Effect of Resveratrol Supplementation on Intestinal Oxidative Stress, Immunity and Gut Microbiota in Weaned Piglets Challenged with Deoxynivalenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. DON-Contaminated Diets Preparation
2.2. Animals and Diets
2.3. Histomorphology Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Analyses of Plasma and Intestinal Antioxidant/Oxidant Indices
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Gut Microbiome Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Resveratrol Supplementation on the Redox Status in Piglets Challenged with Deoxynivalenol
3.2. Effect of Resveratrol Supplementation on Pro-Inflammatory Cytokines in Piglets Challenged with Deoxynivalenol
3.3. Effect of Resveratrol Supplementation on Expression of Antibacterial Peptide and Mucin in the Ileum of Piglets Challenged with Deoxynivalenol
3.4. Effect of Resveratrol Supplementation on Ileal Morphology in Piglets Challenged with Deoxynivalenol
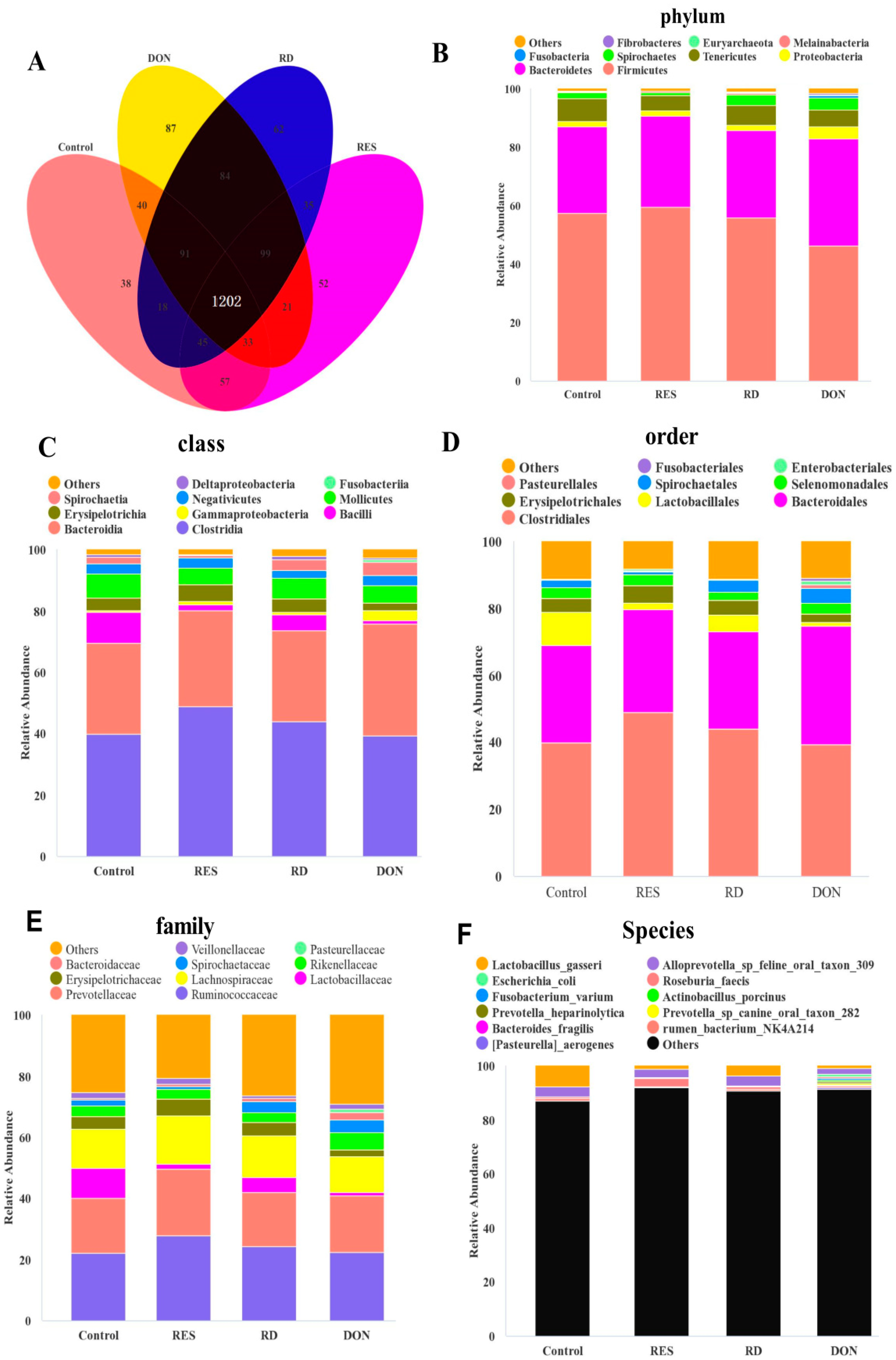
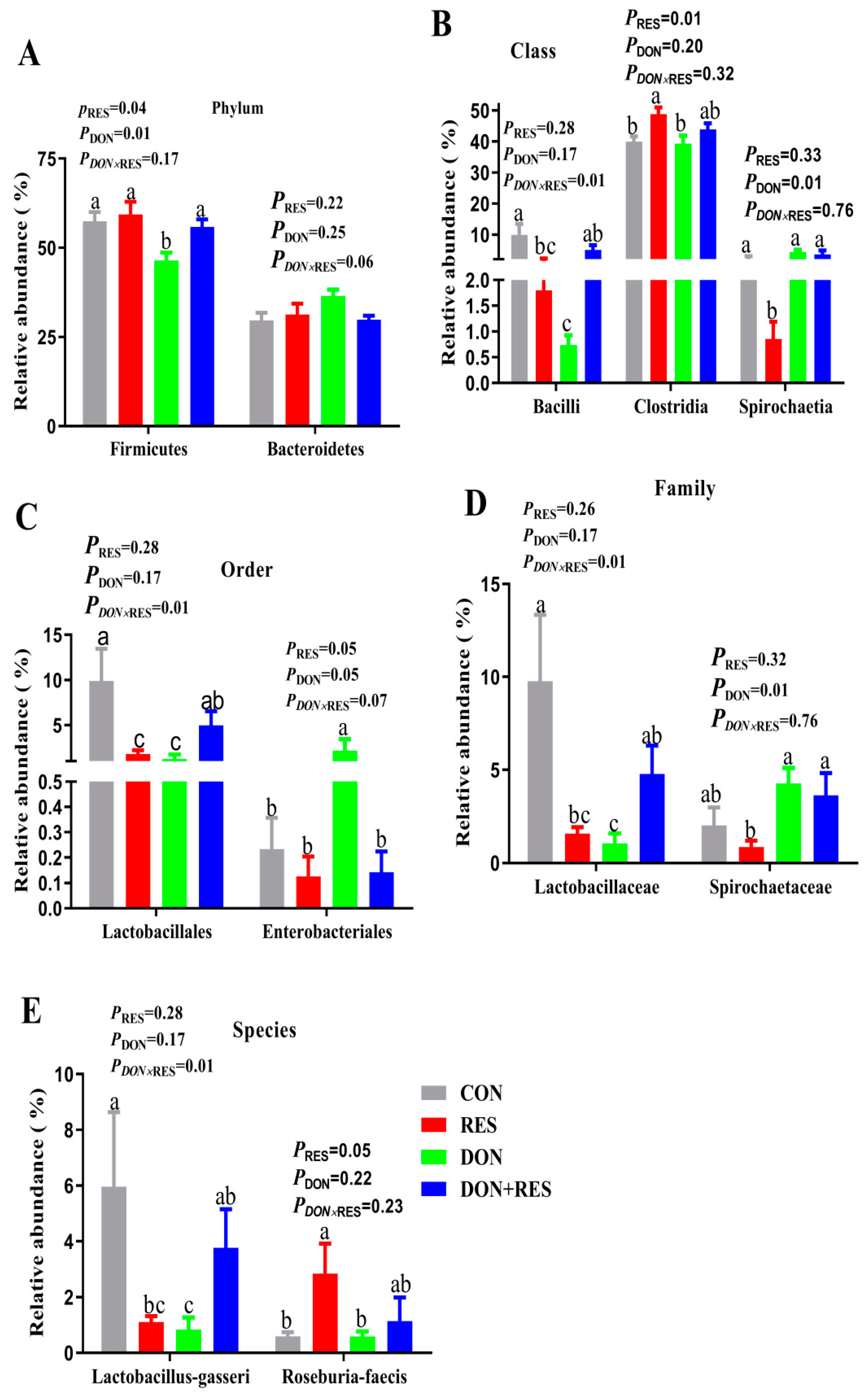
3.5. Effects of Resveratrol Supplementation on the Composition of the Colonic Microbiota of Piglets Challenged with Deoxynivalenol
3.6. Microbiota Functional Prediction Analysis
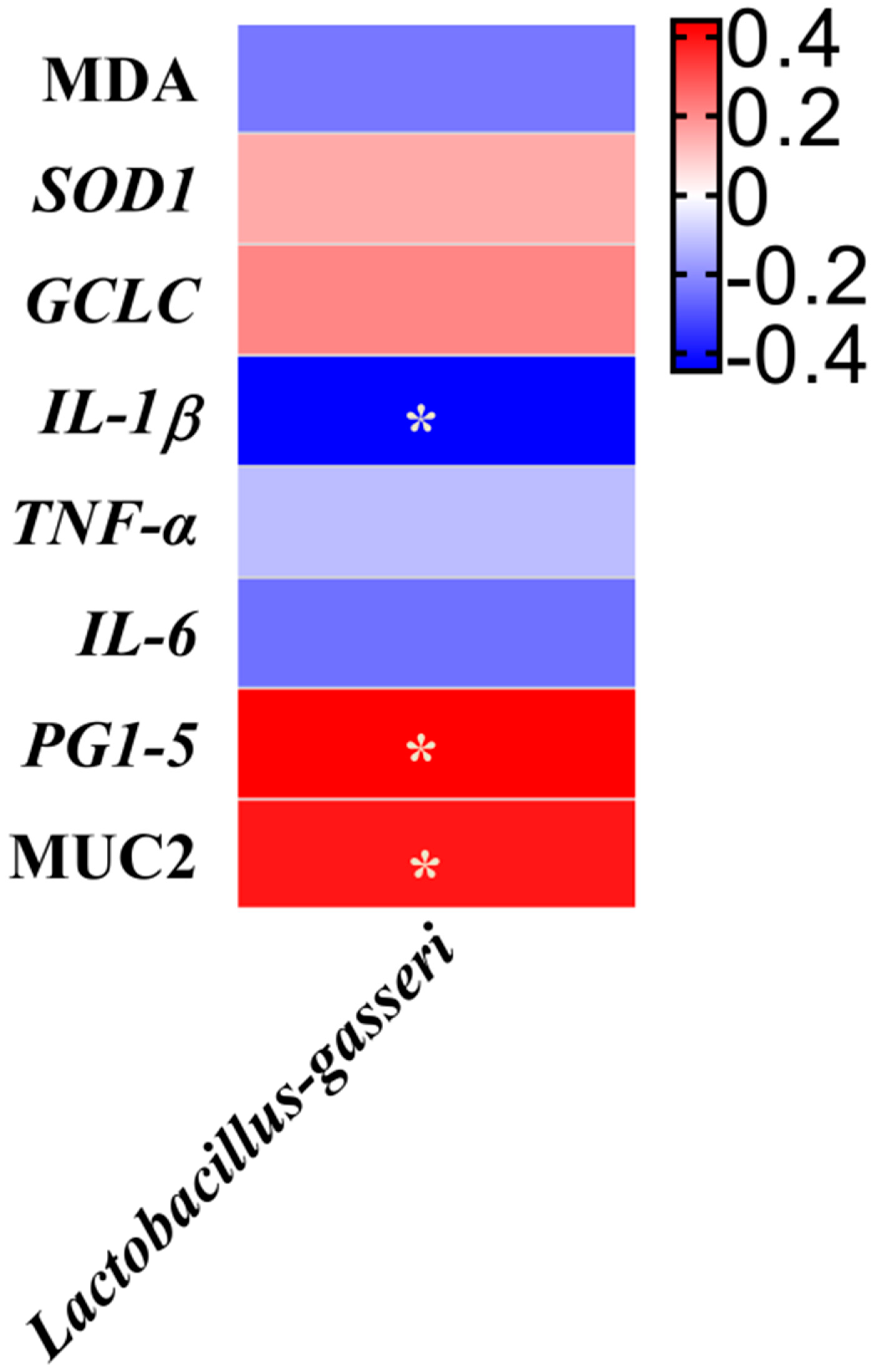
3.7. Correlation Analysis of the Gut Microbiota and Variables Related to Intestinal Barrier Function, Inflammation and Oxidative Damage
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Park, B.J.; Kobayashi Hattori, K.; Tanaka, T.; Chonan, T.; Yoshikawa, K.; Kumagai, S. Effect of cooking process on the deoxynivalenol content and its subsequent cytotoxicity in wheat products. Biosci. Biotechnol. Biochem. 2006, 70, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of mycotoxins on the intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z.Y. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. A 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE 2003, 8, e69014. [Google Scholar] [CrossRef]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-Κb nuclear localization and down regulation of NF-κB and Cyclo-Oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, C.; Ye, J.L.; Lv, Y.T.; Wang, L.; Chen, Z.; Jiang, Z.Y. Resveratrol protects porcine intestinal epithelial cells from deoxynivalenol-induced damage via the Nrf2 signaling pathway. J. Agric. Food Chem. 2019, 67, 1726–1735. [Google Scholar] [CrossRef]
- Meng, Q.W.; Sun, S.H.; Luo, Z.; Shi, B.M.; Shan, A.S.; Cheng, B.J. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, M.; Zhao, L.; Han, S.; Li, Y.; Xiong, B.; Jiang, L. Dietary grape seed procyanidins suppressed weaning stress by improving antioxidant enzyme activity and mRNA expression in weanling piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Chen, Y.; Ji, S.; Jia, P.; Li, Y.; Wang, T. Comparison of the protective effects of resveratrol and pterostilbene against intestinal damage and redox imbalance in weanling piglets. J. Anim. Sci. Biotechnol. 2020, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Silva-Guillen, Y.V.; Arellano, C.; Boyd, R.D.; Martinez, G.; van Heugten, E. Growth performance, oxidative stress and immune status of newly weaned pigs fed peroxidized lipids with or without supplemental vitamin E or polyphenols. J. Anim. Sci. Biotechnol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenolrich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and fecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles and intestinal gene expression in pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Cao, S.; Shen, Z.; Wang, C.; Zhang, Q.; Hong, Q.; He, Y.; Hu, C. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. 2019, 10, 344–354. [Google Scholar] [CrossRef]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.P.; He, J.H. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappaB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef]
- Jin, X.; Wang, K.; Liu, H.; Hu, F.; Zhao, F.; Liu, J. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxid Med. Cell Longev. 2016, 2016, 2572175. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Leclercq, G.; Goossens, V.; Remijsen, Q.; Vandenabeele, P.; Motterlini, R.; Lefebvre, R.A. Antioxidant potential of CORM-A1 and resveratrol during TNF-α/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-K cells. Toxicol. Appl. Pharm. 2015, 288, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.C.; Zhang, B.Y.; Zhang, L.; Wu, K.T.; Yang, A. Potential link between gut microbiota and deoxynivalenol-induced feed refusal in weaned piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Zha, A.D.; Cui, Z.J.; Qi, M.; Liao, S.M.; Yin, J.; Tan, B.E. Baicalin-copper complex modulates gut microbiota, inflammatory responses, and hormone secretion in DON-challenged piglets. Animals 2020, 10, 1535. [Google Scholar] [CrossRef]
- Robert, H.; Payros, D.; Pinton, P.; Théodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55, 44–55. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Wu, L.; Wang, W.C.; Yao, K.; Zhou, T.; Yin, J.; Li, T.J. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS ONE 2013, 8, e69502. [Google Scholar] [CrossRef]
- Juan, C.; Oueslati, S.; Mañes, J.; Berrada, H. Multimycotoxin determination in tunisian farm animal feed. J. Food Sci. 2019, 84, 3885–3893. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Qiu, Y.; Liu, S.; Hou, L.; Li, K.; Wang, L.; Gao, K.; Yang, X.; Jiang, Z. Supplemental choline modulates growth performance and gut inflammation by altering the gut microbiota and lipid metabolism in weaned piglets. J. Nutr. 2021, 1511, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiao, H.; Ren, W.; Yin, J.; Tan, B.; Liu, G.; Li, L.; Nyachoti, C.M.; Xiong, X.; Wu, G. Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS ONE 2014, 9, e100591. [Google Scholar] [CrossRef]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. BBA-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhang, B.; Wu, K.; Yang, A.; Li, C.; Zhang, J.; Zhang, C.; Rajput, S.A.; Zhang, N.; et al. Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned piglets and IPEC-J2 cells. Toxins 2018, 10, 541. [Google Scholar] [CrossRef]
- Xu, X.; Hua, H.W.; Wang, L.W.; He, P.W.; Zhang, L.; Qin, Q.; Yu, C.; Wang, X.Y.; Zhang, G.L.; Liu, Y.L. Holly polyphenols alleviate intestinal inflammation and alter microbiota composition in lipopolysaccharide-challenged pigs. Br. J. Nutr. 2020, 123, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Fu, Q.; Zhao, X.; Song, X.; Yu, J.; Yang, Y.; Sun, K.; Bai, L.; Tian, Y.; Chen, S.; et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS ONE 2018, 13, e0192692. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Liu, Y.; Luan, C.; Xia, X. Antibacterial activity, cytotoxicity and mechanisms of action of cathelicidin peptides against enteric pathogens in weaning piglets. Int. J. Pept. Res. 2021, 17, 175–184. [Google Scholar] [CrossRef]
- Lin, L.; Wen, S.H.; Guo, S.Z.; Su, X.Y.; Wu, H.J.; Chong, L.; Zhang, H.L.; Zhang, W.X.; Li, C.C. Role of SIRT1 in Streptococcus pneumoniae-induced human β-defensin-2 and interleukin-8 expression in A549 cell. Mol. Cell Biochem. 2014, 394, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Janssens, G.P.; De Baere, S.; Mountzouris, K.C.; Su, S.; Wong, E.A.; De Meulenaer, B.; et al. Mycotoxins deoxynivalenol and fumonisins alter the extrinsic component of intestinal barrier in broiler chickens. J. Agric. Food Chem. 2015, 63, 10846–10855. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, D.H.; Lee, K. Homeostasis effects of fermented Maillard reaction products by Lactobacillus gasseri 4M13 in dextran sulfate sodium-induced colitis mice. J. Sci. Food Agric. 2022, 102, 434–444. [Google Scholar] [CrossRef]

| Item, μg/kg | Basal Feed | Contaminated Feed | Limit of Detection |
|---|---|---|---|
| DON | 247 | 3790 | 100 |
| AFB | undetected | undetected | 2 |
| ZEN | 94 | 102 | 10 |
| OTA | 16 | 21 | 10 |
| Item | |
|---|---|
| Ingredient composition, % | |
| Corn | 34.00 |
| Expanded corn | 14.72 |
| Soybean meal | 10.0 |
| Expanded soybean | 8.50 |
| Fishmeal | 4.00 |
| Low protein whey powder | 11.00 |
| Soybean hull | 5.00 |
| Plasma protein powder | 4.00 |
| Soybean oil | 1.35 |
| Sucrose | 2.00 |
| CaHPO4 | 1.20 |
| Limestone powder | 0.65 |
| NaCL | 0.45 |
| Choline chloride 50% | 0.20 |
| L-Lysine HCl | 0.82 |
| DL-Methionine | 0.25 |
| L-Threonine | 0.30 |
| Trp | 0.06 |
| Premix 1 | 1.50 |
| Total | 100 |
| Nutrient levels 2 | |
| DE, kcal/kg | 3516.62 |
| CP% | 19.26 |
| SID Lys% | 1.55 |
| SID Met + Cys% | 0.78 |
| SID Thr% | 0.88 |
| SID Trp% | 0.25 |
| Genes | Sequences, 5′–3′ | Product Size, Bp | GenBank Accession | |
|---|---|---|---|---|
| β-actin | Forward Reverse | CATCGTCCACCGCAAAT TGTCACCTTCACCGTTCC | 210 | NC_010445 |
| SOD1 | Forward Reverse | GAGACCTGGGCAATGTGACT CTGCCCAAGTCATCTGGTTT | 139 | NM_001190422.1 |
| GCLC | Forward Reverse | CAAACCATCCTACCCTTTGG ATTGTGCAGAGAGCCTGGTT | 172 | XM_003482164.4 |
| GCLM | Forward Reverse | GATGCCGCCCGATTTAACTG ACAATGACCGAGTACCGCAG | 177 | XM_001926378.4 |
| HO-1 | Forward Reverse | CGCTCCCGAATGAACACTCT GCGAGGGTCTCTGGTCCTTA | 148 | NM_001004027.1 |
| NQO-1 | Forward Reverse | ATCACAGGTAAACTGAAGGACCC TGGCAGCGTATGTGTAAGCA | 229 | NM_001159613.1 |
| IL-1β | Forward Reverse | CTCCAGCCAGTCTTCATTGTTC TGCCTGATGCTCTTGTTCCA | 230 | NM_214055.1 |
| IL-6 | Forward Reverse | TACATCCTCGGCAAAATC TCTCATCAAGCAGGTCTCC | 168 | NM_001252429.1 |
| Muc2 | Forward Reverse | CTGCTCCGGGTCCTGTGGGA CCCGCTGGCTGGTGCGATAC | 100 | XM_007465997.1 |
| pBD1 | Forward Reverse | ACCGCCTCCTCCTTGTATTC CACAGGTGCCGATCTGTTTC | 150 | NM_213838.1 |
| pBD2 | Forward Reverse | CCAGAGGTCCGACCACTACA GGTCCCTTCAATCCTGTTGAA | 88 | AY506573.1 |
| PG1-5 | Forward Reverse | GTAGGTTCTGCGTCTGTGTCG CAAATCCTTCACCGTCTACCA | 166 | XM_005669497.2 |
| Variable | Treatments | Pooled SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DON | RES | DON + RES | DON | RES | RES × DON | ||
| The levels of antioxidant/oxidant indices in the plasma | ||||||||
| MDA (nmol/mL) | 1.96 c | 3.31 a | 1.80 c | 2.58 b | 0.12 | <0.01 | <0.01 | 0.02 |
| T-SOD (U/mL) | 60.57 | 53.67 | 62.39 | 61.84 | 2.91 | 0.21 | 0.10 | 0.28 |
| GSH (mg/L) | 4.24 b | 2.80 c | 5.59 a | 3.38 c | 0.24 | <0.01 | <0.01 | 0.17 |
| T-AOC (U/mL) | 2.31 b | 1.56 c | 3.17 a | 2.01 b | 0.15 | <0.01 | <0.01 | 0.20 |
| The contents of antioxidant/oxidant indices in the ileum, μmol/g protein | ||||||||
| MDA | 0.52 c | 1.00 a | 0.43 c | 0.68 b | 0.04 | <0.01 | <0.01 | 0.01 |
| T-SOD | 58.24 b | 39.57 d | 77.00 a | 50.77 c | 2.17 | <0.01 | <0.01 | 0.09 |
| GSH | 5.13 ab | 4.21 c | 5.68 a | 4.38 bc | 0.26 | <0.01 | 0.17 | 0.48 |
| T-AOC | 0.49 ab | 0.31 c | 0.56 a | 0.44 b | 0.03 | <0.01 | <0.01 | 0.29 |
| The expression of antioxidative genes in the ileum | ||||||||
| SOD1 | 1.01 b | 0.56 c | 2.31 a | 1.03 b | 0.10 | <0.01 | <0.01 | <0.01 |
| GCLC | 1.01 b | 0.72 b | 1.94 a | 0.82 b | 0.14 | <0.01 | 0.01 | <0.01 |
| GCLM | 1.01 b | 0.41 c | 1.73 a | 0.78 b | 0.13 | <0.01 | <0.01 | 0.17 |
| HO-1 | 1.01 b | 0.70 c | 1.40 a | 0.78 c | 0.08 | <0.01 | <0.01 | 0.07 |
| NQO-1 | 1.01b | 0.69 c | 1.54 a | 0.89 bc | 0.10 | <0.01 | 0.01 | 0.11 |
| Variable | Treatments | Pooled SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DON | RES | DON + RES | DON | RES | RES × DON | ||
| The protein levels of pro-inflammation cytokines levels in the plasma, ng/L | ||||||||
| IL-1β | 194.74c | 227.01a | 189.00c | 211.92b | 4.38 | <0.01 | 0.03 | 0.30 |
| IL-6 | 398.28 | 406.59 | 394.42 | 410.25 | 14.80 | 0.42 | 0.10 | 0.80 |
| TNF-α | 61.37 | 63.83 | 59.06 | 60.09 | 2.11 | 0.41 | 0.16 | 0.73 |
| The pro-inflammation cytokines contents in the ileum, ng/g protein | ||||||||
| IL-1β | 20.40c | 38.41a | 17.45c | 32.13b | 2.21 | <0.01 | 0.05 | 0.03 |
| IL-6 | 25.44c | 38.09a | 21.45c | 32.80b | 2.56 | <0.01 | 0.03 | 0.04 |
| TNF-α | 14.12b | 19.44a | 9.75c | 15.45b | 1.25 | <0.01 | <0.01 | 0.03 |
| The gene expression of pro-inflammation in the ileum | ||||||||
| IL-1β | 1.01bc | 1.69a | 0.86c | 1.22b | 0.07 | <0.01 | <0.01 | 0.04 |
| IL6 | 1.01c | 2.01a | 0.66d | 1.25b | 0.08 | <0.01 | <0.01 | 0.01 |
| TNF-α | 1.02b | 1.66a | 0.78c | 1.08b | 0.07 | <0.01 | <0.01 | 0.02 |
| Variable | Treatments | Pooled SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DON | RES | DON + RES | DON | RES | RES × DON | ||
| Gene expression | ||||||||
| pBD-1 | 1.02 a | 0.44 b | 1.05 a | 0.57 b | 0.06 | <0.01 | 0.16 | 0.42 |
| pBD-2 | 1.02 a | 0.41 c | 1.08 a | 0.71 b | 0.06 | <0.01 | <0.01 | 0.07 |
| pG1-5 | 1.01 a | 0.54 c | 0.96 a | 0.77 b | 0.05 | <0.01 | 0.09 | <0.01 |
| Muc2 | 1.01 ab | 0.71 c | 1.07 a | 0.92 b | 0.05 | <0.01 | <0.01 | 0.18 |
| Protein expressions | ||||||||
| Muc2 | 9.23 a | 6.89 b | 10.09 a | 8.54 a | 0.51 | <0.01 | 0.02 | 0.04 |
| sIgA | 491.59 a | 354.63 c | 517.31 a | 421.63 b | 20.47 | <0.01 | 0.03 | 0.32 |
| Variable | Treatments | Pooled SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DON | RES | DON + RES | DON | RES | DON × RES | ||
| Villus height, μm | 382 b | 354 b | 438 a | 359 b | 11 | <0.01 | 0.01 | 0.03 |
| crypt depth, μm | 292 a | 278 a | 238 b | 266 ab | 12 | 0.54 | 0.01 | 0.09 |
| VCR | 1.33 b | 1.29 b | 1.85 a | 1.36 b | 0.08 | <0.01 | <0.01 | <0.01 |
| Variable | Treatments | Pooled SEM | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DON | RES | DON + RES | DON | RES | DON × RES | ||
| Alpha-diversity index | ||||||||
| ACE | 916 b | 1028 a | 890 b | 969 ab | 88 | <0.01 | 0.17 | 0.60 |
| Shannon | 7.25 b | 7.67 a | 7.01 b | 7.37 ab | 0.38 | 0.01 | 0.17 | 0.53 |
| species | 883 b | 970 a | 829 b | 914 ab | 82 | <0.01 | 0.17 | 0.66 |
| Chao1 | 1005 ab | 1023 a | 886 b | 964 b | 84 | 0.01 | 0.17 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Nie, X.; Yang, J.; Wang, L.; Zhu, C.; Yang, X.; Jiang, Z. Effect of Resveratrol Supplementation on Intestinal Oxidative Stress, Immunity and Gut Microbiota in Weaned Piglets Challenged with Deoxynivalenol. Antioxidants 2022, 11, 1775. https://doi.org/10.3390/antiox11091775
Qiu Y, Nie X, Yang J, Wang L, Zhu C, Yang X, Jiang Z. Effect of Resveratrol Supplementation on Intestinal Oxidative Stress, Immunity and Gut Microbiota in Weaned Piglets Challenged with Deoxynivalenol. Antioxidants. 2022; 11(9):1775. https://doi.org/10.3390/antiox11091775
Chicago/Turabian StyleQiu, Yueqin, Xinzhi Nie, Jun Yang, Li Wang, Cui Zhu, Xuefen Yang, and Zongyong Jiang. 2022. "Effect of Resveratrol Supplementation on Intestinal Oxidative Stress, Immunity and Gut Microbiota in Weaned Piglets Challenged with Deoxynivalenol" Antioxidants 11, no. 9: 1775. https://doi.org/10.3390/antiox11091775
APA StyleQiu, Y., Nie, X., Yang, J., Wang, L., Zhu, C., Yang, X., & Jiang, Z. (2022). Effect of Resveratrol Supplementation on Intestinal Oxidative Stress, Immunity and Gut Microbiota in Weaned Piglets Challenged with Deoxynivalenol. Antioxidants, 11(9), 1775. https://doi.org/10.3390/antiox11091775










