Maqui (Aristotelia chilensis (Mol.) Stuntz): A Natural Antioxidant to Improve Quality of Meat Patties
Abstract
:1. Introduction
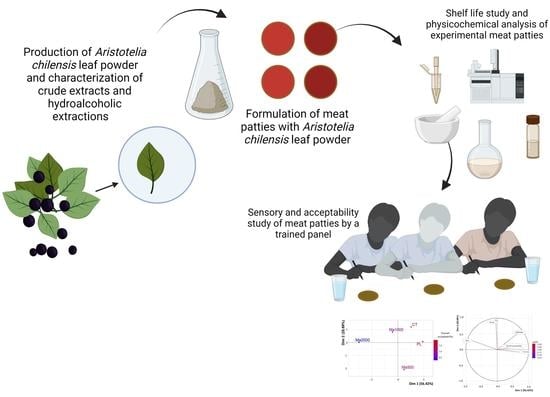
2. Materials and Methods
2.1. Plant Material
2.2. Maqui Leaf Powder Characterization: Total Phenolic Content, Main Phenolic Compounds and Antioxidant Activity
2.3. Preparation of the Beef Patties
2.4. Proximate Composition
2.5. Determination of pH and Color of the Beef Patties
2.6. Fatty Acid Profile
2.7. Lipid Oxidation (TBARs)
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenol Content and Antioxidant Activity of Maqui Leaf Powder
3.2. Proximate Composition
3.3. pH and Color
3.4. Lipid Oxidation
3.5. Fatty Acid Profile
3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Recent Research Advances in Meat Products. Foods 2021, 10, 1303. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of Oils Using Hydrogels as Strategy to Replace Animal Fats and Improve the Healthiness of Meat Products. Curr. Opin. Food Sci. 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Domínguez, R.; Bohrer, B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Recent Discoveries in the Field of Lipid Bio-Based Ingredients for Meat Processing. Molecules 2021, 26, 190. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of Tiger Nut (Cyperus esculentus L.) Oil Emulsion as Animal Fat Replacement in Beef Burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy Beef Burgers: Effect of Animal Fat Replacement by Algal and Wheat Germ Oil Emulsions. Meat Sci. 2021, 173, 108396. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Vegetable Oil as Fat Replacer Inhibits Formation of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Reduced Fat Pork Patties. Food Control 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastião, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat Replacement by Oleogel Rich in Oleic Acid and Its Impact on the Technological, Nutritional, Oxidative, and Sensory Properties of Bologna-Type Sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef]
- Botella-Martinez, C.; Lucas-González, R.; Lorenzo, J.M.; Santos, E.M.; Rosmini, M.; Sepúlveda, N.; Teixeira, A.; Sayas-Barberá, E.; Pérez-Alvarez, J.A.; Fernandez-Lopez, J.; et al. Cocoa Coproducts-Based and Walnut Oil Gelled Emulsion as Animal Fat Replacer and Healthy Bioactive Source in Beef Burgers. Foods 2021, 10, 2706. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, F.A.L.; Munekata, P.E.S.; Lopes de Oliveira, A.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) Extract on Oxidative Stability, Physicochemical and Sensory Properties of Fresh Lamb Sausage with Fat Replacement by Tiger Nut (Cyperus esculentus L.) Oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries Extracts as Natural Antioxidants in Meat Products: A Review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of Pitanga Leaf Extracts on Lipid and Protein OxidaInfluencetion of Pork Burger during Shelf-Life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Jamwal, A.; Kumar, S.; Bhat, Z.F.; Kumar, A.; Kaur, S. Nutrition & Food Science Article Information. Nutr. Food Sci. 2015, 45, 39–53. [Google Scholar]
- Dua, S.; Bhat, Z.F.; Kumar, S. Effect of Oleuropein on the Oxidative Stability and Storage Quality of Tabaq-Maz, Fried Mutton Ribs; Elsevier: Amsterdam, The Netherlands, 2015; Volume 12, ISBN 9188036189. [Google Scholar]
- Kaur, S.; Kumar, S.; Bhat, Z.F.; Kumar, A. Effect of Pomegranate Seed Powder, Grape Seed Extract and Tomato Powder on the Quality Characteristics of Chicken Nuggets. Nutr. Food Sci. 2015, 45, 583–594. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.; Bekhit, A.E.D.A.; Bhat, H.F. Obesity and Neurological Disorders: Dietary Perspective of a Global Menace. Crit. Rev. Food Sci. Nutr. 2019, 59, 1294–1310. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; da Silva Barretto, A.C. Red Pitaya Extract as Natural Antioxidant in Pork Patties with Total Replacement of Animal Fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana Seed Extracts as a Useful Strategy to Extend the Shelf Life of Pork Patties: UHPLC-ESI/QTOF Phenolic Profile and Impact on Microbial Inactivation, Lipid and Protein Oxidation and Antioxidant Capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of Guarana (Paullinia cupana) Seed and Pitanga (Eugenia uniflora L.) Leaf Extracts on Lamb Burgers with Fat Replacement by Chia Oil Emulsion during Shelf Life Storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Bravo, S.; Inostroza, K.; Lorenzo, J.M.; Sepúlveda, G.; Domínguez, R.; Scheuermann, E.; Paz, E.A.; Quiñones, J.; Santos, E.M.; Andrés, S.C.; et al. Influence of Murta (Ugni molinae Turcz) Powder on the Frankfurters Quality. Appl. Sci. 2021, 11, 8610. [Google Scholar] [CrossRef]
- Kalem, I.K.; Bhat, Z.F.; Kumar, S.; Wang, L.; Mudiyanselage, R.J.; Bhat, H.F. Tinospora Cordifolia: A Novel Bioactive Ingredient for Edible Films for Improved Lipid Oxidative and Microbial Stability of Meat Products. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Velázquez, L.; Quiñones, J.; Díaz, R.; Pateiro, M.; Lorenzo, J.M.; Sepúlveda, N. Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status. Antioxidants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Ipinza Carmona, R.; Magni Díaz, C.; Gutiérrez Caro, B.; Torres Cuadros, J. La Domesticación Del Maqui (Aristotelia chilensis): Un Estudio de Caso en Chile. INFOR 2019, 19–24. [Google Scholar]
- López, J.; Vera, C.; Bustos, R.; Florez-Mendez, J. Native Berries of Chile: A Comprehensive Review on Nutritional Aspects, Functional Properties, and Potential Health Benefits. J. Food Meas. Charact. 2021, 15, 1139–1160. [Google Scholar] [CrossRef]
- Vidal, J.L.; Avello, L.M.; Loyola, C.C.; Campos, P.J.; Aqueveque, M.P.; Dungan, R.S.; Galotto, L.M.; Guarda, M.A. Microencapsulation of Maqui (Aristotelia chilensis [Molina] Stuntz) Leaf Extracts to Preserve and Control Antioxidant Properties. Chil. J. Agric. Res. 2013, 73, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red Beetroot. A Potential Source of Natural Additives for the Meat Industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; López, E.M.S.; Rodríguez, J.A.; Barros, L.; Lorenzo, J.M. Potential Use of Elderberry (Sambucus nigra L.) as Natural Colorant and Antioxidant in the Food Industry. A Review. Foods 2021, 10, 2713. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols And. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 1442; International Standards Meat and Meat Products—Determination of Moisture Content. International Organization for Standarization: Geneva, Switzerland, 1997.
- ISO 936; International Standards Meat and Meat Products—Determination of Ash Content. International Organization for Standarization: Geneva, Switzerland, 1998.
- Folch, J.; Lees, M.; Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Vyncke, W. Evaluation of the Direct Thiobarbituric Acid Extraction Method for Determining Oxidative Rancidity in Mackerel. Fette Seifen Anstrichm. 1975, 77, 239–240. [Google Scholar] [CrossRef]
- NCh-ISO6658:2016; Análisis Sensorial de Alimentos—Metodología—Guía General. Instituto Nacional de Normalizacion: Santiago, Chile, 2016; pp. 1–27.
- Resconi, V.C.; Pascual-Alonso, M.; Aguayo-Ulloa, L.; Miranda-De La Lama, G.C.; Alierta, S.; Campo, M.M.; Olleta, J.L.; Villarroel, M.; María, G.A. Effect of Dietary Grape Pomace and Seed on Ewe Milk and Meat Quality of Their Suckling Lambs. J. Food Qual. 2018, 2018, 2371754. [Google Scholar] [CrossRef] [Green Version]
- Vaissie, P.; Monge, A.; Husson, F. Factoshiny: Perform Factorial Analysis from “FactoMineR” with a Shiny Application. R Package Version 2.4. Available online: https://CRAN.R-project.org/package=Factoshiny (accessed on 1 June 2022).
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and r-Glucosidase/r-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Yalcin, H.; Çapar, T.D. Bioactive Compounds of Fruits and Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer Science + Business Media LLC: New York, NY, USA, 2017; pp. 723–745. ISBN 978-1-4939-7016-2. [Google Scholar]
- Water, E.; Phenolic, M.; Activities, A.; Barba, F.J.; Alc, C.; Abdelkebir, R.; Bäuerl, C.; Gaspar, P.; Lorenzo, J.M.; Garc, J.V. Ultrasonically-Assisted and Conventional Extraction. Molecules 2020, 25, 1759. [Google Scholar]
- Rubilar, M.; Pinelo, M.; Ihl, M.; Scheuermann, E.; Sineiro, J.; Nuñez, M.J. Murta Leaves (Ugni Molinae Turcz) as a Source of Antioxidant Polyphenols. J. Agric. Food Chem. 2006, 54, 59–64. [Google Scholar] [CrossRef]
- Muñoz, O.; Christen, P.; Cretton, S.; Backhouse, N.; Torres, V.; Correa, O.; Costa, E.; Miranda, H.; Delporte, C. Chemical Study and Anti-Inflammatory, Analgesic and Antioxidant Activities of the Leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011, 63, 849–859. [Google Scholar] [CrossRef]
- Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; del Mar Torres-Martínez, B.; Torrescano-Urrutia, G.R.; Lorenzo, J.M.; Sánchez-Escalante, A. Inclusion of Ethanol Extract of Mesquite Leaves to Enhance the Oxidative Stability of Pork Patties. Foods 2019, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Boruzi, A.I.; Nour, V. Walnut (Juglans regia L.) Leaf Powder as a Natural Antioxidant in Cooked Pork Patties. CyTA J. Food 2019, 17, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.J.; Kim, H.J.; Lee, N.; Lee, C.H. Characterization of Pork Patties Containing Dry Radish (Raphanus sativus) Leaf and Roots. Asian-Australas. J. Anim. Sci. 2019, 32, 413–420. [Google Scholar] [CrossRef]
- Elhadi, D.A.E.; Elgasim, E.A.; Mohamed Ahmed, I.A. Microbial and Oxidation Characteristics of Refrigerated Chicken Patty Incorporated with Moringa (Moringa oleifera) Leaf Powder. CyTA J. Food 2016, 15, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of Hull, Bur and Leaf Chestnut Extracts on the Shelf-Life of Beef Patties Stored under MAP: Evaluation of Their Impact on Physicochemical Properties, Lipid Oxidation, Antioxidant, and Antimicrobial Potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, S.; Miranda-Romero, L.A.; Fujikawa, H.; de Jesús Maldonado-Simán, E.; Alarcón-Zuñiga, B. Antibacterial Activity of Lactic Acid Bacteria to Improve Shelf Life of Raw Meat. Biocontrol Sci. 2019, 24, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GILL, C.O. Meat Spoilage and Evaluation of the Potential Storage Life of Fresh Meat. J. Food Prot. 1983, 46, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef]
- Oyagüe, M. Estabilidad Del Color de La Carne Fresca. Nacameh 2007, 1, 67–74. [Google Scholar]
- Tomasevic, I.; Djekic, I.; Font-i-Furnols, M.; Terjung, N.; Lorenzo, J.M. Recent Advances in Meat Color Research. Curr. Opin. Food Sci. 2021, 41, 81–87. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic Extracts of Cherry (Prunus cerasus L.) and Blackcurrant (Ribes nigrum L.) Leaves as Natural Preservatives in Meat Products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Choe, J.H.; Jang, A.; Lee, E.S.; Choi, J.H.; Choi, Y.S.; Han, D.J.; Kim, H.Y.; Lee, M.A.; Shim, S.Y.; Kim, C.J. Oxidative and Color Stability of Cooked Ground Pork Containing Lotus Leaf (Nelumbo nucifera) and Barley Leaf (Hordeum vulgare) Powder during Refrigerated Storage. Meat Sci. 2011, 87, 12–18. [Google Scholar] [CrossRef]
- Faustman, C.; Cassens, R.G. The Biochemical Basis for Discoloration in Fresh Meat: A Review. J. Muscle Foods 1990, 1, 217–243. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Ambrosiadis, I.; Fletouris, D.; Botsoglou, N. Effect of Olive Leaf (Olea europea L.) Extracts on Protein and Lipid Oxidation of Long-Term Frozen n-3 Fatty Acids-Enriched Pork Patties. Meat Sci. 2014, 98, 150–157. [Google Scholar] [CrossRef]
- Shin, D.-J.; Choe, J.; Hwang, K.-E.; Kim, C.-J.; Jo, C. Antioxidant Effects of Lotus (Nelumbo nucifera) Root and Leaf Extracts and Their Application on Pork Patties as Inhibitors of Lipid Oxidation, Alone and in Combination. Int. J. Food Prop. 2019, 22, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Markovi, Z.; Djorovic, J.; Markovic, J.M.D.; Biocanin, R.; Amic, D. Comparative Density Functional Study of Antioxidative Activity of the Hydroxybenzoic Acids and Their Anions. Turk. J. Chem. 2016, 40, 499–509. [Google Scholar] [CrossRef]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the Antiradical Properties of Phenolic Acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, F.D.; Sinclair, A.J.; Mann, N.J.; Turner, A.H.; Abedin, L.; Li, D. A Stearic Acid-Rich Diet Improves Thrombogenic and Atherogenic Risk Factor pro® Les in Healthy Males. Eur. J. Clin. Nutr. 2001, 55, 88–96. [Google Scholar]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M.; Childs, L. Cardiovascular Disease Risk of Dietary Stearic Acid Compared with Trans, Other Saturated, and Unsaturated Fatty Acids: A Systematic Review. Am. J. Clin. Nutr. 2010, 91, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Ulbricht & Southgate Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar]
- Czernichow, S.; Thomas, D.; Bruckert, E. N-6 Fatty Acids and Cardiovascular Health: A Review of the Evidence for Dietary Intake Recommendations. Br. J. Nutr. 2010, 104, 788–796. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, M.; Naveena, B.M.; Vaithiyanathan, S.; Sen, A.R.; Sureshkumar, K. Effect of Incorporation of Moringa Oleifera Leaves Extract on Quality of Ground Pork Patties. J. Food Sci. Technol. 2014, 51, 3172–3180. [Google Scholar] [CrossRef] [Green Version]


| Polyphenol content | |
| Total phenolic content (TPC)* (mg GAE/g dm) | 148.76 ± 19.03 |
| Hydroxybenzoic acids (mg GAE/g) 1 | 82.5 ± 3.11 |
| Hydroxycinnamic acids (mg CAE/g) 2 | 3.65 ± 0.07 |
| Flavonoids (mg QE/g) 3 | 7.1 ± 0.04 |
| Antioxidant activity | |
| DPPH•(%) | |
| 325 µg/mL | 94 ± 0.21 |
| 155 µg/mL | 82 ± 0.57 |
| 10 µg/mL | 15 ± 0.88 |
| IC50 DPPH (µg/mL) | |
| Treatment | Moisture (%) | Fat (%) | Ash (%) |
|---|---|---|---|
| CT | 63.22 ± 1.58 | 22.50 ± 0.71 | 2.23 ± 0.41 |
| PL | 61.10 ± 1.66 | 22.00 ± 0.50 | 2.30 ± 0.11 |
| Ma500 | 64.25 ± 3.82 | 21.50 ± 0.71 | 1.91 ± 0.23 |
| Ma1000 | 61.39 ± 1.76 | 22.50 ± 0.72 | 1.78 ± 0.10 |
| Ma2000 | 63.16 ± 2.74 | 21.50 ± 0.70 | 2.10 ± 0.12 |
| Parameter | Treatment | Storage Time | |
|---|---|---|---|
| Day 0 | Day 7 | ||
| pH | CT | 5.50 ± 0.08 a1 | 5.65 ± 0.07 a2 |
| PL | 5.76 ± 0.01 b1 | 5.27 ± 0.07 b2 | |
| Ma500 | 5.73 ± 0.01 b1 | 5.33 ± 0.05 c2 | |
| Ma1000 | 5.62 ± 0.13 a1 | 5.30 ± 0.03 d2 | |
| Ma2000 | 5.75 ± 0.07 b1 | 5.39 ± 0.06 e2 | |
| Color | |||
| a* | CT | 23.70 ± 1.28 a1 | 7.62 ± 0.64 a2 |
| PL | 23.55 ± 2.65 a1 | 8.33 ± 0.96 a2 | |
| Ma500 | 22.90 ± 2.02 a1 | 6.78 ± 1.30 a2 | |
| Ma1000 | 19.15 ± 3.43 b1 | 6.12 ± 0.82 b2 | |
| Ma2000 | 18.45 ± 1.46 c1 | 4.77 ± 1.50 c2 | |
| b* | CT | 20.30 ± 1.23 a | 18.62 ± 2.42 a |
| PL | 19.97 ± 2.31 a | 18.08 ± 2.42 a | |
| Ma500 | 18.72 ± 1.08 a1 | 13.88 ± 1.28 b2 | |
| Ma1000 | 19.35 ± 1.68 a1 | 12.68 ± 1.45 b2 | |
| Ma2000 | 18.05 ± 2.36 a1 | 11.15 ± 1.42 b2 | |
| L* | CT | 45.80 ± 2.17 a | 42.47 ± 2.84 a |
| PL | 47.58 ± 4.82 a | 42.35 ± 2.05 a | |
| Ma500 | 42.45 ± 1.78 a1 | 34,72 ± 1.66 b2 | |
| Ma1000 | 46.07 ± 5.76 a1 | 34.12 ± 2.61 b2 | |
| Ma2000 | 43.60 ± 2.96 a1 | 34.83 ± 3.08 b2 | |
| Day 0 | Day 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FA (%) | C | PL | Ma500 | Ma1000 | Ma2000 | C | PL | Ma500 | Ma1000 | Ma2000 |
| C14:0 | 1.55 ± 0.05 a1 | 1.60 ± 0.00 bc1 | 1.63 ± 0.00 c1 | 1.64 ± 0.02 c1 | 1.57 ± 0.00 ab1 | 1.49 ± 0.00 a2 | 1.53 ± 0.00 b2 | 1.58 ± 0.00 c2 | 1.62 ± 0.00 e1 | 1.60 ± 0.00 d2 |
| C14:1 n − 7 | 0.16 ± 0.00 a1 | 0.15 ± 0.00 a1 | 0.15 ± 0.00 b1 | 0.14 ± 0.00 b1 | 0.12 ± 0.07 c1 | 0.15 ± 0.00 a2 | 0.14 ± 0.00 b2 | 0.13 ± 0.00 b2 | 0.14 ± 0.00 b1 | 0.14 ± 0.00 b2 |
| C16:0 | 21.95 ± 0.04 a1 | 22.26 ± 0.02 ab1 | 22.81 ± 0.01 b1 | 22.81 ± 0.33 b1 | 22.59 ± 0.06 b1 | 22.61 ± 0.05 a2 | 22.79 ± 0.01 ab2 | 22.97 ± 0.01 bc2 | 23.11 ± 0.11 c1 | 23.87 ± 0.09 d2 |
| C16:1 n − 7 | 3.15 ± 0.00 a1 | 3.18 ± 0.04 a1 | 2.91 ± 0.00 b1 | 3.11 ± 0.04 a1 | 2.77 ± 0.02 c1 | 2.98 ± 0.01 a2 | 2.98 ± 0.01 a2 | 2.83 ± 0.00 b2 | 2.98 ± 0.03 a1 | 2.68 ± 0.00 c2 |
| C17:0 | 0.48 ± 0.00 a1 | 0.47 ± 0.04 a1 | 0.42 ± 0.00 ab1 | 0.46 ± 0.00 ab1 | 0.38 ±0.03 b1 | 0.48 ± 0.00 a2 | 0.41 ± 0.00 b1 | 0.41 ± 0.00 b2 | 0.44 ± 0.03 ab1 | 0.44 ± 0.01 ab1 |
| C17:1 n − 7 | 0.45 ± 0.00 a1 | 0.45 ± 0.00 a1 | 0.35 ± 0.00 b1 | 0.36 ± 0.00 b1 | 0.31 ± 0.00 c1 | 0.43 ± 0.00 a2 | 0.41 ± 0.00 b2 | 0.34 ± 0.00 c2 | 0.34 ± 0.00 c2 | 0.35 ± 0.00 c2 |
| C18:0 | 12.97 ± 0.06 a1 | 13.15 ± 0.02 a1 | 14.27 ± 0.02 c1 | 13.80 ± 0.19 b1 | 14.14 ± 0.01 bc1 | 13.24 ± 0.01 a2 | 13.21 ± 0.01 a1 | 14.18 ± 0.00 b2 | 14.19 ± 0.03 b1 | 14.98 c ± 0.05 c2 |
| C18:1 n − 9 | 44.07 ± 0.21 ab1 | 44.24 ± 0.01 a1 | 43.15 ± 0.00 bc1 | 42.67 ± 0.52 c1 | 43.48 ± 0.01 abc1 | 44.03 ± 0.05 a1 | 44.10 ± 0.02 a2 | 42.88 ± 0.03 b2 | 42.37 ± 0.16 c1 | 42.65 ± 0.19 bc2 |
| 9 t-C18:1 | 0.20 ± 0.00 a1 | 0.21 ± 0.00 b1 | 0.16 ± 0.00 c1 | 0.17 ± 0.00 d1 | 0.15 ± 0.07 e1 | 0.19 ± 0.00 a2 | 0.20 ± 0.00 a2 | 0.16 ± 0.00 b2 | 0.15 ± 0.01 b1 | 0.17 ± 0.01 ab1 |
| C18:2 n − 6 | 11.93 ± 0.25 a1 | 11.32 ± 0.00 b1 | 11.22 ± 0.02 b1 | 11.88 ± 0.11 a1 | 11.68 ± 0.04 ab1 | 11.54 ± 0.00 bc1 | 11.40 ± 0.05 b1 | 11.71 ± 0.05 cd2 | 11.77 ± 0.08 d1 | 10.31 ± 0.03 a2 |
| C18:3 n − 6 | 0.21 ± 0.00 a1 | 0.20 ± 0.00 ab1 | 0.20 ± 0.00 bc1 | 0.19 ± 0.00 c1 | 0.21 ± 0.00 a1 | 0.21 ± 0.00 a2 | 0.21 ± 0.00 b2 | 0.20 ± 0.00 c2 | 0.20 ± 0.00 c1 | 0.22 ± 0.00 a2 |
| C20:0 | 0.67 ± 0.00 a1 | 0.59 ± 0.01 b1 | 0.59 ± 0.02 b1 | 0.64 ± 0.01 ab1 | 0.61 ± 0.00 b1 | 0.62 ± 0.00 a2 | 0.58 ± 0.00 c1 | 0.60 ± 0.00 b1 | 0.61 ± 0.00 a1 | 0.54 ± 0.00 d2 |
| C18:3 n − 3 | 0.71 ± 0.05 a1 | 0.69 ± 0.05 a1 | 0.60 ± 0.00 a1 | 0.59 ± 0.01 a1 | 0.64 ± 0.00 a1 | 0.66 ± 0.00 a1 | 0.65 ± 0.00 b1 | 0.61 ± 0.00 c2 | 0.57 ± 0.00 d1 | 0.58 ± 0.00 d2 |
| CLA | 0.13 ± 0.01 a1 | 0.11 ± 0.01 ab1 | 0.12 ± 0.00 ab1 | 0.12 ± 0.00 ab1 | 0.11 ± 0.00 b1 | 0.12 ± 0.00 b1 | 0.11 ± 0.00 a1 | 0.11 ± 0.00 a2 | 0.13 ± 0.00 c2 | 0.13 ± 0.00 c2 |
| C21:0 | 0.52 ± 0.00 a1 | 0.50 ± 0.00 b1 | 0.48 ± 0.00 c1 | 0.49 ± 0.00 bc1 | 0.50 ± 0.00 b1 | 0.51 ± 0.00 a1 | 0.50 ± 0.00 a1 | 0.50 ± 0.00 a2 | 0.49 ± 0.00 a1 | 0.50 ± 0.00 a1 |
| C20:2 n − 6 | 0.11 ± 0.00 a1 | 0.11 ± 0.00 a1 | 0.12 ± 0.00 b1 | 0.12 ± 0.00 b1 | 0.11 ± 0.00 a1 | 0.10 ± 0.00 a2 | 0.11 ± 0.00 b2 | 0.11 ± 0.00 c2 | 0.12 ± 0.00 c2 | 0.11 ± 0.00 bc1 |
| C22:0 | 0.35 ± 0.06 ab1 | 0.38 ± 0.00 ab1 | 0.40 ± 0.00 b1 | 0.41 ± 0.01 b1 | 0.29 ± 0.00 a1 | 0.28 ± 0.01 a1 | 0.33 ± 0.05 ab1 | 0.29 ± 0.00 ab2 | 0.38 ± 0.00 b2 | 0.36 ± 0.00 ab2 |
| C20:5 n − 3 | 0.10 ± 0.00 a1 | 0.10 ± 0.00 a1 | 0.10 ± 0.00 a1 | 0.10 ± 0.00 a1 | 0.09 ± 0.00 b1 | 0.09 ± 0.00 ab2 | 0.10 ± 0.00 bc1 | 0.10 ± 0.00 bc2 | 0.10 ± 0.00 c1 | 0.10 ± 0.00 a1 |
| C24:1 n − 9 | 0.13 ± 0.01 a1 | 0.12 ± 0.01 a1 | 0.13 ± 0.00 a1 | 0.13 ± 0.01 a1 | 0.12 ± 0.00 a1 | 0.13 ± 0.00 b1 | 0.11 ± 0.00 a1 | 0.11 ± 0.00 a2 | 0.12 ± 0.00 ab2 | 0.11 ± 0.00 a1 |
| SFA | 38.55 ± 0.02 a1 | 39.01 ± 0.02 a1 | 40.67 ± 0.01 b1 | 40.30 ± 0.57 b1 | 40.13 ± 0.04 b1 | 39.27 ± 0.05 a2 | 39.40 ± 0.05 a2 | 40.60 ± 0.01 b2 | 40.90 ± 0.11 b1 | 42.36 ± 0.13 c2 |
| MUFA | 48.16 ± 0.21 a1 | 48.35 ± 0.04 a1 | 46.86 ± 0.01 b1 | 46.58 ± 0.48 b1 | 46.94 ± 0.01 b1 | 47.90 ± 0.04 a1 | 47.94 ± 0.01 a2 | 46.46 ± 0.03 b2 | 46.10 ± 0.20 b1 | 46.10 ± 0.18 b2 |
| PUFA | 13.30 ± 0.19 a1 | 12.63 ± 0.06 bc1 | 12.47 ± 0.02 c | 13.13 ± 0.09 a1 | 12.93 ± 0.03 ab1 | 12.83 ± 0.01 bc1 | 12.66 ± 0.05 b1 | 12.94 ± 0.04 c2 | 13.01 ± 0.09 c1 | 11.55 ± 0.04 a2 |
| n − 3 | 0.84 ± 0.06 a1 | 0.82 ± 0.05 a1 | 0.74 ± 0.00 a1 | 0.73 ± 0.01 a1 | 0.76 ± 0.01 a1 | 0.79 ± 0.00 a1 | 0.78 ± 0.00 a1 | 0.75 ± 0.00 b2 | 0.71 ± 0.01 c1 | 0.71 ± 0.00 c2 |
| n − 6 | 12.32 ± 0.25 a1 | 11.70 ± 0.01 b1 | 11.61 ± 0.02 b1 | 12.28 ± 0.10 a1 | 12.06 ± 0.04 ab1 | 11.92 ± 0.01 bc1 | 11.77 ± 0.05 b1 | 12.09 ± 0.04 cd2 | 12.16 ± 0.08 d1 | 10.71 ± 0.04 a2 |
| n − 6/n − 3 | 14.67 ± 1.25 a1 | 14.30 ± 0.93 a1 | 15.63 ± 0.01 a1 | 16.79 ± 0.41 a1 | 15.79 ± 0.17 a1 | 15.14 ± 0.01 a1 | 15.11 ± 0.09 a1 | 16.20 ± 0.07 b2 | 17.10 ± 0.01 c1 | 15.19 ± 0.04 a2 |
| AI | 0.46 ± 0.00 a | 0.47 ± 0.00 ab | 0.50 ± 0.00 c | 0.49 ± 0.01 c | 0.48 ± 0.00 bc | 0.47 ± 0.00 a2 | 0.48 ± 0.00 a2 | 0.50 ± 0.00 b2 | 0.50 ± 0.00 b1 | 0.53 ± 0.00 c2 |
| TI | 1.11 ± 0.01 a | 1.14 ± 0.00 a | 1.23 ± 0.00 b | 1.21 ± 0.03 b | 1.20 ± 0.00 b | 1.15 ± 0.00 a2 | 1.16 ± 0.00 a2 | 1.23 ± 0.00 b1 | 1.24 ± 0.01 b1 | 1.32 ± 0.01 c2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez, L.; Quiñones, J.; Inostroza, K.; Sepúlveda, G.; Díaz, R.; Scheuermann, E.; Domínguez, R.; Lorenzo, J.M.; Velásquez, C.; Sepúlveda, N. Maqui (Aristotelia chilensis (Mol.) Stuntz): A Natural Antioxidant to Improve Quality of Meat Patties. Antioxidants 2022, 11, 1405. https://doi.org/10.3390/antiox11071405
Velázquez L, Quiñones J, Inostroza K, Sepúlveda G, Díaz R, Scheuermann E, Domínguez R, Lorenzo JM, Velásquez C, Sepúlveda N. Maqui (Aristotelia chilensis (Mol.) Stuntz): A Natural Antioxidant to Improve Quality of Meat Patties. Antioxidants. 2022; 11(7):1405. https://doi.org/10.3390/antiox11071405
Chicago/Turabian StyleVelázquez, Lidiana, John Quiñones, Karla Inostroza, Gastón Sepúlveda, Rommy Díaz, Erick Scheuermann, Rubén Domínguez, José M. Lorenzo, Carla Velásquez, and Néstor Sepúlveda. 2022. "Maqui (Aristotelia chilensis (Mol.) Stuntz): A Natural Antioxidant to Improve Quality of Meat Patties" Antioxidants 11, no. 7: 1405. https://doi.org/10.3390/antiox11071405