Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Ethics
2.2. Birds, Husbandry, and Experimental Design
2.3. Diets
2.4. Experimental Procedures
2.5. Laboratory Analysis
2.5.1. Diets Composition
2.5.2. Total Phenolics and Terpenes Analysis of the Plant Mixture
2.5.3. Plasma Oxidative Status
2.5.4. Drip Losses in Muscle Samples
2.5.5. Determination of the Oxidative Stability of the Muscle by the TBARs Method
2.5.6. Instrumental Color Analysis
2.6. Statistical Analysis
3. Results
3.1. Performance Parameters
3.2. Carcass Parameters
3.3. Plasma Oxidative Status
3.4. TBARs Production in Meat, Drip Losses and Changes in Color Parameters
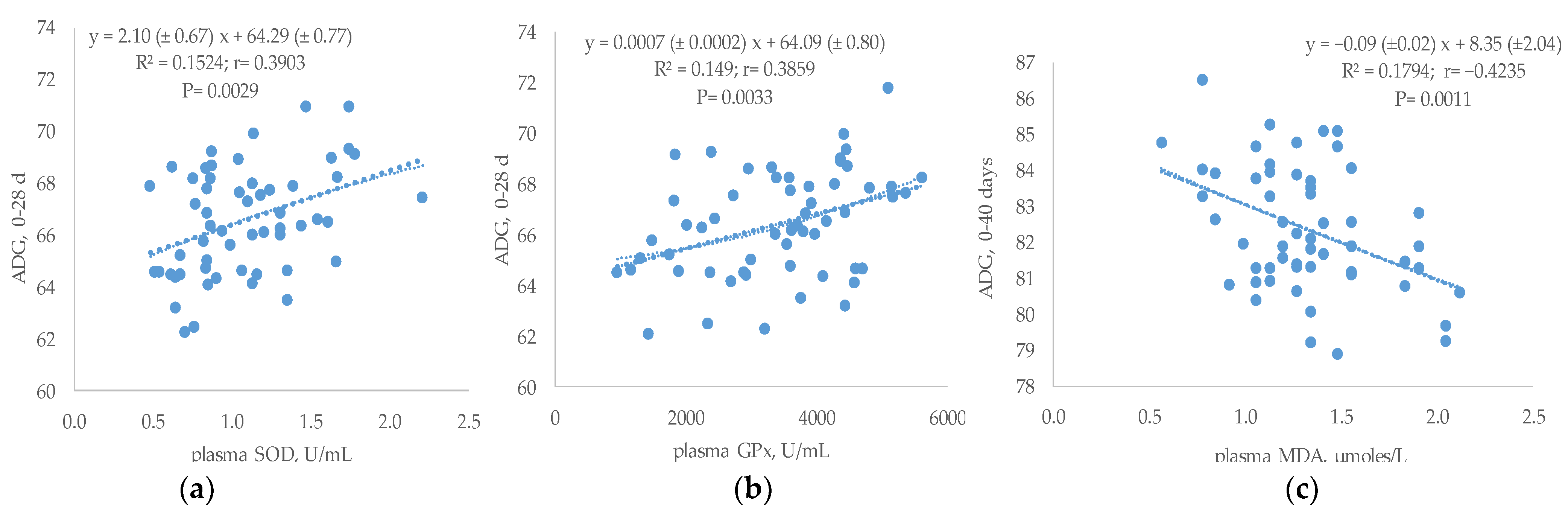
3.5. Relationship between Plasma Antioxidant Status and Performances or Breast TBARs
4. Discussion
4.1. Effect of PSB and OLG-Mix Supplementation in Diets on Performance Parameters
4.2. Effect of PSB and OLG-Mix Supplementation in Diets on Carcass Parameters
4.3. Effect of Dietary PSB and OLG-Mix Supplementation on Oxidative Status of Broilers
4.4. Effect of Dietary PSB and OLG-Mix Supplementation on Meat Lipid Stability
4.5. Effect of PSB and OLG-Mix Supplementation in Diets on Breast Meat Color and Drip Losses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Starter | Grower | Finisher | |
|---|---|---|---|
| (0–12 Days) | (13–28 Days) | (29–40 Days) | |
| Ingredients (g/kg) | |||
| Corn | 250.0 | 150.0 | 100.0 |
| Wheat | 373.5 | 485.5 | 584.5 |
| Molasses | 0.0 | 5.0 | 5.0 |
| Soybean meal, 47% | 331.7 | 304.3 | 248.9 |
| Soybean oil | 10.5 | 26.3 | 36.0 |
| Calcium carbonate | 10.2 | 9.1 | 8.2 |
| Monocalcium phosphate | 9.0 | 6.6 | 4.4 |
| Salt | 3.0 | 2.9 | 2.9 |
| DL-Methionine | 3.0 | 2.5 | 2.3 |
| L-Lysine HCl | 2.7 | 1.9 | 2.0 |
| L-Threonine | 1.4 | 0.9 | 0.8 |
| Vitamin and mineral premix 1 | 5.0 | 5.0 | 5.0 |
| Calculated composition (g/kg of feed) 2 | |||
| Dry matter | 883.5 | 886.1 | 888.1 |
| Ash | 53.4 | 49.6 | 44.5 |
| AMEn (MJ/kg) | 121.4 | 125.7 | 130.0 |
| Crude protein | 217.6 | 207.4 | 187.9 |
| Ether extract | 29.6 | 43.3 | 51.7 |
| Crude fiber | 29.6 | 43.3 | 51.7 |
| Starch | 38.5 | 389.7 | 418.0 |
| Calcium | 9.6 | 8.7 | 7.9 |
| Total phosphorus | 6.0 | 5.3 | 4.6 |
| Available phosphorus | 4.8 | 4.4 | 3.9 |
| Sodium salt | 1.4 | 1.4 | 1.4 |
| Digestible amino acids | |||
| Lys | 11.9 | 10.6 | 9.4 |
| Met | 5.8 | 5.1 | 4.7 |
| Met + Cys | 8.9 | 8.1 | 7.5 |
| Thr | 8.0 | 7.1 | 6.3 |
| Trp | 2.3 | 2.2 | 2.0 |
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Magdelaine, P.; Spiess, M.P.; Valceschini, E. Poultry meat consumption trends in Europe. World’s Poult. Sci. J. 2008, 64, 53–64. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Bosetti, G.E.; Griebler, L.; Aniecevski, E.; Facchi, C.S.; Baggio, C.; Rossatto, G.; Leite, F.; Valentini, F.D.A.; Santo, A.D.; Pagnusatt, H.; et al. Microencapsulated carvacrol and cinnamaldehyde replace growth-promoting antibiotics: Effect on performance and meat quality in broiler chickens. An. Acad. Bras. Cienc. 2020, 92, 1–14. [Google Scholar] [CrossRef]
- Bedford, A.; Gond, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, W.; Gao, F.; Zhou, G. Effect of sodium butyrate on intestinal inflammatory response to lipopolysaccharide in broiler chickens. Can. J. Anim. Sci. 2015, 95, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Gálfi, P.; Neogrády, S. The pH-dependent inhibitory action of n-butyrate on gastrointestinal epithelial cell division. Food Res. Int. 2001, 34, 581–586. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of sodium butyrate on intestinal health of poultry—A review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019, 98, 2858–2865. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Levy, A.W.; Kessler, J.W.; Fuller, L.; Williams, S.; Mathis, G.F.; Lumpkins, B.; Valdez, F. Effect of feeding an encapsulated source of butyric acid (ButiPEARL) on the performance of male Cobb broilers reared to 42 d of age. Poult. Sci. 2015, 94, 1864–1870. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Li, S.; Chang, Q.; An, L.; Zhao, Z. Sodium butyrate enhances growth performance and intestinal development in broilers. Czech J. Anim. Sci. 2020, 65, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.H.; Gao, F.; Zhu, Q.F.; Li, C.; Jiang, Y.; Dai, S.F.; Zhou, G.H. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 2011, 90, 2592–2599. [Google Scholar] [CrossRef]
- Lan, R.; Zhao, Z.; Li, S.; An, L. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions. Poult. Sci. 2020, 99, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Y.; Liu, K.; Fan, R.; Zhou, Z. Dietary sodium butyrate and /or vitamin D3 supplementation alters growth performance, meat quality, chemical composition, and oxidative stability in broilers. Food Chem. 2022, 390, 133138. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, P.J.A.; Morrissey, P.A.; Buckley, D.J. Advances in Research and Application of Vitamin E as an Antioxidant for Poultry Meat. In Proceedings of XII Eur. Symp. Qual. Poult. Meat, Zaragoza, Spain, 1995. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_PS/RossPSHandBook2018.pdf (accessed on 1 September 2022).
- İpçak, H.H.; Alçiçek, A. Addition of Capsicum oleoresin, Carvacrol, Cinnamaldehyde and their mixtures to the broiler diet II: Effects on meat quality. J. Anim. Sci. Technol. 2018, 60, 9. [Google Scholar] [CrossRef]
- Oluwafemi, R.A.; Olawale, A.I.; Alagbe, J.O. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition—A review. Agric. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Rajat, G. A study on antioxidant properties of different bioactive compounds. J. Drug Deliv. Ther. 2014, 4, 105–115. [Google Scholar]
- Rey, A.I.; Hopia, A.; Kivikari, R.; Kahkonen, M. Use of natural food/plant extract: Cloudberry (Rubus Chamaemorus), beetroot (Beta Vulgaris ‘‘Vulgaris’’) or willow herb (Epilobium angustifolium) to reduce lipid oxidation of cooked pork patties. LWT 2004, 38, 363–370. [Google Scholar] [CrossRef]
- Luna, A.; Lábaque, M.C.; Zygadlo, J.A.; Marin, R.H. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 2010, 89, 366–370. [Google Scholar] [CrossRef]
- Šojić, B.; Tomović, V.; Džinić1, N.; Jokanović, M.; Ikonić, P.; Škaljac, S.; Pavlić, B. Plant extracts as natural antioxidants in meat processing. Bulg. J. Agric. Sci. 2019, 25, 27–30. [Google Scholar]
- Rey, A.I.; de-Cara, A.; Segura, J.F.; Martí, P.; Hechavarría, T.; Calvo, L. Dietary oleuropein extract supplementation and their combination with α-tocopheryl acetate and selenium modifies free fatty acid profile and improves stability of pork. J. Sci. Food Agric. 2020, 101, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- BOE. RD 53/2013, de 21 de octubre por la que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia, Spain. Boletín Oficial del Estado. 2013, 252, 34367–34391. [Google Scholar]
- EC. Council Regulation (EC) No 2009/1099/CE of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union. 2009, 303, 1–27. [Google Scholar]
- Ross, an Aviagen brand. Broiler Management Handbook; Aviagen. 2018, pp. 1–147. Available online: https://ap.aviagen.com/?persist_locale=1 (accessed on 1 September 2022).
- de Blas, C.; García-Rebollar, P.; Mateos, G.G. Revisión de las Normas FEDNA Para la Fabricación de Piensos Compuestos; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2017. [Google Scholar]
- de Blas, C.; García-Rebollar, P.; Gorrachategui, M.; Mateos, G.G. Tablas FEDNA de Composición y Valor Nutritivo de Alimentos Para la Fabricación de Piensos Compuestos, 4th ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2019. [Google Scholar]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Ratanpaul, V.; Williams, B.A.; Black, J.L.; Gidley, M.J. Apparent amylase diffusion rates in milled cereal grains determined in vitro: Potential relevance to digestion in the small intestine of pigs. J. Cereal Sci. 2018, 82, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Vander Auwera, J.; Bologne, G.; Roelandts, I.; Duchesne, J.C. Inductively coupled plasma-mass spectrometric (ICP-MS) analysis of silicate rocks and minerals. Geol. Belg. 1998, 1, 49–53. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; Palazzo de Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinda, Á.; Lanzón, A.; Rios, J.J.; Albi, T. Aislamiento y cuantificación de los componentes de la hoja del olivo: Extracto de hexano. Grasas y Aceites. 2002, 53, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Rey, A.I.; de-Cara, A.; Calvo, L.; Puig, P.; Hechavarría, T. Changes in plasma fatty acids, free amino acids, antioxidant defense, and physiological stress by Oleuropein supplementation in pigs prior to slaughter. Antioxidants 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Nirungsan, K.; Thongnopnua, P. Simple and rapid high-performance liquid chromatographic method for endogenous α-tocopherol determination in human plasma. Biomed. Chromatogr. 2006, 20, 774–781. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method. Enzymol. 1999, 299, 15–27. [Google Scholar]
- Slaughter, M.R.; O’Brien, P.J. Fully-automated spectrophotometric method for measurement of antioxidant activity of catalase. Clin. Biochem. 2000, 33, 525–534. [Google Scholar] [CrossRef]
- Honikel, K.O.; Kim, C.J.; Hamm, R.; Roncales, P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986, 16, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.D.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]
- CIE. International commission on illumination, recommendations on uniform colour spaces, colour difference equations, psychometric colour terms. CIE Publ. No. 15 (E-1.3. 1) 1971/(TO-1.3) 1978, 2, 5–6. [Google Scholar]
- Van der Wielen, P.W.J.J.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; Van Kapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guo, Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim. Feed Sci. Technol. 2007, 132, 240–249. [Google Scholar] [CrossRef]
- Ogwuegbu, M.C.; Oyeagu, C.E.; Edeh, H.O.; Dim, C.E.; Ani, A.O.; Lewu, F.B. Effects of sodium butyrate and rosemary leaf meal on general performance, carcass traits, organ sizes and nutrient digestibility of broiler chickens. Iran. J. Appl. Anim. Sci. 2021, 11, 365–379. [Google Scholar]
- Ravindran, V.; Abdollahi, M.R. Nutrition and digestive physiology of the broiler chick: State of the art and outlook. Animals 2021, 11, 2795. [Google Scholar] [CrossRef]
- Sierzant, K.; Orda, J.; Korzeniowska, M.; Malicki, A. Effect of dietary supplementation with extracts of rosemary, olive leaves, pine bark and quercetin on selected performance indices of broiler chickens and microbiological status of their ileum. Med. Weter. 2019, 75, 247–252. [Google Scholar] [CrossRef]
- Xie, P.; Deng, Y.; Huang, L.; Zhang, C. Effect of olive leaf (Olea europaea L.) extract addition to broiler diets on the growth performance, breast meat quality, antioxidant capacity and caecal bacterial populations. Ital. J. Anim. Sci. 2022, 21, 1246–1258. [Google Scholar] [CrossRef]
- Sarica, S.; Urkmez, D. The use of grape seed, olive leaf and pomegranate peel extracts as alternative natural antimicrobial feed additives in broiler diets. Europ. Poult. Sci. 2016, 80, 1–13. [Google Scholar]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, B.; Robinson, K.; Belem, T.; Lyu, W.; Deng, Z.; Ramanathan1, R.; Zhang, G. Butyrate in combination with forskolin alleviates necrotic enteritis, increases feed efficiency, and improves carcass composition of broilers. J. Anim. Sci. Biotechnol. 2022, 13, 3. [Google Scholar] [CrossRef]
- Mátis, G.; Petrilla, J.; Kulcsár, A.; van den Bighelaar, H.; Boomsma, B.; Neogrády, Z.; Fébel, H. Effects of dietary butyrate supplementation and crude protein level on carcass traits and meat composition of broiler chickens. Arch. Anim. Breed. 2019, 62, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Yu, H.; Lepp, D.; Shi, X.; Yang, X.; Hu, J.; Leeson, S.; Yang, C.; Nie, S.; Hou, Y.; et al. Transcriptome analysis reveals regulation of gene expression for lipid catabolism in young broilers by butyrate glycerides. PLoS ONE. 2016, 11, e0160751. [Google Scholar]
- Shafey, T.M.; Almufarij, S.I.; Albatshan, H.A. Effect of feeding olive leaves on the performance, intestinal and carcass characteristics of broiler chickens. Int. J. Agric. Biol. 2013, 13, 585–589. [Google Scholar]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Vlaicu, P.A.; Badea, I.A.; Mironeasa, S. Effects of grape seed oil supplementation to broilers diets on growth performance, meat fatty acids, health lipid indices and lipid oxidation parameters. Agriculture 2021, 11, 404. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Evans, M.D.; Griffiths, H.R.; Lunec, J. Reactive oxygen species and their cytotoxic mechanisms. Adv. Mol. Cell Biol. 1997, 20, 25–73. [Google Scholar]
- Xing, X.; Jiang, Z.; Tang, X.; Wang, P.; Li, Y.; Sun, Y.; Le, G.; Zou, S. Sodium butyrate protects against oxidative stress in HepG2 cells through modulation Nrf2 pathway and mitochondrial function. J. Physiol. Biochem. 2017, 73, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jia, Y.; Zhao, N.; Hu, Y.; Hong, J.; Gao, S.; Zhao, R. Sodium butyrate protects against high-fat diet-induced oxidative stress in rat liver by promoting expression of nuclear factor E2-related factor 2. Br. J. Nutr. 2019, 122, 400–410. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.A.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAM8 mice by induction of Nrf2-dependent gene ex-pression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition—A review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Hou, C.; Yang, L.; Li, H.; Guo, J.; Huo, C.; Wang, M.; Miao, Y.; Liu, J.; et al. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nu-cleus of spontaneously hypertensive rats. Neuropharmacology 2017, 113, 556–566. [Google Scholar] [CrossRef]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef]
- Aljebory, A.F.O.; Ibrahim, D.K. Effect of adding two levels of Resveratrol and Oleuropein to the diet on some biochemical traits of blood and gene expression for heat shock protein (HSP70) in the liver for broiler chickens reared under heat stress conditions. Ann. Romanian Soc. Cell Biol. 2021, 25, 13996–14011. [Google Scholar]
- El-Damrawy, S.Z.; Khalifah, M.M.; Fares, W.A. Dietary olive leaf and antioxidative status in chickens "performance, some physiological traits and immunological responses of mandarah chicks supplemented olive leaves powder in their diets". Egypt. Poult. Sci. 2012, 33, 279–287. [Google Scholar]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrolos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Tufarelli, V.; Laudadio, V.; Casalino, E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. 2016, 23, 6197–6204. [Google Scholar] [CrossRef]
- Wang, M.L.; Suo, X.; Gu, J.H.; Zhang, W.W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Blount, J.D.; Forbes, S.; Royle, N.J. Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct. Ecol. 2010, 24, 365–373. [Google Scholar] [CrossRef]
- Hung, Y.T.; Hanson, A.R.; Shurson, G.C.; Urriola, P.E. Peroxidized lipids reduce growth performance of poultry and swine: A meta-analysis. Anim. Feed Sci. Technol. 2017, 231, 47–58. [Google Scholar] [CrossRef]
- Shah, M.A.; Don Bosco, S.J.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Romero, C.; Nardoia, M.; Arija, I.; Viveros, A.; Rey, A.I.; Prodanov, M.; Chamorro, S. Feeding broiler chickens with grape seed and skin meals to enhance α- and γ-tocopherol content and meat oxidative stability. Antioxidants. 2021, 10, 699. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Soica, C.; Iuga, M.; Mironeasa, S. Effects of supplementing grape pomace to broilers fed polyunsaturated fatty acids enriched diets on meat quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Sarica, S.; Urkmez, D. Comparison of the effects of dietary supplementation of natural antimicrobial feed additives on lipid oxidation, microbial content and quality of broiler raw meat. Turk. J. Agric. 2018, 6, 1537–1543. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, A.I.; Menoyo, D.; Segura, J.; López-Bote, C.J.; Calvo, L. Combination of dietary glycaemic index and fasting time prior to slaughter as strategy to modify quality of pork. Meat Sci. 2020, 161, 108013. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Martino, C.; Grotta, L.; Martino, G. Evaluation of chemical composition and meat quality of breast muscle in broilers reared under light-emitting diode. Animals 2021, 11, 1505. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the color of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, Y.; Wu, W.; Yin, D.; Sun, X.; Guo, X.; Chen, J.; Mahmood, T.; Yan, L.; Yuan, J. Effects of nicotinamide and sodium butyrate on meat quality and muscle ubiquitination degradation genes in broilers reared at a high stocking density. Poult. Sci. 2020, 99, 1462–1470. [Google Scholar] [CrossRef]
- Grashorn, M.A. Use of phytobiotics in broiler nutrition—An alternative to infeed antibiotics? J. Anim. Feed Sci. 2010, 19, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Fernandes da Silva, D.C.; Varela de Arruda, A.M.; Gonçalves, A.A. Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J. Food Sci. Technol. 2017, 54, 1818–1826. [Google Scholar] [CrossRef]
- Warriss, D.; Bevis, E.A.; Ekins, P.J. The relationships between glycogen stores and muscle ultimate pH in commercially slaughtered pigs. Br. Vet. J. 1989, 145, 378–383. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Balzan, S.; Cardazzo, B.; Novelli, E.; Carraro, L.; Fontana, F.; Currò, S.; Laghetto, M.; Trocino, A.; Xiccato, G.; Taticchi, A.; et al. Employment of phenolic compounds from olive vegetation water in broiler chickens: Effects on gut microbiota and on the shelf life of breast fillets. Molecules 2021, 26, 4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Zhao, X.; Chen, X.; Wang, L.; Geng, Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 2018, 98, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.; Toldrá, F.; Aristoy, M.C.; López-Bote, C.J.; Rey, A.I. Effect of dietary organic selenium on muscle proteolytic activity and water-holding capacity in pork. Meat Sci. 2016, 121, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Calculated Composition | OLG-Mix |
|---|---|
| Ash | 139.0 |
| Crude protein | 56.0 |
| Ether extract | 92.0 |
| Crude fiber | 329.0 |
| Digestible Lysine | 1.0 |
| Analyzed composition | OLG-mix |
| (mg GAE/kg) | |
| Polyphenols | 7000 |
| Terpenes | 70,000 |
| PSB (g/kg) | OLG-Mix (g/kg) | 0–12 Days | 13–28 Days | 29–40 Days | 0–40 Days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADG | ADFI | FCR | ADG | ADFI | FCR | ADG | ADFI | FCR | ADG | ADFI | FCR | |||||
| 0 | 0 | 33.1 b | 39.2 | 1.19 a | 91.1 | 127 | 1.40 | 121 a | 211 | 1.75 b | 82.1 | 125 | 1.52 | |||
| 2 | 0 | 33.6 a | 38.8 | 1.16 b | 92.5 | 127 | 1.38 | 117 b | 209 | 1.79 a | 81.8 | 124 | 1.52 | |||
| 0 | 2 | 32.5 c | 38.6 | 1.19 a | 90.5 | 127 | 1.40 | 121 a | 212 | 1.75 b | 82.1 | 125 | 1.53 | |||
| 2 | 2 | 33.3 ab | 38.5 | 1.16 b | 91.9 | 127 | 1.38 | 117 b | 210 | 1.80 a | 81.3 | 124 | 1.53 | |||
| Main effects | ||||||||||||||||
| PSB | ||||||||||||||||
| 0 | 32.8 | 38.9 | 1.19 | 90.8 | 127 | 1.40 | 121 | 211 | 1.75 | 82.1 | 125 | 1.53 | ||||
| 2 | 33.4 | 38.7 | 1.16 | 92.2 | 127 | 1.38 | 117 | 210 | 1.79 | 81.5 | 124 | 1.52 | ||||
| OLG-mix | ||||||||||||||||
| 0 | 33.3 | 39.0 | 1.17 | 91.8 | 127 | 1.39 | 119 | 210 | 1.77 | 82.0 | 125 | 1.52 | ||||
| 2 | 32.9 | 38.6 | 1.17 | 91.2 | 127 | 1.39 | 119 | 211 | 1.77 | 81.7 | 125 | 1.53 | ||||
| SD 1 | 0.936 | 1.109 | 0.023 | 3.627 | 3.075 | 0.040 | 6.454 | 6.378 | 0.074 | 1.479 | 2.355 | 0.021 | ||||
| p-value 2 | ||||||||||||||||
| General | 0.0131 | 0.4172 | < 0.0001 | 0.4605 | 0.9474 | 0.2641 | 0.1583 | 0.7201 | 0.1495 | 0.3700 | 0.369 | 0.858 | ||||
| PSB | 0.0068 | 0.4712 | < 0.0001 | 0.1376 | 0.9918 | 0.0507 | 0.0253 | 0.3038 | 0.0253 | 0.122 | 0.086 | 0.726 | ||||
| OLG-mix | 0.0696 | 0.1472 | 0.5616 | 0.5563 | 0.5526 | 0.7688 | 0.8669 | 0.6128 | 0.8116 | 0.533 | 0.957 | 0.448 | ||||
| PSBxOLG-mix | 0.5085 | 0.6575 | 0.7432 | 0.9912 | 0.9408 | 0.9314 | 0.7804 | 0.9401 | 0.6589 | 0.561 | 0.703 | 0.819 | ||||
| PSB (g/kg) | OLG-Mix (g/kg) | Mortality (%) | EBI | |||||
|---|---|---|---|---|---|---|---|---|
| 0–12 Days | 13–28 Days | 29–40 Days | 0–40 Days | 0–40 Days | ||||
| 0 | 0 | 0.91 | 1.55 | 0.65 | 3.03 | 522.12 | ||
| 2 | 0 | 1.52 | 1.07 | 1.07 | 3.85 | 513.58 | ||
| 0 | 2 | 0.61 | 1.23 | 0.65 | 2.42 | 521.09 | ||
| 2 | 2 | 0.97 | 1.24 | 0.85 | 3.31 | 512.21 | ||
| Main effects | ||||||||
| PSB | ||||||||
| 0 | 0.76 | 1.39 | 0.65 | 2.73 | 521.60 | |||
| 2 | 1.24 | 1.15 | 0.96 | 3.58 | 512.90 | |||
| OLG-mix | ||||||||
| 0 | 1.21 | 1.31 | 0.86 | 3.44 | 517.85 | |||
| 2 | 0.79 | 1.23 | 0.75 | 2.87 | 516.65 | |||
| SD 1 | 2.278 | 2.136 | 1.833 | 4.011 | 26.283 | |||
| p-value 2 | ||||||||
| General | 0.744 | 0.9447 | 0.9242 | 0.8233 | 0.3903 | |||
| PSB | 0.4155 | 0.6923 | 0.5489 | 0.4443 | 0.2329 | |||
| OLG-mix | 0.4801 | 0.9022 | 0.8258 | 0.6048 | 0.8684 | |||
| PSBxOLG-mix | 0.8418 | 0.676 | 0.8306 | 0.9763 | 0.9812 | |||
| PSB (g/kg) | OLG-Mix (g/kg) | CY | BY | TY | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BW | CY | BW | CY | |||||||
| 0 | 0 | 799 | 209 | 262 | 202 a | 253 a | ||||
| 2 | 0 | 798 | 206 | 258 | 194 b | 244 b | ||||
| 0 | 2 | 799 | 206 | 258 | 198 ab | 248 ab | ||||
| 2 | 2 | 799 | 205 | 257 | 194 b | 243 b | ||||
| Main effects | ||||||||||
| PSB | ||||||||||
| 0 | 799 | 208 | 260 | 200 | 250 | |||||
| 2 | 798 | 206 | 258 | 194 | 243 | |||||
| OLG-mix | ||||||||||
| 0 | 799 | 208 | 260 | 198 | 248 | |||||
| 2 | 799 | 206 | 257 | 196 | 245 | |||||
| SD 1 | 0.998 | 1.087 | 1.372 | 1.105 | 1.303 | |||||
| p-value 2 | ||||||||||
| General | 0.9837 | 0.7215 | 0.7526 | 0.1470 | 0.129 | |||||
| PSB | 0.7616 | 0.4443 | 0.5053 | 0.0413 | 0.0364 | |||||
| OLG-mix | 0.8889 | 0.4780 | 0.4619 | 0.4005 | 0.3598 | |||||
| PSBxOLG-mix | 0.8300 | 0.6316 | 0.6520 | 0.4834 | 0.5006 | |||||
| PSB (g/kg) | OLG-mix (g/kg) | MDA (µmol/L) 1 | FRAP (mmol/L) 2 | SOD (U/mL) 3 | CAT (U/mL) 4 | GPx (U/L) 5 | Vit E (µg/mL) 6 |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 1.33 | 0.76 | 0.83 b | 0.05 | 3.58 | 1.04 |
| 2 | 0 | 1.35 | 0.72 | 1.14 a | 0.10 | 3.76 | 1.10 |
| 0 | 2 | 1.34 | 0.80 | 1.13 a | 0.04 | 3.07 | 1.12 |
| 2 | 2 | 1.32 | 0.73 | 1.21 a | 0.08 | 3.62 | 1.12 |
| Main effects | |||||||
| PSB | |||||||
| 0 | 1.33 | 0.78 | 0.98 | 0.04 | 3.33 | 1.08 | |
| 2 | 1.34 | 0.73 | 1.17 | 0.09 | 3.69 | 1.11 | |
| OLG-mix | |||||||
| 0 | 1.34 | 0.74 | 0.98 | 0.08 | 3.67 | 1.07 | |
| 2 | 1.33 | 0.77 | 1.17 | 0.06 | 3.35 | 1.12 | |
| SD 7 | 0.361 | 0.236 | 0.354 | 0.120 | 1.004 | 0.245 | |
| p-value 8 | |||||||
| General | 0.9955 | 0.8104 | 0.0260 | 0.4837 | 0.3241 | 0.7835 | |
| PSB | 0.9507 | 0.4107 | 0.0434 | 0.1518 | 0.1895 | 0.6493 | |
| OLG-mix | 0.9328 | 0.6526 | 0.0471 | 0.6007 | 0.2442 | 0.4040 | |
| PSBx OLG-mix | 0.8176 | 0.7734 | 0.2131 | 0.7818 | 0.5006 | 0.6719 |
| PSB (g/kg) | OLG-Mix (g/kg) | 0 Days 1 | 3 Days 2 | 6 Days 3 |
|---|---|---|---|---|
| 0 | 0 | 0.36 a | 1.00 a | 1.79 a |
| 2 | 0 | 0.30 b | 0.67 b | 1.09 b |
| 0 | 2 | 0.35 a | 0.91 ba | 1.25 b |
| 2 | 2 | 0.31 b | 0.73 b | 1.16 b |
| Main effects | ||||
| PSB | ||||
| 0 | 0.36 | 0.95 | 1.52 | |
| 2 | 0.30 | 0.70 | 1.12 | |
| OLG-mix | ||||
| 0 | 0.33 | 0.83 | 1.44 | |
| 2 | 0.33 | 0.82 | 1.20 | |
| SD 4 | 0.080 | 0.356 | 0.475 | |
| p-value 5 | ||||
| General | 0.0950 | 0.0520 | 0.0005 | |
| PSB | 0.0138 | 0.0082 | 0.0018 | |
| OLG-mix | 0.9185 | 0.8044 | 0.0519 | |
| PSBxOLG-mix | 0.6775 | 0.4287 | 0.0188 |
| PSB (g/kg) | OLG-Mix (g/kg) | L* 1 | a* 2 | b* 3 | hue_angle 4 | Chroma 5 | Drip Losses |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 54.64 | −0.25 | 7.09 b | −0.46 | 7.21 b | 56.0 ab |
| 2 | 0 | 56.94 | 0.24 | 8.03 ba | 0.49 | 8.11 ba | 67.2 a |
| 0 | 2 | 57.51 | −0.07 | 8.73 ba | −0.08 | 8.83 ba | 43.0 b |
| 2 | 2 | 56.04 | 0.68 | 9.35 a | 0.25 | 9.47 a | 56.9 ab |
| Main effects | |||||||
| PSB | |||||||
| 0 | 56.14 | −0.16 | 7.91 | −0.27 | 8.02 | 49.5 | |
| 2 | 56.50 | 0.46 | 8.69 | 0.37 | 8.79 | 62.1 | |
| OLG-mix | |||||||
| 0 | 55.77 | −0.002 | 7.56 | 0.02 | 7.66 | 61.6 | |
| 2 | 56.78 | 0.31 | 9.04 | 0.09 | 9.15 | 50.0 | |
| SD 6 | 4.390 | 1.300 | 2.624 | 0.165 | 2.622 | 2.335 | |
| p-value 7 | |||||||
| General | 0.3112 | 0.2413 | 0.1174 | 0.3105 | 0.1166 | 0.0792 | |
| PSB | 0.7143 | 0.0730 | 0.2557 | 0.0912 | 0.2625 | 0.0484 | |
| OLG-mix | 0.3881 | 0.3645 | 0.0332 | 0.8577 | 0.0318 | 0.0668 | |
| PSBxOLG-mix | 0.1019 | 0.7096 | 0.8058 | 0.4140 | 0.8504 | 0.8299 |
| Y | Treatment | Intercept | Variable X | r | R2 | RSD 1 | p (Lineal) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma Vitamin E (x) | |||||||||||||
| Plasma MDA | PSB-0 | 2.02 | ± | 0.17 | −0.64 | a | ± | 0.15 | −0.5255 | 0.2572 | 0.26 | 0.0001 | |
| Plasma MDA | PSB-2 | 2.08 | ± | 0.26 | −0.68 | a | ± | 0.23 | −0.5099 | 0.2600 | 0.30 | 0.0078 | |
| Plasma MDA | OLG-mix-0 | 2.33 | ± | 0.22 | −0.91 | a | ± | 0.20 | −0.6846 | 0.4686 | 0.25 | 0.0001 | |
| Plasma MDA | OLG-mix 2 | 2.05 | ± | 0.23 | −0.60 | b | ± | 0.20 | −0.5511 | 0.3037 | 0.21 | 0.0064 | |
| Plasma SOD (x) | |||||||||||||
| Muscle MDA | PSB-0 | 3.45 | ± | 0.32 | −0.74 | a | ± | 0.32 | −0.4762 | 0.2268 | 0.44 | 0.0216 | |
| Muscle MDA | PSB-2 | 2.63 | ± | 0.23 | −0.48 | b | ± | 0.18 | −0.5102 | 0.2603 | 0.36 | 0.0129 | |
| Muscle MDA | OLG-mix 0 | 3.40 | ± | 0.39 | −0.89 | a | ± | 0.37 | −0.4542 | 0.2063 | 0.58 | 0.0258 | |
| Muscle MDA | OLG-mix 2 | 2.67 | ± | 0.24 | −0.40 | b | ± | 0.20 | −0.3752 | 0.1407 | 0.41 | 0.0500 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de-Cara, A.; Saldaña, B.; Vázquez, P.; Rey, A.I. Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants 2023, 12, 201. https://doi.org/10.3390/antiox12010201
de-Cara A, Saldaña B, Vázquez P, Rey AI. Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants. 2023; 12(1):201. https://doi.org/10.3390/antiox12010201
Chicago/Turabian Stylede-Cara, Almudena, Beatriz Saldaña, Patricia Vázquez, and Ana I Rey. 2023. "Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers" Antioxidants 12, no. 1: 201. https://doi.org/10.3390/antiox12010201
APA Stylede-Cara, A., Saldaña, B., Vázquez, P., & Rey, A. I. (2023). Dietary Protected Sodium Butyrate and/or Olive Leaf and Grape-Based By-Product Supplementation Modifies Productive Performance, Antioxidant Status and Meat Quality in Broilers. Antioxidants, 12(1), 201. https://doi.org/10.3390/antiox12010201








