Paeonol Attenuates Hepatic Ischemia/Reperfusion Injury by Modulating the Nrf2/HO-1 and TLR4/MYD88/NF-κB Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Animals
2.3. Experimental Protocol
2.4. Induction of HIR
2.5. Samples Preparation
2.6. Liver Function Tests
2.7. Oxidative Stress Parameters
2.8. Real-Time Polymerase Chain Reaction (PCR)
2.9. Histopathological Examination and Scoring
2.10. Immunohistochemical Examination and Scoring
2.11. Statistical Analysis
3. Results
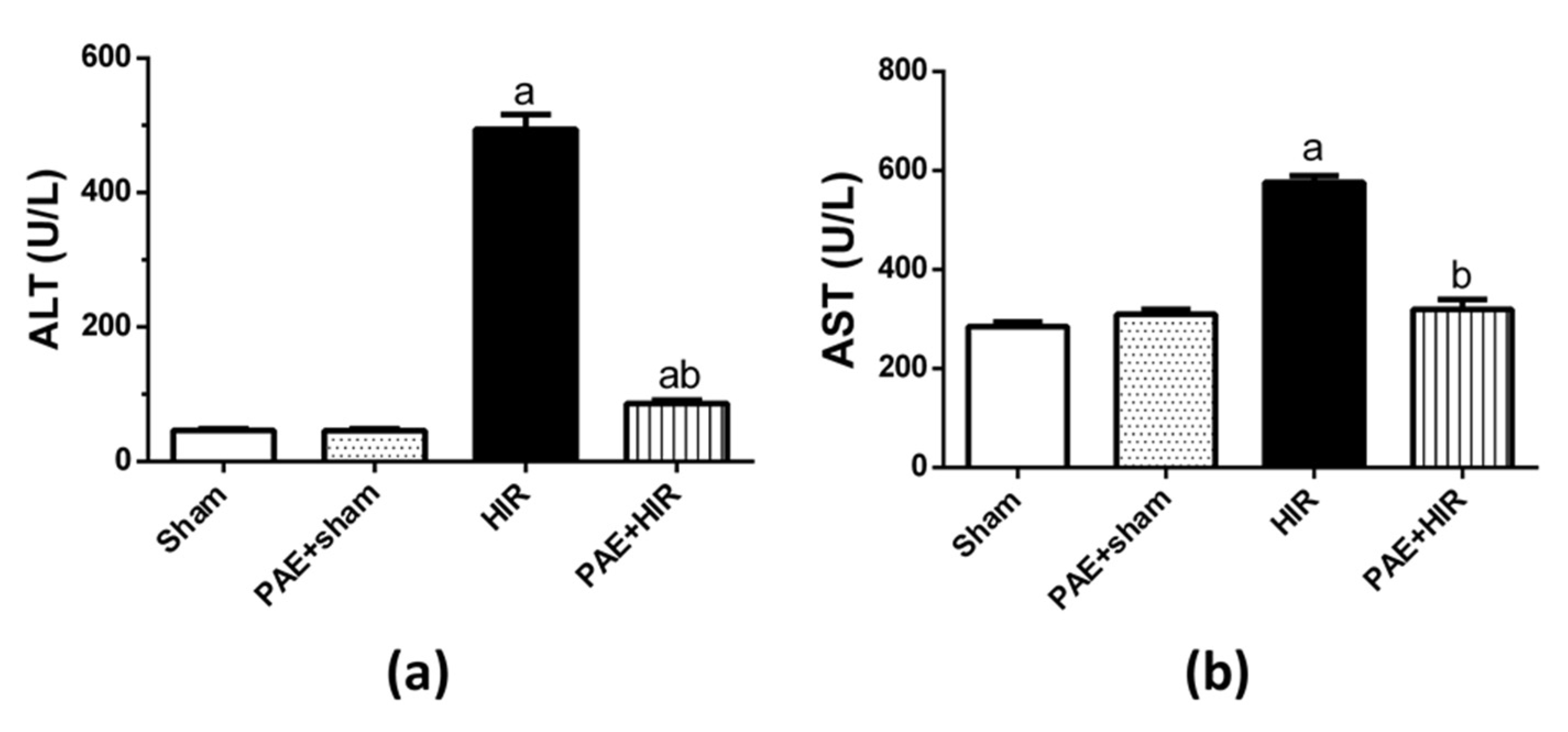
3.1. Effect of Paeonol on Serum ALT and AST
3.2. Effect of Paeonol on Hepatic Histopathology
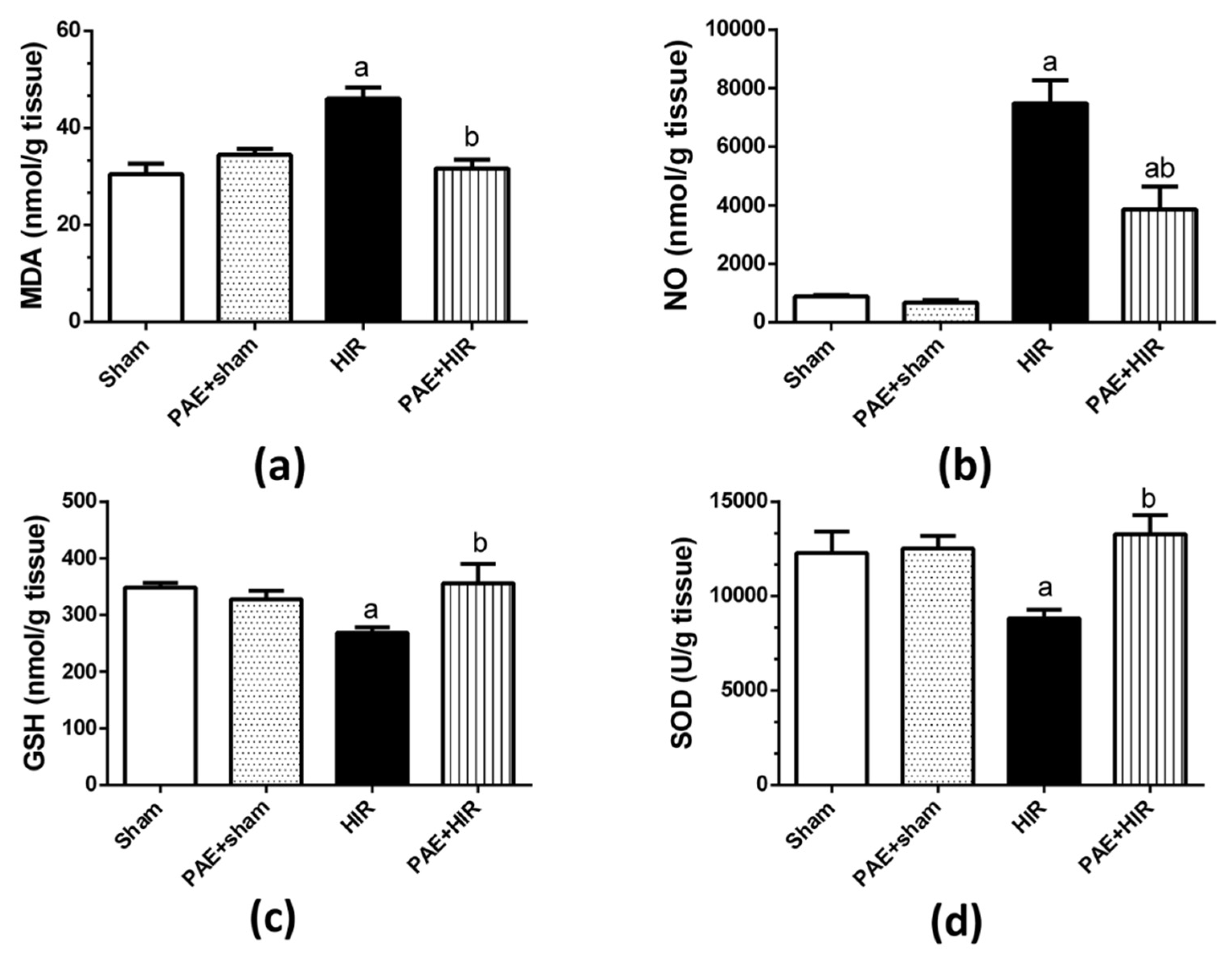
3.3. Effect of Paeonol on Oxidative Parameters and Antioxidant Profile
3.3.1. Effect of Paeonol on Hepatic MDA, NO, GSH, and SOD
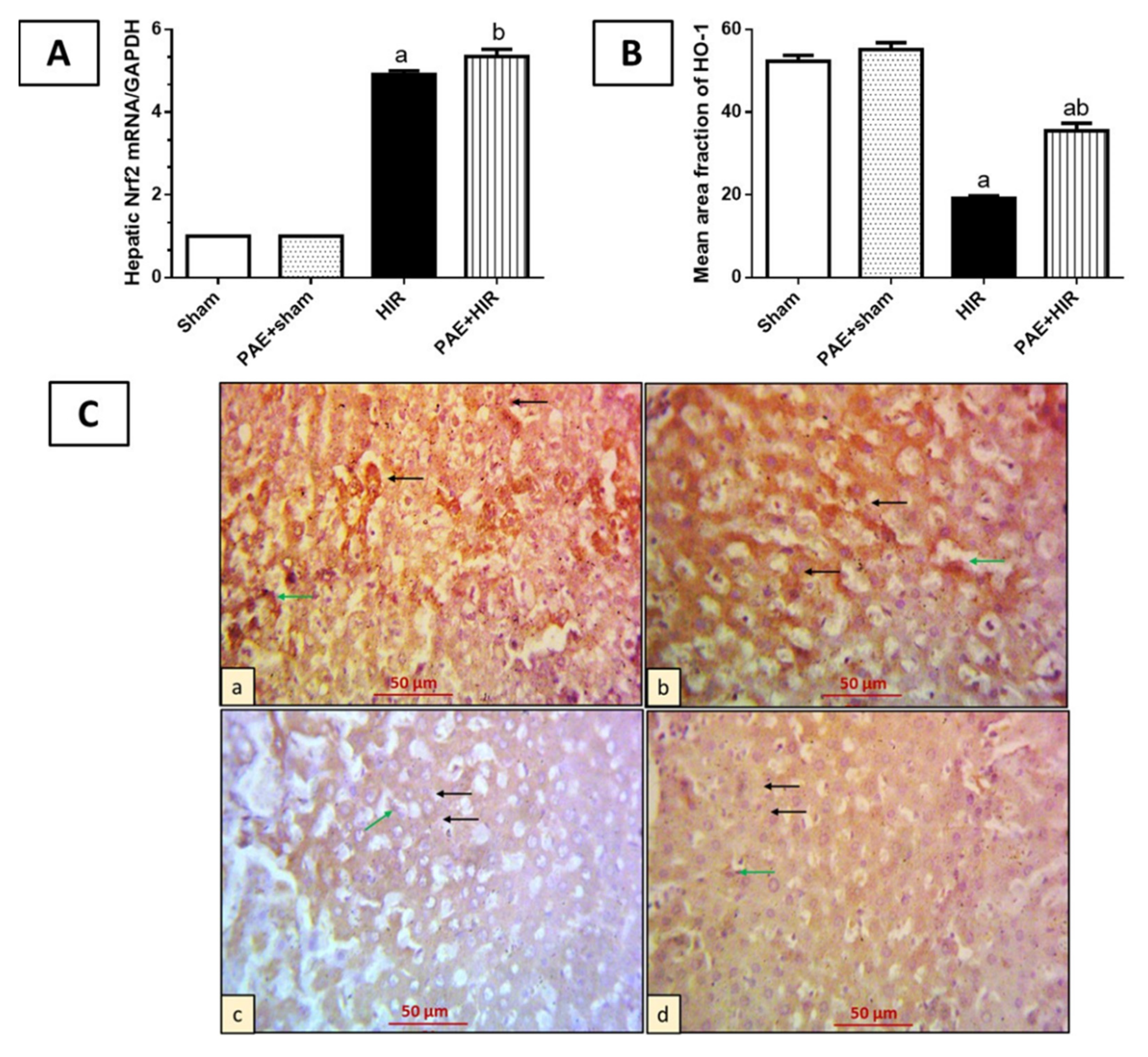
3.3.2. Effect of Paeonol on Nrf2/HO-1 Pathway
3.4. Effect of Paeonol on Hepatic Inflammatory Mediators
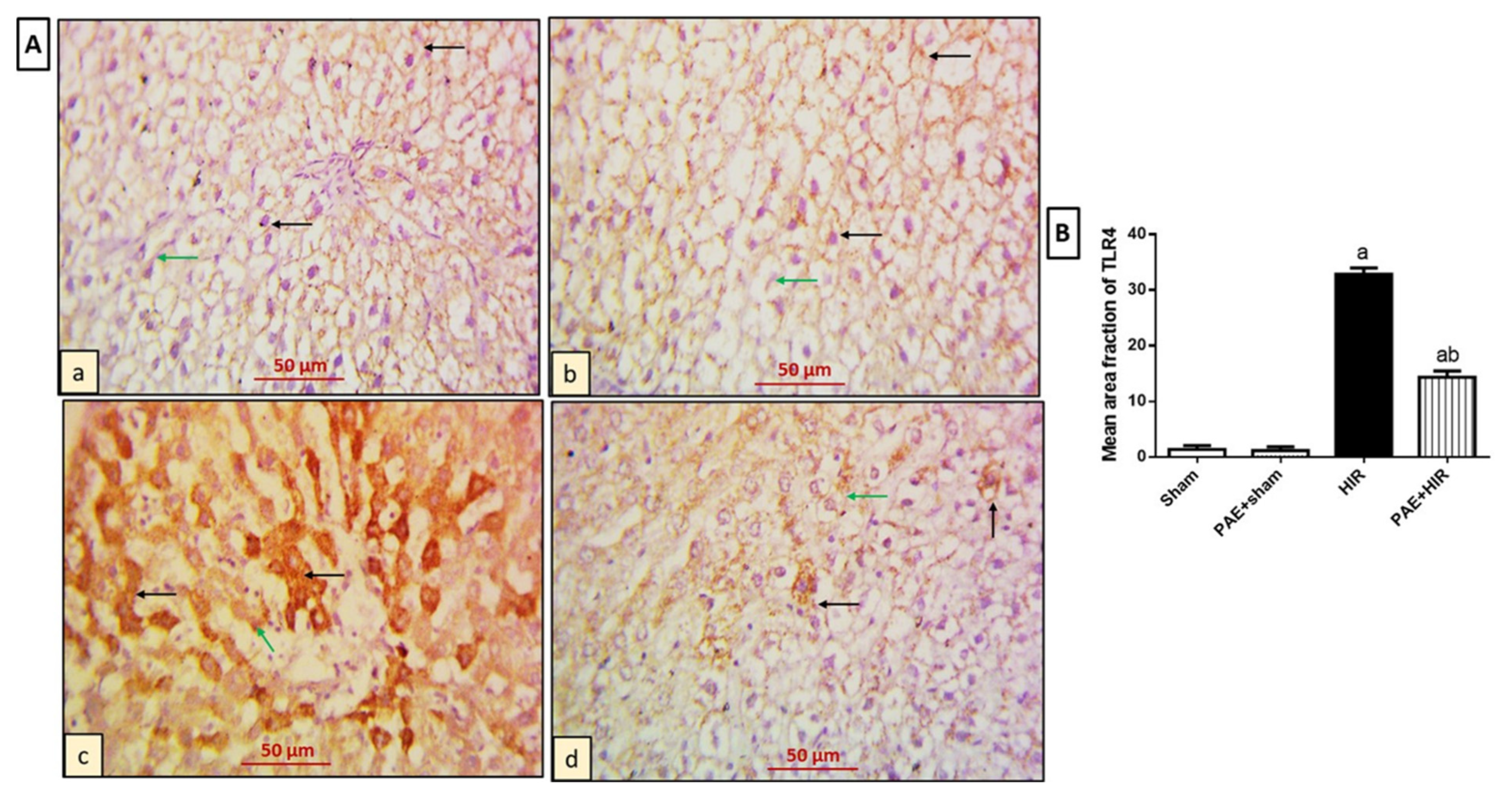
3.4.1. Effect of Paeonol on Hepatic TLR4 and MYD88
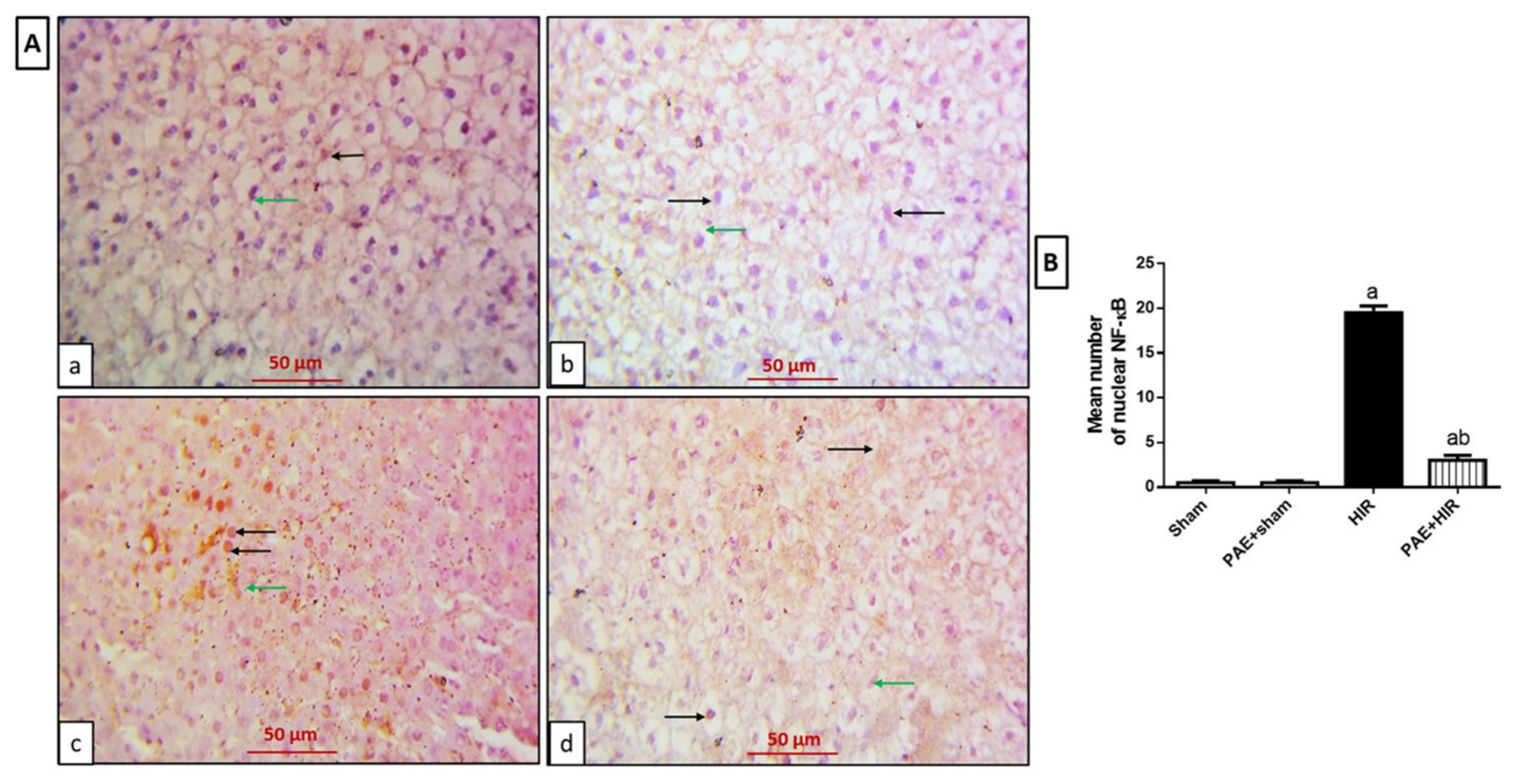
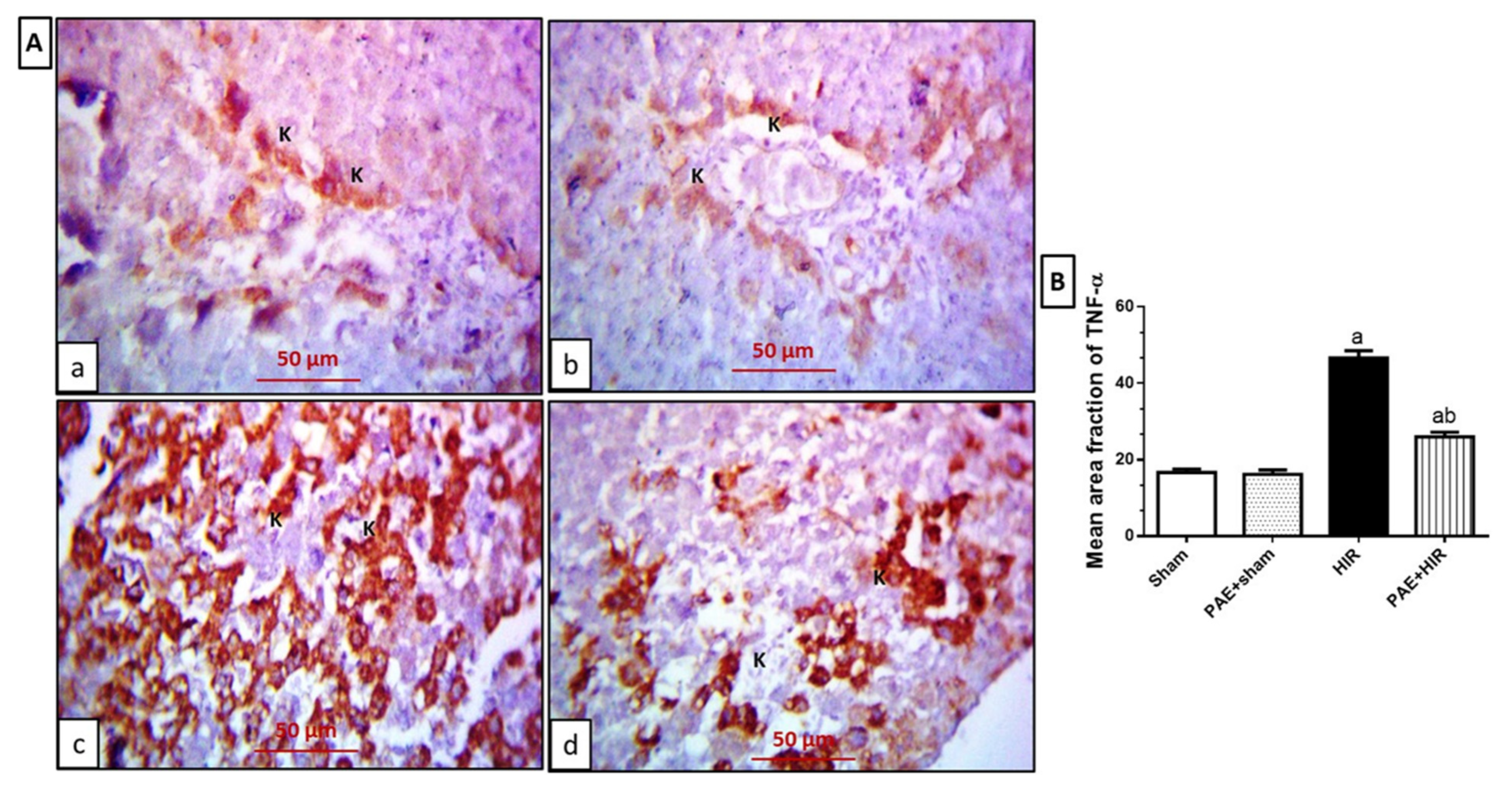
3.4.2. Effect of Paeonol on Hepatic NF-κB and TNF-α
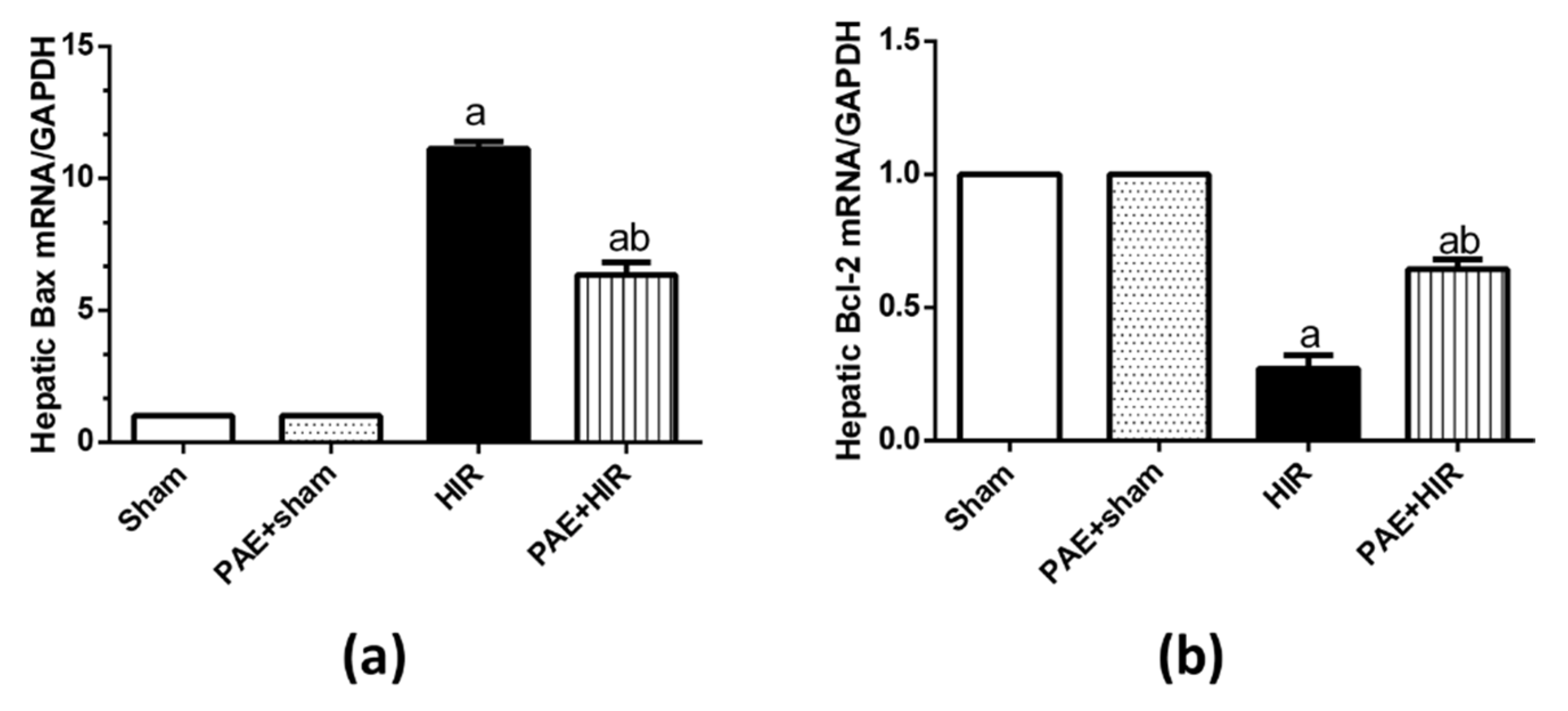
3.5. Effect of Paeonol on Apoptosis in HIR Injury in Rats
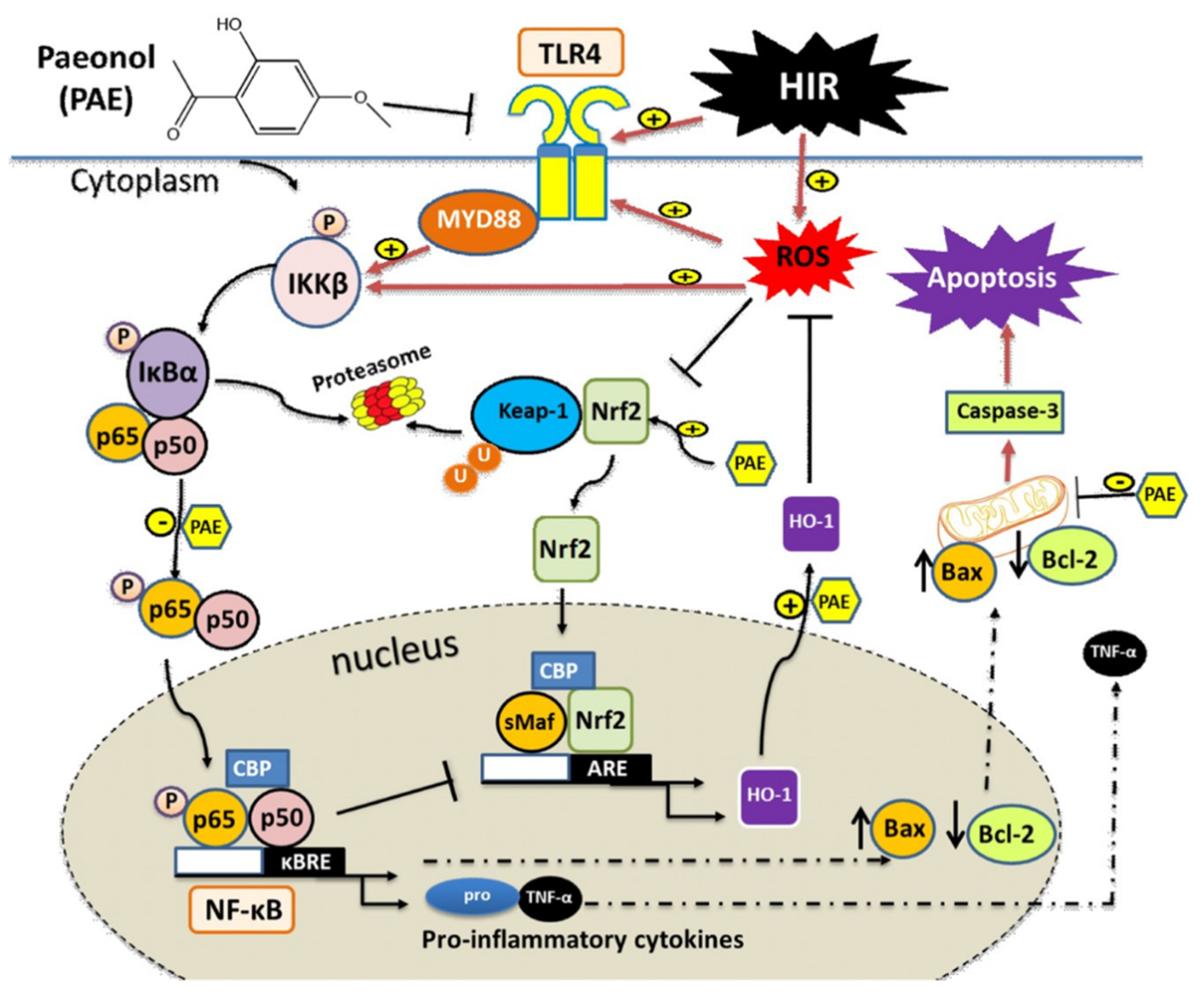
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weigand, K.; Brost, S.; Steinebrunner, N.; Büchler, M.; Schemmer, P.; Müller, M. Ischemia/Reperfusion injury in liver surgery and transplantation: Pathophysiology. HPB Surg. 2012, 2012, 176723. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.Y.; Fu, P.Y.; Li, P.D.; Li, Z.N.; Liu, H.Y.; Xin, M.G.; Li, W. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J. Gastrointest. Surg. 2014, 6, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Molinari, A.F.; Corazzi, F.; Rocchi, L.; Zito, P.; Cimino, M.; Costa, G.; Raimondi, F.; Torzilli, G. Pharmacological Modulation of Ischemic-Reperfusion Injury during Pringle Maneuver in Hepatic Surgery. A Prospective Randomized Pilot Study. World J. Surg. 2016, 40, 2202–2212. [Google Scholar] [PubMed]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019, 39, 788–801. [Google Scholar] [CrossRef]
- Elias-Miró, M.; Jiménez-Castro, M.B.; Rodés, J.; Peralta, C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic. Res. 2013, 47, 555–568. [Google Scholar] [CrossRef]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.-K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global consequences of liver ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef]
- Montalvo-Jave, E.E.; Escalante-Tattersfield, T.; Ortega-Salgado, J.A.; Piña, E.; Geller, D.A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 2008, 147, 153–159. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Abe, Y.; Hines, I.N.; Zibari, G.B.; Pavlick, K.; Gray, L.; Kitagawa, Y.; Grisham, M.B. Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic. Biol. Med. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Z.; Gao, W.; Duan, Y.; Fan, G.; Geng, X.; Wu, B.; Li, K.; Liu, K.; Peng, C. Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-κB Signaling Pathway, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2020, 11, 544124. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdel-Gaber, S.A.; Amin, E.F.; Ibrahim, S.A.; Mohammed, R.K.; Abdelrahman, A.M. Molecular mechanisms contributing to the protective effect of levosimendan in liver ischemia-reperfusion injury. Eur. J. Pharmacol. 2014, 741, 64–73. [Google Scholar] [CrossRef]
- Morsy, M.A. Protective effect of lisinopril on hepatic ischemia/reperfusion injury in rats. Indian J. Pharmacol. 2011, 43, 652–655. [Google Scholar]
- Ikeda, T.; Yanaga, K.; Kishikawa, K.; Kakizoe, S.; Shimada, M.; Sugimachi, K. Ischemic injury in liver transplantation: Difference in injury sites between warm and cold ischemia in rats. Hepatology 1992, 16, 454–461. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; De Cuyper, I.M.; Branco-Madeira, F.; de Bleser, P.; Kool, M.; Meinders, M.; Hoogenboezem, M.; Mul, E.; Wolkers, M.C.; Salerno, F.; et al. GATA1-Deficient Dendritic Cells Display Impaired CCL21-Dependent Migration toward Lymph Nodes Due to Reduced Levels of Polysialic Acid. J. Immunol. 2016, 197, 4312–4324. [Google Scholar] [CrossRef]
- Liu, S.; Khemlani, L.S.; A Shapiro, R.; Johnson, M.L.; Liu, K.; A Geller, D.; Watkins, S.C.; Goyert, S.M.; Billiar, T.R. Expression of CD14 by hepatocytes: Upregulation by cytokines during endotoxemia. Infect. Immun. 1998, 66, 5089–5098. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Chen, Z.-L.; Zhang, S.-J.; Wang, Y.-Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, S.; Tang, H.; Zhang, X.; Tao, R.; Yan, Z.; Shi, J.; Guo, W.; Zhang, S. Veratric acid alleviates liver ischemia/reperfusion injury by activating the Nrf2 signaling pathway. Int. Immunopharmacol. 2021, 101, 108294. [Google Scholar] [CrossRef]
- Bardallo, R.G.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2021. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell. Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef]
- Li, P.M.; Li, Y.L.; Liu, B.; Wang, W.J.; Wang, Y.Z.; Li, Z. Curcumin inhibits MHCC97H liver cancer cells by activating ROS/TLR-4/caspase signaling pathway. Asian Pac. J. Cancer Prev. 2014, 15, 2329–2334. [Google Scholar] [CrossRef]
- Li, Y.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Roles of Toll-Like Receptors in Nitroxidative Stress in Mammals. Cells 2019, 8, 576. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, R.Q.; Wei, X.G.; Ren, X.M.; Gao, X.Q. Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac. J. Trop. Med. 2016, 9, 503–507. [Google Scholar] [CrossRef]
- Kadono, K.; Uchida, Y.; Hirao, H.; Miyauchi, T.; Watanabe, T.; Iida, T.; Ueda, S.; Kanazawa, A.; Mori, A.; Okajima, H.; et al. Thrombomodulin Attenuates Inflammatory Damage Due to Liver Ischemia and Reperfusion Injury in Mice in Toll-Like Receptor 4-Dependent Manner. Am. J. Transplant. 2017, 17, 69–80. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Wang, F.; Liang, X.; Zeng, Z.; Zhao, J.; Zheng, H.; Jiang, X.; Zhang, Y. Improvement in cerebral ischemia-reperfusion injury through the TLR4/NF-κB pathway after Kudiezi injection in rats. Life Sci. 2017, 191, 132–140. [Google Scholar] [CrossRef]
- Qi, M.; Zheng, L.; Qi, Y.; Han, X.; Xu, Y.; Xu, L.; Yin, L.; Wang, C.; Zhao, Y.; Sun, H.; et al. Dioscin attenuates renal ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling pathway via up-regulation of HSP70. Pharmacol. Res. 2015, 100, 341–352. [Google Scholar] [CrossRef]
- Tao, X.; Sun, X.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Zhao, Y.; Wang, C.; Peng, J. Total Flavonoids from Rosa laevigata Michx Fruit Ameliorates Hepatic Ischemia/Reperfusion Injury through Inhibition of Oxidative Stress and Inflammation in Rats. Nutrients 2016, 8, 418. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.T.; Jin, L.; Lin, J.; Fan, Z.L.; Zeng, Z.; Huang, H.F. Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF-κB signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 551–560. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Gaber, S.A.; Rifaai, R.A.; Mohammed, M.M.; Nair, A.B.; Abdelzaher, W.Y. Protective mechanisms of telmisartan against hepatic ischemia/reperfusion injury in rats may involve PPARγ-induced TLR4/NF-κB suppression. Biomed. Pharmacother. 2022, 145, 112374. [Google Scholar] [CrossRef]
- Du, P.; Zhang, X.; Luo, K.; Li, Y.; Fu, C.; Xiao, J.; Xiao, Q. Curculigoside mitigates hepatic ischemia/reperfusion-induced oxidative stress, inflammation, and apoptosis via activation of the Nrf-2/HO-1 pathway. Hum. Exp. Toxicol. 2022, 41, 09603271221087146. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Q.; Xu, Y.; Chen, Y.; Deng, Y.; Zhi, F.; Qian, K. Attenuating Oxidative Stress by Paeonol Protected against Acetaminophen-Induced Hepatotoxicity in Mice. PLoS ONE 2016, 11, e0154375. [Google Scholar]
- Li, H.; Song, F.; Duan, L.R.; Sheng, J.J.; Xie, Y.H.; Yang, Q.; Chen, Y.; Dong, Q.Q.; Zhang, B.L.; Wang, S.W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Xia, Z.-M.; Shang, C.-H.; Chao, Q.-L.; Liu, Y.-R.; Fan, H.-Y.; Chen, D.-Q.; Qiu, F.; Zhao, F. Anti-inflammatory and Anti-oxidative Activities of Paeonol and Its Metabolites Through Blocking MAPK/ERK/p38 Signaling Pathway. Inflammation 2016, 39, 434–446. [Google Scholar] [CrossRef]
- Hafez, H.; Morsy, M.; Mohamed, M.; Zenhom, N. Mechanisms underlying gastroprotective effect of paeonol against indomethacin-induced ulcer in rats. Hum. Exp. Toxicol. 2019, 38, 510–518. [Google Scholar] [CrossRef]
- Al-Taher, A.Y.; Morsy, M.A.; Rifaai, R.A.; Zenhom, N.M.; Abdel-Gaber, S.A. Paeonol Attenuates Methotrexate-Induced Cardiac Toxicity in Rats by Inhibiting Oxidative Stress and Suppressing TLR4-Induced NF-κB Inflammatory Pathway. Mediators Inflamm. 2020, 2020, 8641026. [Google Scholar] [CrossRef]
- Liao, W.-Y.; Tsai, T.-H.; Ho, T.-Y.; Lin, Y.-W.; Cheng, C.-Y.; Hsieh, C.-L. Neuroprotective Effect of Paeonol Mediates Anti-Inflammation via Suppressing Toll-Like Receptor 2 and Toll-Like Receptor 4 Signaling Pathways in Cerebral Ischemia-Reperfusion Injured Rats. Evid.-Based Complement. Altern. Med. 2016, 2016, 3704647. [Google Scholar] [CrossRef]
- Ma, L.; Chuang, C.-C.; Weng, W.; Zhao, L.; Zheng, Y.; Zhang, J.; Zuo, L. Paeonol Protects Rat Heart by Improving Regional Blood Perfusion during No-Reflow. Front. Physiol. 2016, 7, 298. [Google Scholar] [CrossRef]
- Mohamed, M.Z.; Morsy, M.A.; Mohamed, H.H.; Hafez, H.M. Paeonol protects against testicular ischaemia-reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia 2020, 52, e13599. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Sastry, K.V.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gaber, S.A.; Geddawy, A.; Moussa, R.A. The hepatoprotective effect of sitagliptin against hepatic ischemia reperfusion-induced injury in rats involves Nrf-2/HO-1 pathway. Pharmacol. Rep. 2019, 71, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Itoh, A.; Pleasure, D.; Itoh, T. MEK-ERK Signaling Is Involved in Interferon-γ-induced Death of Oligodendroglial Progenitor Cells. J. Biol. Chem. 2006, 281, 20095–20106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refaie, M.M.M.; El-Hussieny, M.; Zenhom, N.M. Protective role of nebivolol in cadmium-induced hepatotoxicity via downregulation of oxidative stress, apoptosis and inflammatory pathways. Environ. Toxicol. Pharmacol. 2018, 58, 212–219. [Google Scholar] [CrossRef]
- Wimmer, I.; Tröscher, A.R.; Brunner, F.; Rubino, S.J.; Bien, C.G.; Weiner, H.L.; Lassmann, H.; Bauer, J. Systematic evaluation of RNA quality, microarray data reliability and pathway analysis in fresh, fresh frozen and formalin-fixed paraffin-embedded tissue samples. Sci. Rep. 2018, 8, 6351. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Elsevier Health Sciences: London, UK, 2007. [Google Scholar]
- Ahmed, A.-S.F.; Bayoumi, A.M.; Eltahir, H.M.; Hafez, S.M.A.; Abouzied, M.M. Amelioration of Sepsis-induced liver and lung injury by a superoxide dismutase mimetic; role of TNF-α and Caspase-3. J. Adv. Biomed. Pharm. Sci. 2020, 3, 31–39. [Google Scholar] [CrossRef]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Suyasunanont, D.; Klaikeaw, N. Curcumin decreased oxidative stress, inhibited NF-κB activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009, 2009, 981963. [Google Scholar] [CrossRef]
- Du, Y.; Qian, B.; Gao, L.; Tan, P.; Chen, H.; Wang, A.; Zheng, T.; Pu, S.; Xia, X.; Fu, W. Aloin Preconditioning Attenuates Hepatic Ischemia/Reperfusion Injury via Inhibiting TLR4/MyD88/NF-κB Signal Pathway In Vivo and In Vitro. Oxid. Med. Cell. Longev. 2019, 2019, 3765898. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Wan, R.Z.; Zhang, P.; Zhang, B. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure in mice. Int. Immunopharmacol. 2017, 46, 124–132. [Google Scholar] [CrossRef]
- Ko, J.S.; Gwak, M.S.; Kim, G.S.; Shin, Y.H.; Ryu, S.; Kim, J.S.; Kim, S.J.; Kim, S.T. The protective effect of ischemic preconditioning against hepatic ischemic-reperfusion injury under isoflurane anesthesia in rats. Transplant. Proc. 2013, 45, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, J.W.; Han, S.; Gwak, M.S.; Kim, G.S.; Jeon, S.Y.; Ryu, S.; Hahm, T.S.; Ko, J.S. Ischemic Preconditioning Protects Against Hepatic Ischemia-Reperfusion Injury Under Propofol Anesthesia in Rats. Transplant. Proc. 2020, 52, 2964–2969. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, P.; Yao, L.P.; Wang, W.; Gao, Y.M.; Zhang, J.; Fu, Y.J. Paeonol alleviated acute alcohol-induced liver injury via SIRT1/Nrf2/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, X.; Simonaro, C.M.; Yi, B.; Schuchman, E.H. Acid Ceramidase Protects Against Hepatic Ischemia/Reperfusion Injury by Modulating Sphingolipid Metabolism and Reducing Inflammation and Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 633657. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, Y.; Wang, X.; Gao, B.; Li, Y.; Li, R.; Wang, J. Protective Effects of Fisetin on Hepatic Ischemia-reperfusion Injury Through Alleviation of Apoptosis and Oxidative Stress. Arch. Med. Res. 2021, 52, 163–173. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Konishi, T.; Lentsch, A.B. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017, 17, 277–287. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Xiong, Y.; Ma, X.; Qu, L. Effect of sevoflurane pretreatment in relieving liver ischemia/reperfusion-induced pulmonary and hepatic injury. Acta Cir. Bras. 2019, 34, e201900805. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhu, J.; Xie, X.; Hao, M.; Zhao, Y. The protective effect of glycyrrhizin on hepatic ischemia-reperfusion injury in rats and possible related signal pathway. Iran. J. Basic Med. Sci. 2020, 23, 1232–1238. [Google Scholar] [PubMed]
- Chen, B.; Ning, M.; Yang, G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 2012, 17, 4672–4683. [Google Scholar] [CrossRef] [Green Version]
- Song, M.Y.; Lee, D.Y.; Chun, K.S. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Arch. Biochem. Biophys. 2019, 678, 108186. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, G.Y.; Pei, W.M.; Zhou, B.; Zhang, Q.Q.; Pan, B.B. Dexmedetomidine alleviates hepatic injury via the inhibition of oxidative stress and activation of the Nrf2/HO-1 signaling pathway. Eur. Cytokine Netw. 2019, 30, 88–97. [Google Scholar]
- Li, C.Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551. [Google Scholar] [CrossRef]
- Zhong, Q.; Mishra, M.; Kowluru, R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3941–3948. [Google Scholar] [CrossRef]
- Wu, J.; Xu, L.; Sun, C.; Zhang, B.; Li, J.; Sun, J.; Zhang, Y.; Sun, D. Paeonol alleviates epirubicin-induced renal injury in mice by regulating Nrf2 and NF-κB pathways. Eur. J. Pharmacol. 2017, 795, 84–93. [Google Scholar] [CrossRef]
- Jaeschke, H.; Farhood, A.; Bautista, A.P.; Spolarics, Z.; Spitzer, J.J. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am. J. Physiol. 1993, 264, G801–G809. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.C.; Soltys, D.; Toledo, A.H.; Toledo-Pereyra, L.H. Tumor necrosis factor-α in liver ischemia/reperfusion injury. J. Investig. Surg. 2011, 24, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramírez, A.; Mosbah, I.B.; Ramalho, F.; Roselló-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; LaPar, D.J.; Stone, M.L.; Zhao, Y.; Kron, I.L.; Laubach, V.E. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am. J. Transplant. 2013, 13, 2255–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somade, O.T.; Ajayi, B.O.; Safiriyu, O.A.; Oyabunmi, O.S.; Akamo, A.J. Renal and testicular up-regulation of pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α, IL-1β, IL-6) following acute edible camphor administration is through activation of NF-kB in rats. Toxicol. Rep. 2019, 6, 759–767. [Google Scholar] [CrossRef]
- Zhao, H.; Perez, J.S.; Lu, K.; George, A.; Ma, D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am. J. Physiol. Physiol. 2014, 306, F801–F811. [Google Scholar] [CrossRef]
- Zhai, K.F.; Duan, H.; Luo, L.; Cao, W.G.; Han, F.K.; Shan, L.L.; Fang, X.M. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology 2017, 25, 523–532. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Yang, X.; Huang, C. Metformin attenuates ischemia-reperfusion injury of fatty liver in rats through inhibition of the TLR4/NF-κB axis. Balk. Med. J. 2020, 37, 196. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Nrf2 | 5′-CCCAGCACATCCAGACAGAC-3′ | 5′-TATCCAGGGCAAGCGACTC-3′ |
| Bax | 5′-AGAGGCAGCGGCAGTGAT-3′ | 5′-AGACACAGTCCAAGGCAGCAG-3′ |
| Bcl-2 | 5′-TATATGGCCCCAGCATGCGA-3′ | 5′-GGGCAGGTTTGTCGACCTCA-3′ |
| GAPDH | 5′-GTCGGTGTGAACGGATTTG-3′ | 5′-CTTGCCGTGGGTAGAGTCAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, M.A.; Ibrahim, Y.F.; Abdel Hafez, S.M.N.; Zenhom, N.M.; Nair, A.B.; Venugopala, K.N.; Shinu, P.; Abdel-Gaber, S.A. Paeonol Attenuates Hepatic Ischemia/Reperfusion Injury by Modulating the Nrf2/HO-1 and TLR4/MYD88/NF-κB Signaling Pathways. Antioxidants 2022, 11, 1687. https://doi.org/10.3390/antiox11091687
Morsy MA, Ibrahim YF, Abdel Hafez SMN, Zenhom NM, Nair AB, Venugopala KN, Shinu P, Abdel-Gaber SA. Paeonol Attenuates Hepatic Ischemia/Reperfusion Injury by Modulating the Nrf2/HO-1 and TLR4/MYD88/NF-κB Signaling Pathways. Antioxidants. 2022; 11(9):1687. https://doi.org/10.3390/antiox11091687
Chicago/Turabian StyleMorsy, Mohamed A., Yasmine F. Ibrahim, Sara Mohamed Naguib Abdel Hafez, Nagwa M. Zenhom, Anroop B. Nair, Katharigatta N. Venugopala, Pottathil Shinu, and Seham A. Abdel-Gaber. 2022. "Paeonol Attenuates Hepatic Ischemia/Reperfusion Injury by Modulating the Nrf2/HO-1 and TLR4/MYD88/NF-κB Signaling Pathways" Antioxidants 11, no. 9: 1687. https://doi.org/10.3390/antiox11091687






