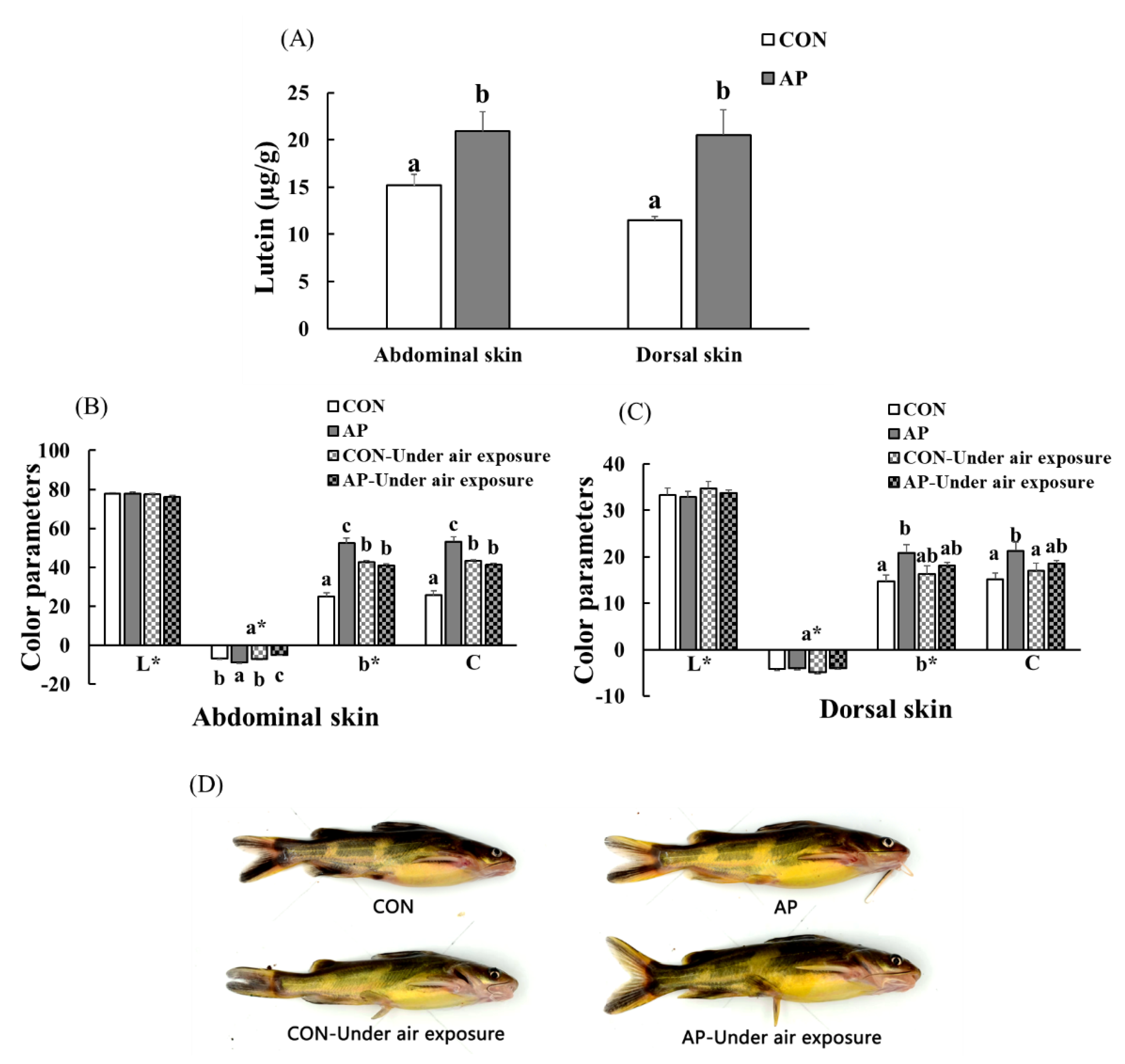
2.4. Fish Skin Color Determination
2.5. Lutein Extraction and Quantification
2.6. Plasma LD, Cortisol, Glucose, and Antioxidant Assays
2.7. Quantitative Real-Time PCR Analysis
2.8. Western Blot Analysis
2.9. Statistical Analyses
3. Results
3.1. Growth and Feed Utilization
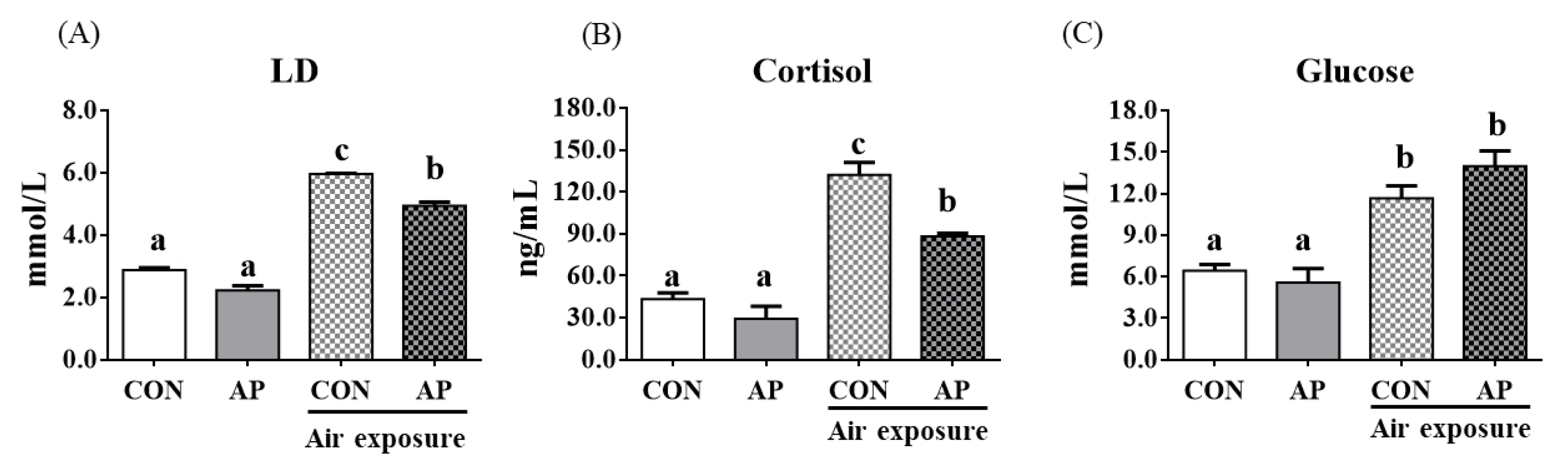
3.2. Stress Response Markers in Plasma
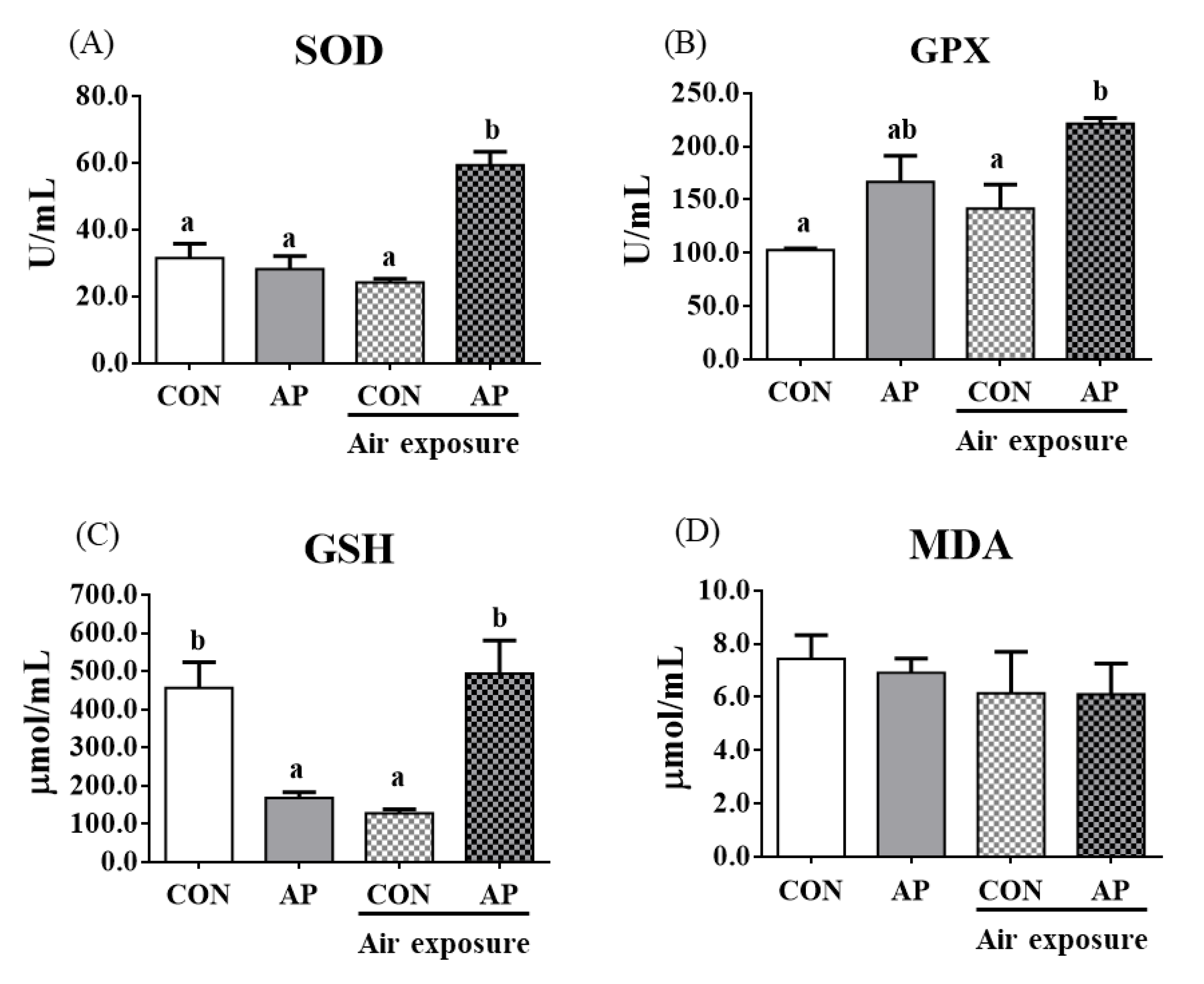
3.3. Antioxidant-Related Parameters in Plasma
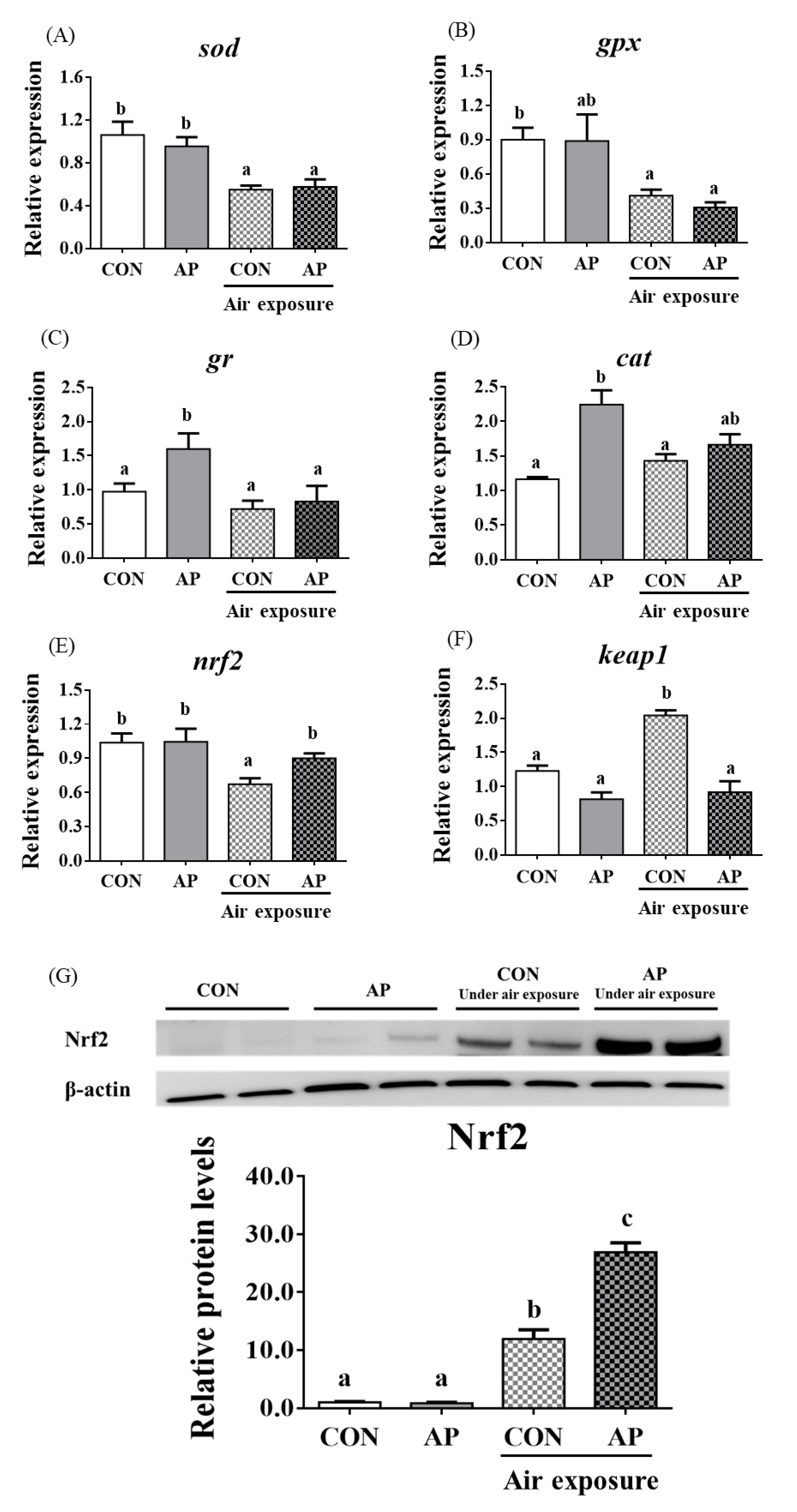
3.4. Antioxidant Related Gene Expression and the Nrf2 Signaling Pathway in the Liver
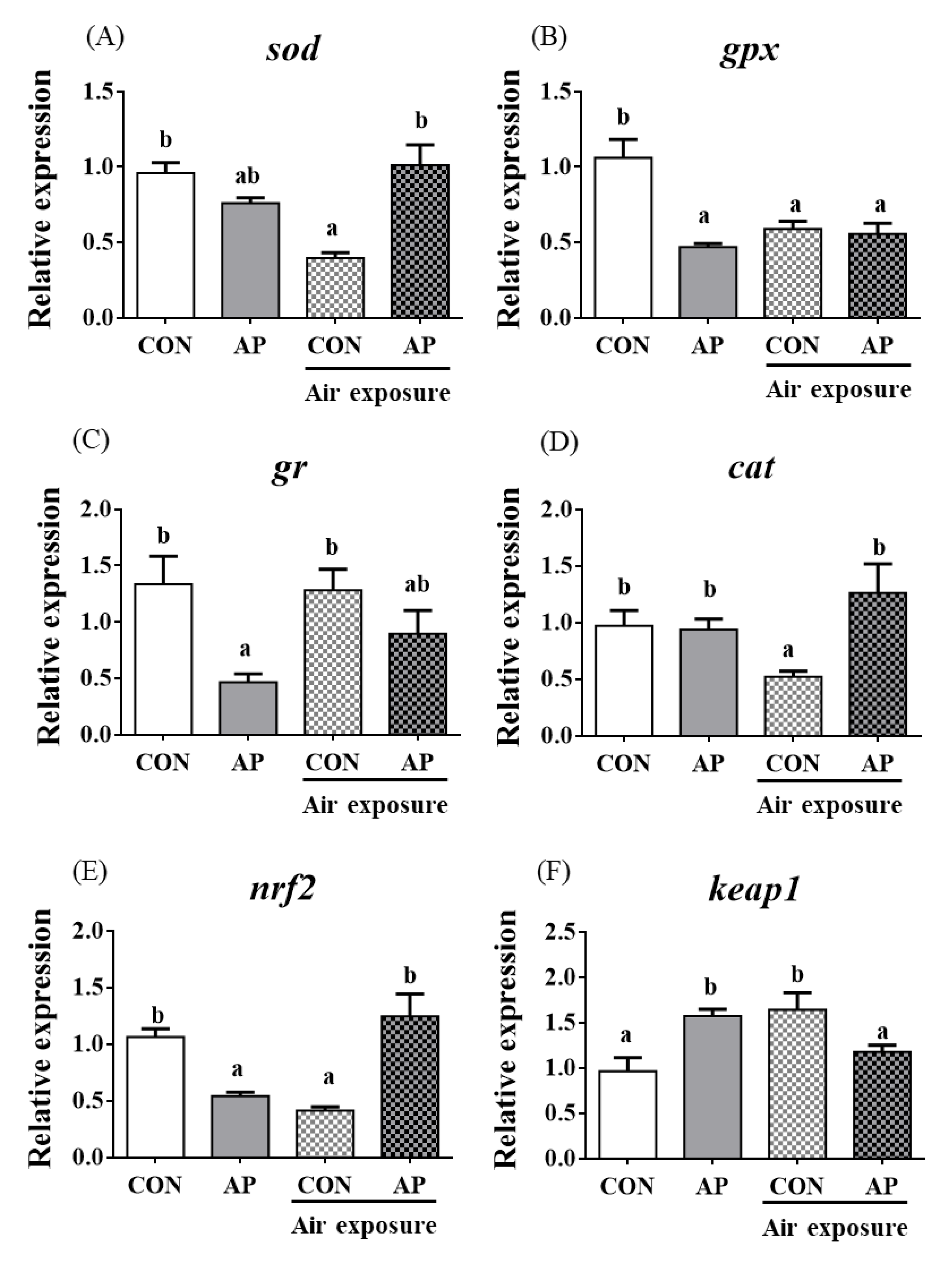
3.5. Antioxidant Related Gene Expression in Kidney
3.6. Skin Lutein Content and Body Color of Fish
3.7. Skin Yellowness Correlation Analysis
4. Discussion
4.1. A. platensis Did Not Affect the Growth Performance and Feed Utilization of Fish
4.2. A. platensis Enhanced Stress Response
4.3. A. platensis Enhanced the Antioxidant Capacity of Fish
4.4. A. platensis Improved Body Color Disorder Caused by Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Míguez, J.M.; Martínez-Rodríguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata), new insights on fish stress response. Front. Physiol. 2018, 9, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Arends, R.; Mancera, J.M.; Muñoz, J.L.; Bonga, S.E.W.; Flik, G. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J. Endocrinol. 1999, 163, 149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Regenstein, J.M.; Xie, D.D.; Lu, W.J.; Ren, X.C.; Yuan, J.J.; Mao, L.C. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Wu, Y.B.; Huang, D.; Ren, X.; Wang, Y. Effect of blood glucose level on acute stress response of grass carp Ctenopharyngodon idella. Fish Physiol. Biochem. 2017, 43, 1433–1442. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Hallermann, J. Effects of air exposure on mortality and growth of undersized pikeperch, Sander lucioperca, at low water temperatures with implications for catch-and-release fishing. Fish Manag. Ecol. 2007, 14, 155–160. [Google Scholar] [CrossRef]
- Duan, Y.F.; Zhang, J.S.; Dong, H.B.; Wang, Y.; Liu, Q.S.; Li, H. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef]
- Paital, B. Modulation of redox regulatory molecules and electron transport chain activity in muscle of air breathing fish Heteropneustes fossilis under air exposure stress. J. Comp. Physiol. B 2014, 184, 65–76. [Google Scholar] [CrossRef]
- de Oliveira, S.T.L.; Soares, R.A.N.; de Negreiros Sousa, S.M.; Fernandes, W.A.C.; Gouveia, G.V.; da Costa, M.M. Natural products as functional food ingredients for Nile tilapia challenged with Aeromonas hydrophila. Aquacult. Int. 2020, 28, 913–926. [Google Scholar] [CrossRef]
- Kumar, V.; Bossier, P. Importance of plant-derived compounds and/or natural products in aquaculture. Aquafeed 2018, 10, 28–31. [Google Scholar]
- Rodriguez, M.G.M.; Pohlenz, C.; Gatlin, D.M., III. Supplementation of organic acids and algae extracts in the diet of red drum Sciaenops ocellatus, immunological impacts. Aquac. Rep. 2017, 48, 1778–1786. [Google Scholar] [CrossRef]
- Şimşek, N.; Karadeniz, A.; Karaca, T. Effects of the Spirulina platensis and Panax ginseng oral supplementation on peripheral blood cells in rats. Rev. Méd. Vét. 2007, 158, 483–488. [Google Scholar]
- Yeganeh, S.; Teimouri, M.; Amirkolaie, A.K. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2015, 101, 84–88. [Google Scholar] [CrossRef]
- Abdelkhalek, N.K.; Ghazy, E.W.; Abdel-Daim, M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus, impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. 2015, 22, 3023–3031. [Google Scholar] [CrossRef]
- Gogoi, S.; Mandal, S.C.; Patel, A.B. Effect of dietary Wolffia arrhiza and Spirulina platensis on growth performance and pigmentation of Queen loach Botia dario (Hamilton, 1822). Aquacult. Nutr. 2018, 24, 285–291. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.K.; Han, D.; Xie, S.Q.; Jin, J.Y.; Yang, Y.X.; Zhu, X.M. Effects of dietary Arthrospira platensis supplementation on the growth performance, antioxidation and immune related-gene expression in yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2020, 17, 100297. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zhang, X.T.; Zhang, G.R.; Shi, Z.C.; Yuan, Y.J.; Zheng, H.; Lin, L.; Wei, K.J.; Ji, W. Expression analysis of nine Toll-like receptors in yellow catfish (Pelteobagrus fulvidraco) responding to Aeromonas hydrophila challenge. Fish Shellfish Immunol. 2017, 63, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, H.Z.; Guo, Q.S.; Yu, Y.B.; Wang, A.M.; Lv, F.; Shen, W.B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, H.K.; Xu, W.J.; Han, D.; Xie, S.Q.; Jin, J.Y.; Yang, Y.X.; Zhu, X.M. Effects of dietary Arthrospira platensis supplementation on the growth, pigmentation, and antioxidation in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2019, 510, 267–275. [Google Scholar] [CrossRef]
- CIE. Recommendations on Uniform Color Spaces, Color Difference Equations, Psychometric Color Terms; Supplement No. 2 to CIE Publication No. 15; Colorimetry; Bureau Central de la CIE; Paris International Commission on Illumination: Paris, France, 1976. [Google Scholar]
- Hunt, R.W. The specification of colour appearance. I. Concepts and terms. Color Res. Appl. 1977, 2, 55–68. [Google Scholar] [CrossRef]
- Karadas, F.; Grammenidis, E.; Surai, P.; Acamovic, T.; Sparks, N.H.C. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br. Poult. Sci. 2006, 47, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Moros, E.; Darnoko, D.; Cheryan, M.; Perkins, E.G.; Jerrell, J. Analysis of xanthophylls in corn by HPLC. J. Agric. Food Chem. 2002, 50, 5787–5790. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Ho, Y.C.; Lu, F.J. Determination of lutein and zeaxanthin and antioxidant capacity of supercritical carbon dioxide extract from daylily (Hemerocallis disticha). Food Chem. 2011, 129, 1813–1818. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 0034.1–0034.11. [Google Scholar] [CrossRef] [Green Version]
- Adel, M.; Yeganeh, S.; Dadar, M.; Sakai, M.; Dawood, M.A.O. Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol 2016, 56, 436–444. [Google Scholar] [CrossRef]
- Barton, B.A. Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress. N. Am. J. Aquacult. 2000, 62, 12–18. [Google Scholar] [CrossRef]
- Tandon, R.; Joshi, B. Blood glucose and lactic acid levels in the fresh water fish, Heteropneustes Fossilis, following Stress 1. Z. Für Tierphysiol. Tierernährung Futterm. 1973, 31, 210–216. [Google Scholar] [CrossRef]
- Lim, H.K.; Hur, J.W. Effects of acute and chronic air exposure on growth and stress response of juvenile olive flounder, Paralichthys olivaceus. Turk. J. Fish. Aquat. Sci. 2018, 18, 143–151. [Google Scholar] [CrossRef]
- Hur, J.W.; Kang, K.H.; Kang, Y.J. Effects of acute air exposure on the hematological characteristics and physiological stress response of olive flounder (Paralichthys olivaceus) and Japanese croaker (Nibea japonica). Aquaculture 2019, 502, 142–147. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; Tang, W.; Zhou, X.; et al. Dietary vitamin A improved the flesh quality of grass carp (Ctenopharyngodon idella) in relation to the enhanced antioxidant capacity through Nrf2/Keap 1a signaling pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Mousavi, S.; Oushani, A.K.; Firouzamandi, M.; Mardani, K. Spirulina platensis in rainbow trout (Oncorhynchus mykiss) feed, effects on growth, fillet composition, and tissue antioxidant mechanisms. Aquacult. Int. 2019, 27, 1613–1623. [Google Scholar] [CrossRef]
- Cao, S.P.; Zhang, P.Y.; Zou, T.; Fei, S.Z.; Han, D.; Jin, J.Y.; Liu, H.K.; Yang, Y.X.; Zhu, X.M.; Xie, S.Q. Replacement of fishmeal by spirulina Arthrospira platensis affects growth, immune related-gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 79, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Ferrari, P.F.; Aliakbarian, B.; Converti, A.; Perego, P. Effect of UV radiation or titanium dioxide on polyphenol and lipid contents of Arthrospira (Spirulina) platensis. Algal Res. 2015, 12, 308–315. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, G.; Chen, T.F.; Wong, Y.S.; Zhao, H.L.; Fan, R.R.; Gu, X.M.; Tong, P.C.; Chan, J.C. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int. J. Biochem. Cell Biol. 2009, 41, 1526–1535. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef]
- Zou, B.; Xiao, G.S.; Xu, Y.J.; Wu, J.J.; Yu, Y.S.; Fu, M.Q. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem. 2018, 255, 23–30. [Google Scholar] [CrossRef]
- Esam, F.; Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Chen, X.L.; Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway, a new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Kawashima, H.; Osada, H.; Toda, E.; Homma, K.; Nagai, N.; Imai, Y.Y.; Tsubota, K.; Ozawa, Y. Dietary Spirulina supplementation protects visual function from photostress by suppressing retinal neurodegeneration in mice. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, H.K.; Surh, Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Roohani, A.M.; Kenari, A.A.; Kapoorchali, M.F.; Borani, M.S.; Zoriezahra, S.J.; Smiley, A.H.; Esmaeili, M.; Rombenso, A.N. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquacult. Nutr. 2019, 25, 633–645. [Google Scholar]
- Teimouri, M.; Amirkolaie, A.K.; Yeganeh, S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 396–399, 14–19. [Google Scholar] [CrossRef]
- Habib, B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; Food & Agriculture Organization of the United Nations: Rome, Italy, 2008; p. 41. [Google Scholar]
- Leema, J.T.M.; Kirubagaran, R.; Vinithkumar, N.V.; Dheenan, P.S.; Karthikayulu, S. High value pigment production from Arthrospira (Spirulina) platensis cultured in seawater. Bioresour. Technol. 2010, 101, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Gherasim, E.C.; Dulf, F.V.; Pintea, A. In vitro bioaccessibility of macular xanthophylls from commercial microalgal powders of Arthrospira platensis and Chlorella pyrenoidosa. Food Sci. Nutr. 2021, 9, 1896–1906. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.H.; Chen, Z.; Yuan, L.; Liu, H.K.; Han, D.; Jin, J.Y.; Yang, Y.X.; Hu, Q.; Zhu, X.M.; et al. Effects of dietary whole and defatted Arthrospira platensis (Cyanobacterium) on growth, body composition and pigmentation of the yellow catfish Pelteobagrus fulvidraco. J. Appl. Phycol. 2021, 33, 2251–2259. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea, algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Tveit, G.M.; Anders, N.; Bondø, M.S.; Mathiassen, J.R.; Breen, M. Atlantic mackerel (Scomber scombrus) change skin colour in response to crowding stress. J. Fish Biol. 2022, 100, 738–747. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P.; Valchářová, T.; Pfauserová, N.; Velíšek, J. Comparative study of stress responses, laterality and familiarity recognition between albino and pigmented fish. Zoology 2022, 150, 125982. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (g kg−1 Dry Matter) | CON | AP |
|---|---|---|
| White fishmeal 1 | 300 | 300 |
| A. platensis 2 | 0 | 20 |
| Soybean meal 3 | 187.8 | 175.8 |
| Rapeseed meal 3 | 200 | 185.5 |
| Wheat flour | 150 | 150 |
| Fish oil | 25 | 25 |
| Soybean oil | 25 | 25 |
| Cellulose | 27.2 | 33.7 |
| Vitamin premix 4 | 3.9 | 3.9 |
| Mineral premix 5 | 50 | 50 |
| CMC 6 | 30 | 30 |
| Choline chloride | 1.1 | 1.1 |
| Chemical composition (g kg−1) | ||
| Moisture | 108.55 | 108.15 |
| In dry matter (g kg−1) | ||
| Crude protein | 418.30 | 416.56 |
| Crude lipid | 80.99 | 75.22 |
| Lutein (µg/g) | 4.96 | 8.08 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Amplicon Size (bp) | Tm (°C) | PCR Efficiency a/b | Accession No |
|---|---|---|---|---|---|---|
| sod | TTGGAGACAATACAAATGGGTG | CATCGGAATCGGCAGTCA | 129 | 57 | 2.007/1.968 | XM_027171881 |
| gpx | ATCTACATTGGCTTGGAAAC | GAAAGTAGGGACTGAGGTGA | 257 | 58 | 1.966/1.950 | XM_027163146 |
| gr | CAGTCGCTTTGTTTGTTCTA | TCCTCCGATACACTTCTCAC | 280 | 57 | 1.992/2.050 | XM_027152663 |
| cat | TCTGTTCCCGTCCTTCATCC | ATATCCGTCAGGCAATCCAC | 151 | 58 | 1.964/2.001 | XM_027163801 |
| nrf2 | TCTCGCCCAGTTACAGCTTG | GTTCCGTGAACGCCACATTC | 128 | 60 | 1.992/1.952 | XM_027164284 |
| keap1 | CGCAGCCGGGCTTTTATTTT | AGGCAGAAACGGGTTCAAGT | 286 | 59 | 1.989/1.959 | XM_027133478.1 |
| β-actin | TTCGCTGGAGATGATGCT | CGTGCTCAATGGGGTACT | 136 | 58 | 2.044/2.003 | EU161066 |
| Indices/Diets | CON | AP |
|---|---|---|
| IBW (g) | 69.8 ± 0.10 | 70.23 ± 0.15 |
| FBW (g) | 103.23 ± 0.35 | 105.32 ± 1.07 |
| Survival rate (%) | 100 | 100 |
| FR (%BW d−1) | 2.52 ± 0.08 | 2.63 ± 0.02 |
| SGR (% d−1) | 0.68 ± 0.05 | 0.62 ± 0.02 |
| FE (%) | 30.51 ± 3.82 | 30.69 ± 0.49 |
| Condition indices | ||
| Condition factor (g cm−3) | 1.89 ± 0.08 | 1.81 ± 0.04 |
| Hepatosomatic index (%) | 1.40 ± 0.17 | 1.68 ± 0.11 |
| Viscerosomatic index (%) | 11.14 ± 0.85 | 10.8 ± 0.56 |
| Yellowness (b*)-Values | ||
|---|---|---|
| Abdominal Skin | Dorsal Skin | |
| Dorsal skin lutein content | 0.82 * | 0.87 * |
| Abdominal skin lutein content | 0.90 ** | 0.67 * |
| Plasma LD content | −0.59 * | −0.66 * |
| Liver sod relative mRNA level | 0.76 | 0.95 * |
| Liver gr relative mRNA level | 0.91 | 0.99 ** |
| Kidney gpx relative mRNA level | 0.96 * | 0.99 |
| Kidney gr relative mRNA level | 0.80 | 0.98 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, H.; Zhu, X.; Han, D.; Jin, J.; Yang, Y.; Xie, S. The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco. Antioxidants 2022, 11, 1100. https://doi.org/10.3390/antiox11061100
Liu C, Liu H, Zhu X, Han D, Jin J, Yang Y, Xie S. The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco. Antioxidants. 2022; 11(6):1100. https://doi.org/10.3390/antiox11061100
Chicago/Turabian StyleLiu, Cui, Haokun Liu, Xiaoming Zhu, Dong Han, Junyan Jin, Yunxia Yang, and Shouqi Xie. 2022. "The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco" Antioxidants 11, no. 6: 1100. https://doi.org/10.3390/antiox11061100
APA StyleLiu, C., Liu, H., Zhu, X., Han, D., Jin, J., Yang, Y., & Xie, S. (2022). The Effects of Dietary Arthrospira platensis on Oxidative Stress Response and Pigmentation in Yellow Catfish Pelteobagrus fulvidraco. Antioxidants, 11(6), 1100. https://doi.org/10.3390/antiox11061100








