Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
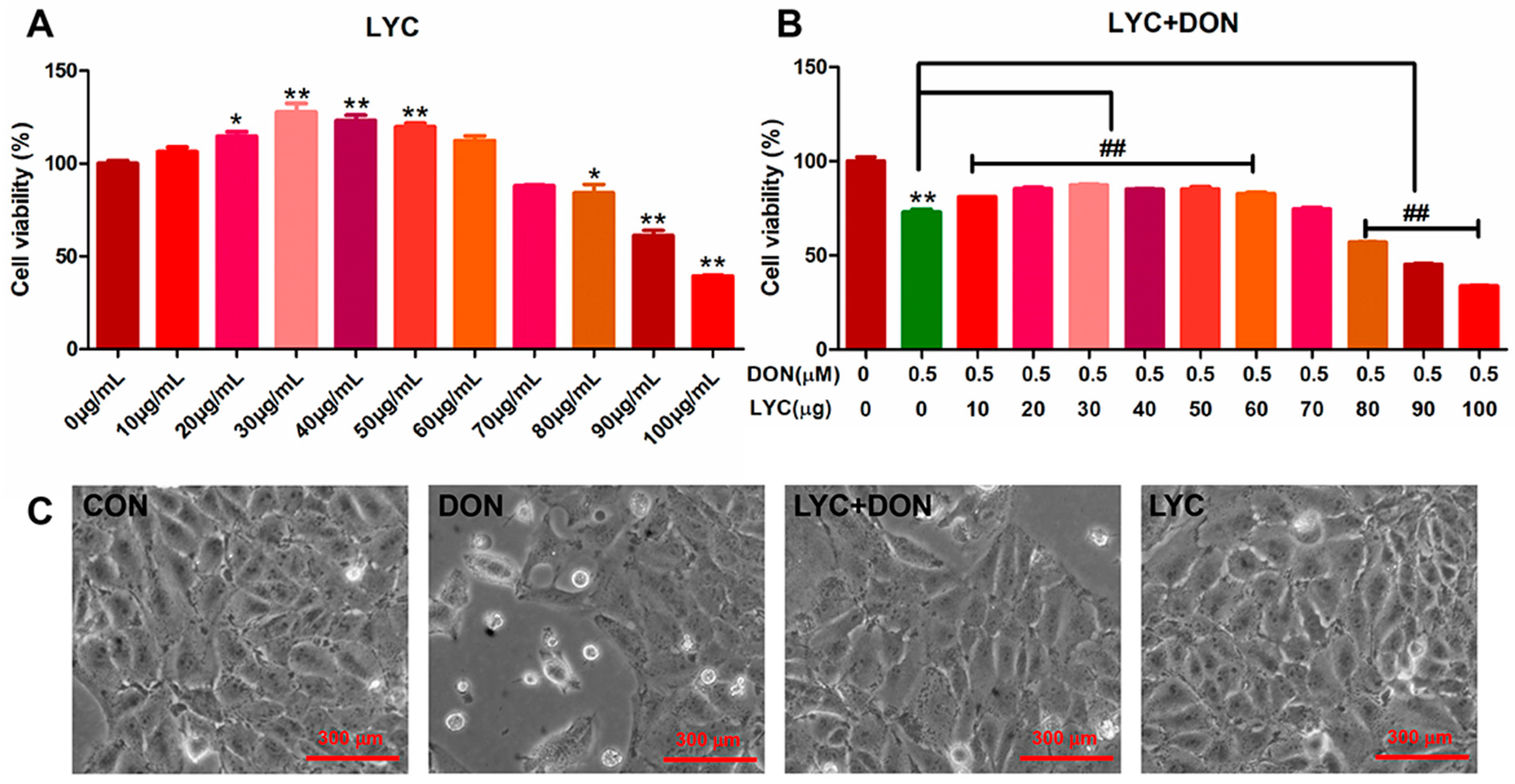
2.2. Cell Viability Assay
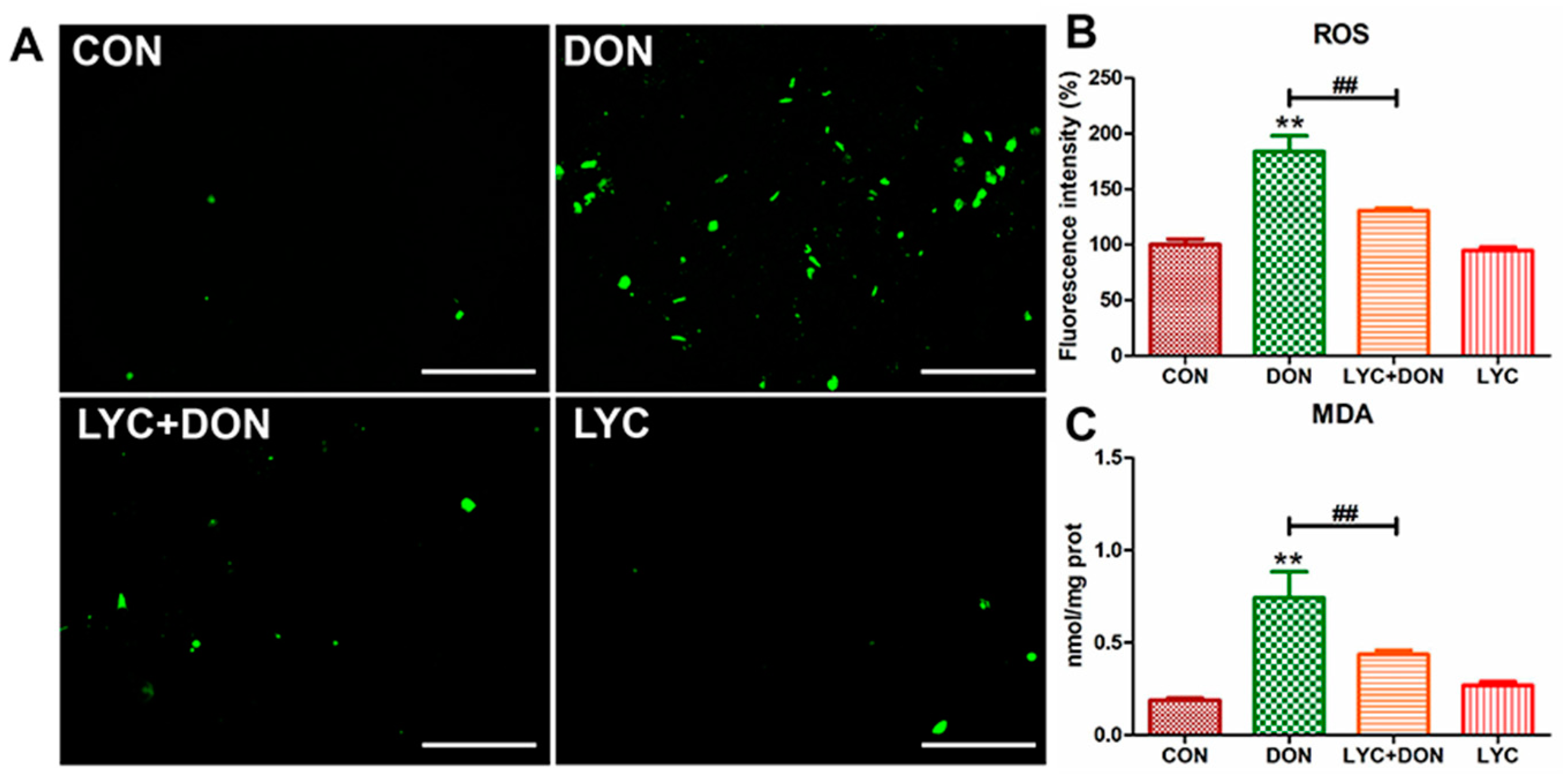
2.3. Malondialdehyde (MDA) Assay
2.4. Reactive Oxygen Species (ROS) Assay
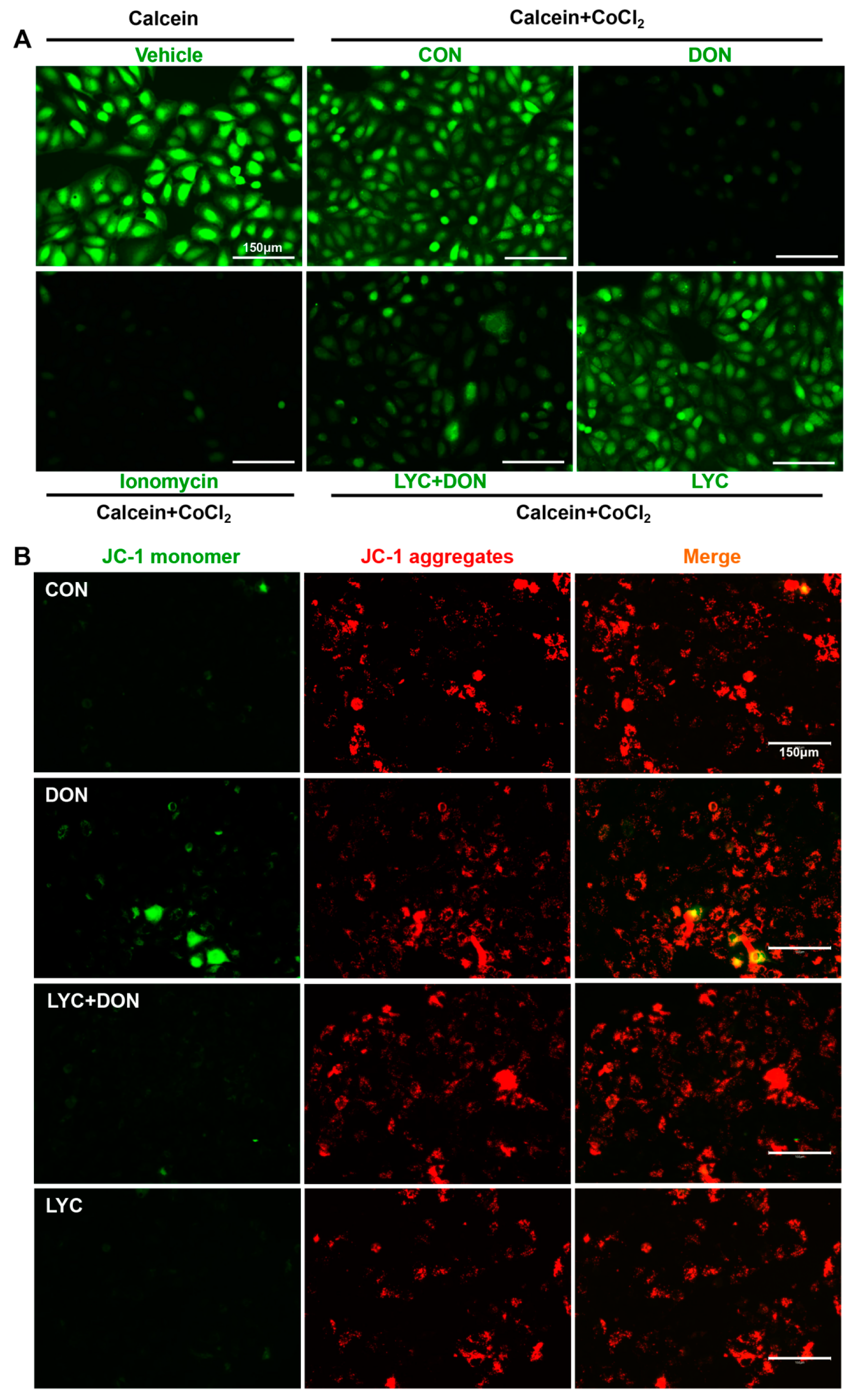
2.5. Mitochondrial Membrane Potential (MMP) Assay
2.6. Mitochondrial Permeability Transition Pore (MPTP) Assay
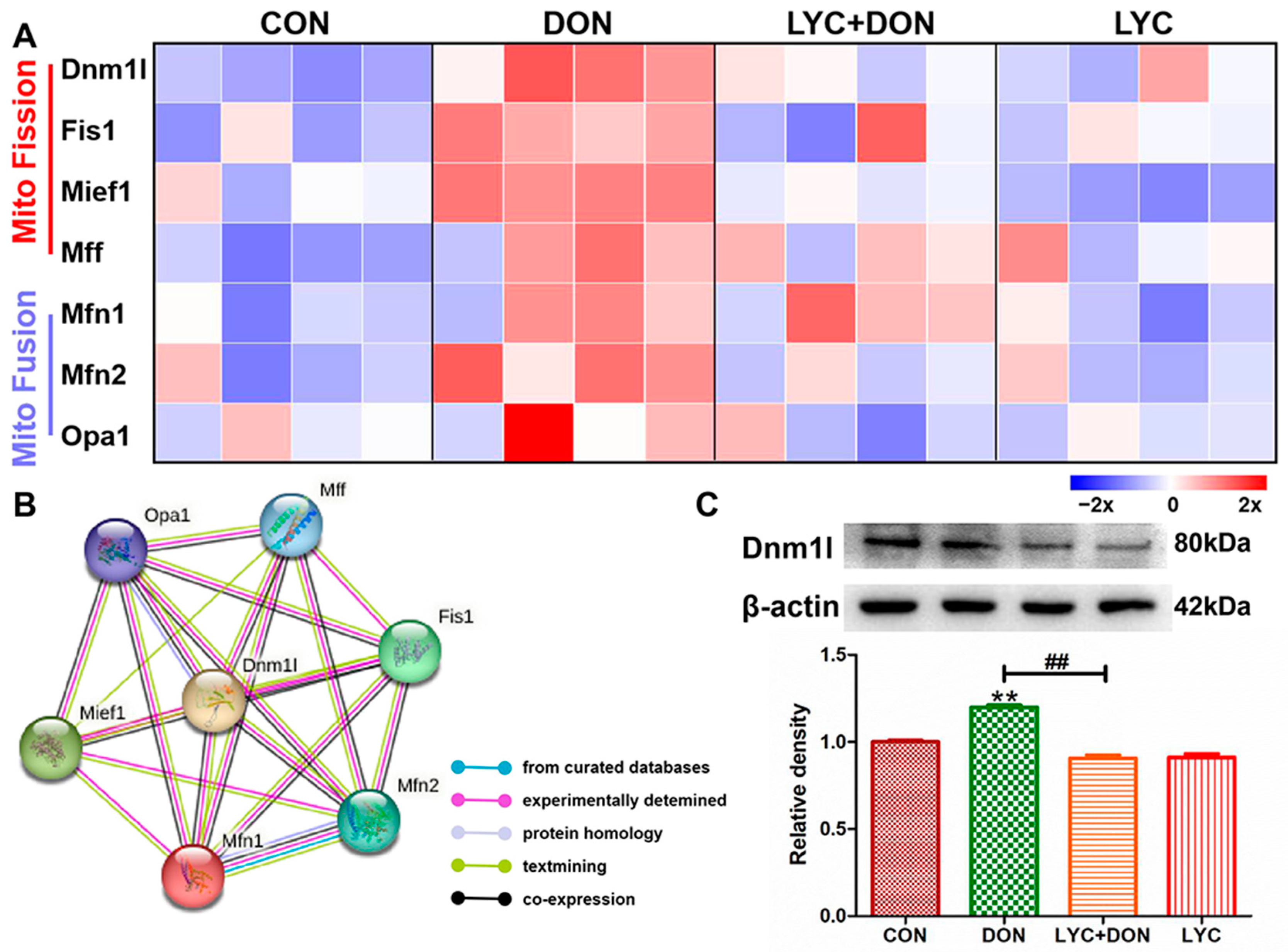
2.7. qRT-PCR Analysis
2.8. Western Blot Analysis
2.9. Data Statistics and Analysis
3. Results
3.1. LYC Alleviated DON Induced IPEC-J2 Cells Damage
3.2. LYC Alleviated DON Induced Intestinal Epithelial Barrier Impairment in IPEC-J2 Cells
3.3. LYC Alleviated DON Induced Oxidative Stress in IPEC-J2 Cells
3.4. LYC Alleviated DON Induced Mitochondrial Impairment in IPEC-J2 Cells
3.5. LYC Alleviated DON Induced Mitochondrial Dynamics Disturbance in IPEC-J2 Cells
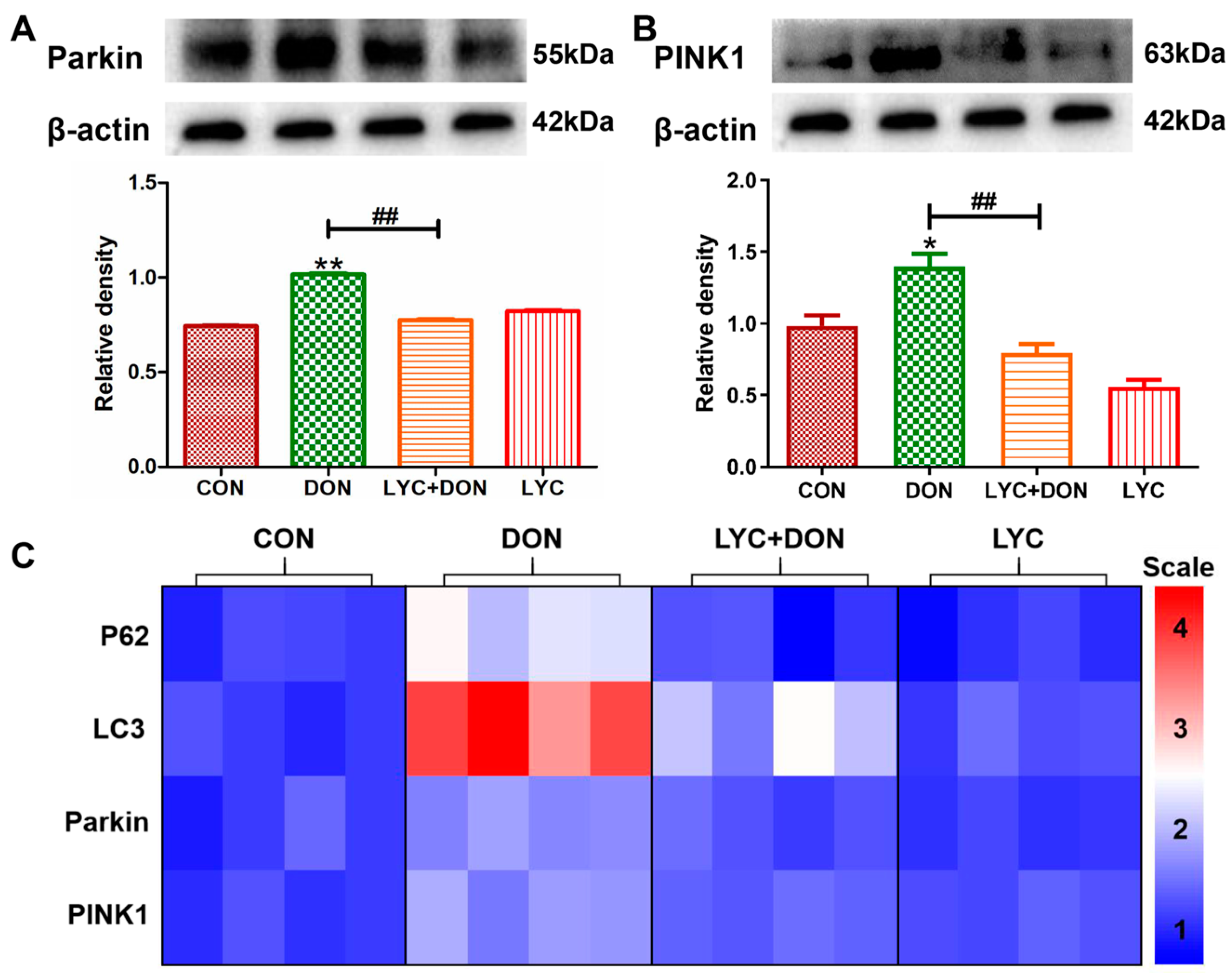
3.6. LYC Alleviated DON Induced Mitophagy in IPEC-J2 Cell
3.7. Correlation and PCA Analysis
4. Discussion
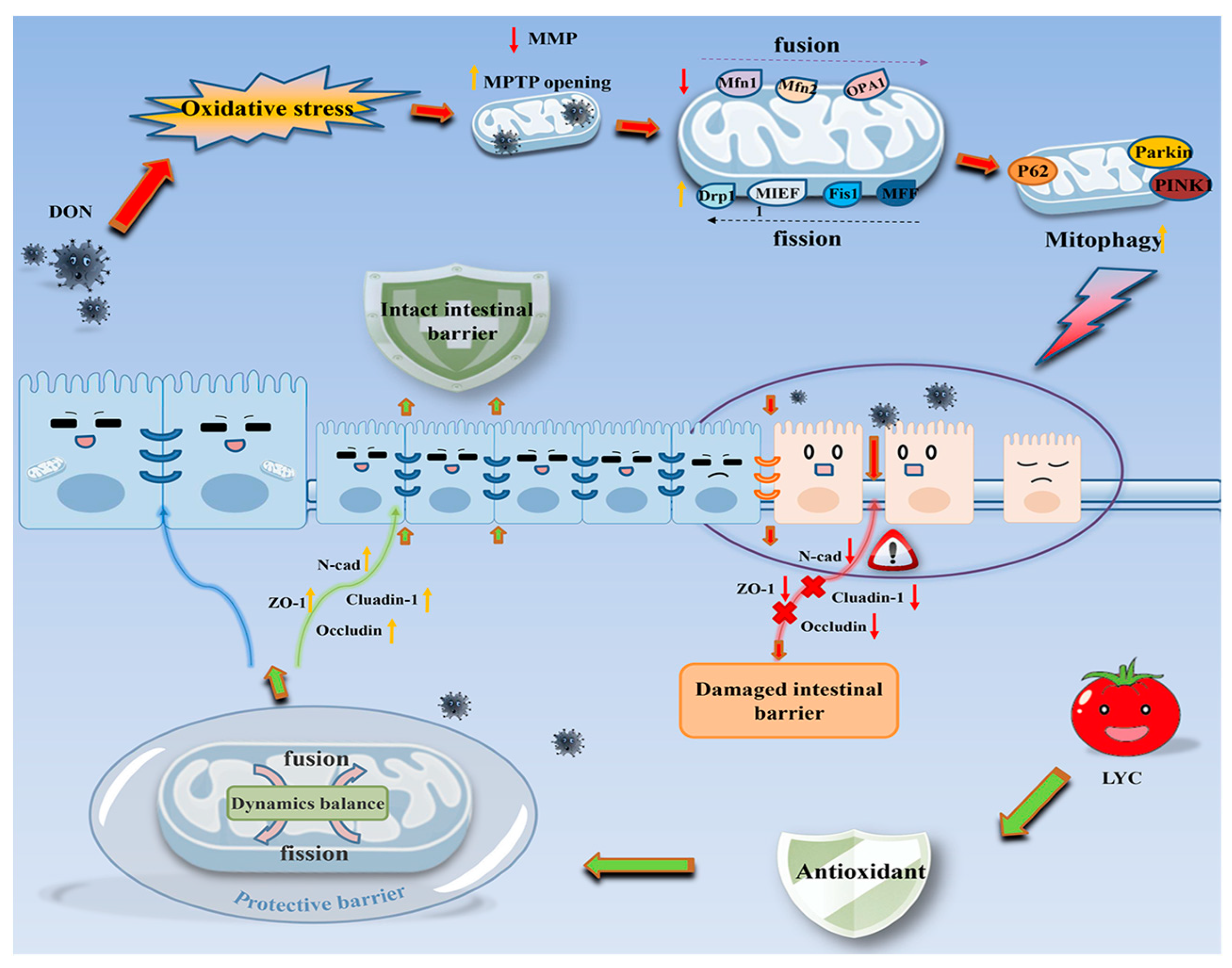
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.-G.; Zhu, M.; Chen, M.-X.; Fan, H.-B.; Fu, H.-L.; Zhou, J.-Y.; Zhai, Z.-Y.; Gao, C.-Q.; Yan, H.-C.; Wang, X.-Q. Acute exposure to deoxynivalenol inhibits porcine enteroid activity via suppression of the Wnt/β-catenin pathway. Toxicol. Lett. 2019, 305, 19–31. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Tahoun, I.F.; Yamani, R.N.; Rend, E.A.; Shehata, A.B. Natural occurrence of deoxynivalenol, nivalenol and deoxynivalenol-3-glucoside in cereal-derived products from Egypt. Food Control 2022, 137, 108974. [Google Scholar] [CrossRef]
- Cao, K.; Xie, C.; Wang, M.; Wang, P.; Gu, Z.; Yang, R. Effects of soaking and germination on deoxynivalenol content, nutrition and functional quality of Fusarium naturally contaminated wheat. LWT 2022, 160, 113324. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, K.; Li, J.; Li, J.; Bi, C.; Shan, A. Deoxynivalenol exposure induces liver damage in mice: Inflammation and immune responses, oxidative stress, and protective effects of Lactobacillus rhamnosus GG. Food Chem. Toxicol. 2021, 156, 112514. [Google Scholar] [CrossRef]
- Chen, C.; Frank, K.; Wang, T.; Wu, F. Global wheat trade and Codex Alimentarius guidelines for deoxynivalenol: A mycotoxin common in wheat. Glob. Food Secur. 2021, 29, 100538. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Zhao, L.; Fan, Y.; Ji, C.; Zhang, J.; Ma, Q. Protective effects of Devosia sp. ANSB714 on growth performance, immunity function, antioxidant capacity and tissue residues in growing-finishing pigs fed with deoxynivalenol contaminated diets. Food Chem. Toxicol. 2018, 121, 246–251. [Google Scholar] [CrossRef]
- Yu, C.; Xu, H.; Zhao, X.; Litke, Q.; Gong, J.; Yang, C.; Liu, S. Developing sodium metabisulfite (SMBS)-containing Eudragit L100-55 microparticles for controlled intestinal release of SMBS to detoxify deoxynivalenol. Food Biosci. 2023. [Google Scholar] [CrossRef]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef]
- Liang, X.; Xie, J.; Liu, H.; Zhao, R.; Zhang, W.; Wang, H.; Pan, H.; Zhou, Y.; Han, W. STIM1 Deficiency in Intestinal Epithelium Attenuates Colonic Inflammation and Tumorigenesis by Reducing ER Stress of Goblet Cells. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 193–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, K.; Meng, J.; Nie, D.; Zhao, Z.; Han, Z. Deoxynivalenol hijacks the pathway of Janus kinase 2/signal transducers and activators of transcription 3 (JAK2/STAT-3) to drive caspase-3-mediated apoptosis in intestinal porcine epithelial cells. Sci. Total Environ. 2023, 864, 161058. [Google Scholar] [CrossRef]
- Auger, C.; Vinaik, R.; Appanna, V.D.; Jeschke, M.G. Beyond mitochondria: Alternative energy-producing pathways from all strata of life. Metabolism 2021, 118, 154733. [Google Scholar] [CrossRef] [PubMed]
- Chamseddine, D.; Mahmud, S.A.; Westfall, A.K.; Castoe, T.A.; Berg, R.E.; Pellegrino, M.W. The mitochondrial UPR regulator ATF5 promotes intestinal barrier function via control of the satiety response. Cell Rep. 2022, 41, 111789. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Bravo Iniguez, A.; Tian, Q.; Du, M.; Zhu, M.-J. PGC-1α in mediating mitochondrial biogenesis and intestinal epithelial differentiation promoted by purple potato extract. J. Funct. Foods 2022, 98, 105291. [Google Scholar] [CrossRef]
- Yang, J.; Bai, Y.; Shen, S.; Tao, X.; Ma, C.; Fu, B.; Dai, Q.; Wu, J.; Meng, Z.; Sun, Q.; et al. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. Chem. Eng. J. 2023, 465, 142940. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.; Zhang, K.; Yu, Y.; Cui, S.W.; Nie, S. Glucomannan from Aloe vera gel maintains intestinal barrier integrity via mitigating anoikis mediated by Nrf2-mitochondria axis. Int. J. Biol. Macromol. 2023, 235, 123803. [Google Scholar] [CrossRef]
- Guerbette, T.; Boudry, G.; Lan, A. Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity. Mol. Metab. 2022, 63, 101546. [Google Scholar] [CrossRef]
- Carvalho, G.C.; de Camargo, B.A.F.; de Araújo, J.T.C.; Chorilli, M. Lycopene: From tomato to its nutraceutical use and its association with nanotechnology. Trends Food Sci. Technol. 2021, 118, 447–458. [Google Scholar] [CrossRef]
- Stevens, Y.; Pinheiro, I.; Salden, B.; Duysburgh, C.; Bolca, S.; Degroote, J.; Majdeddin, M.; Van Noten, N.; Gleize, B.; Caris-Veyrat, C.; et al. Effect of a carotenoid-producing Bacillus strain on intestinal barrier integrity and systemic delivery of carotenoids: A randomised trial in animals and humans. J. Funct. Foods 2021, 80, 104445. [Google Scholar] [CrossRef]
- Alhoshani, N.M.; Al-Johani, N.S.; Alkeraishan, N.; Alarifi, S.; Alkahtani, S. Effect of lycopene as an adjuvant therapy with 5-florouracil in human colon cancer. Saudi J. Biol. Sci. 2022, 29, 103392. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.-X.; Luo, Y.; Cui, J.-G.; Talukder, M.; Li, J.-L. Lycopene mitigates DEHP-induced hepatic mitochondrial quality control disorder via regulating SIRT1/PINK1/mitophagy axis and mitochondrial unfolded protein response. Environ. Pollut. 2022, 292, 118390. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.-F.; Chen, F.-J.; Chen, Y.-H.; Yang, X.; Cai, Z.-H.; Jiang, Y.-B.; Wang, X.-B.; Zhang, G.-P.; Wang, F.-Y. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Margier, M.; Desmarchelier, C.; Halimi, C.; Nowicki, M.; Borel, P.; Meléndez-Martínez, A.J.; Reboul, E. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene. Food Chem. 2019, 300, 125232. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, L.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. L-theanine improves intestinal barrier functions by increasing tight junction protein expression and attenuating inflammatory reaction in weaned piglets. J. Funct. Foods 2023, 100, 105400. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Et Biophys. Acta BBA-Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Vasileva, E.; Spadaro, D.; Rouaud, F.; King, J.M.; Flinois, A.; Shah, J.; Sluysmans, S.; Méan, I.; Jond, L.; Turner, J.R.; et al. Cingulin binds to the ZU5 domain of scaffolding protein ZO-1 to promote its extended conformation, stabilization, and tight junction accumulation. J. Biol. Chem. 2022, 298, 101797. [Google Scholar] [CrossRef]
- Huang, C.; Wu, P.; Jiang, W.-D.; Liu, Y.; Zeng, Y.-Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Deoxynivalenol decreased the growth performance and impaired intestinal physical barrier in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 80, 376–391. [Google Scholar] [CrossRef]
- Zhang, J.-j.; Chen, K.-c.; Zhou, Y.; Wei, H.; Qi, M.-h.; Wang, Z.; Zheng, Y.-n.; Chen, R.-x.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomedicine 2022, 104, 154341. [Google Scholar] [CrossRef]
- Nguepi Tsopmejio, I.S.; Yuan, J.; Diao, Z.; Fan, W.; Wei, J.; Zhao, C.; Li, Y.; Song, H. Auricularia polytricha and Flammulina velutipes reduce liver injury in DSS-induced Inflammatory Bowel Disease by improving inflammation, oxidative stress, and apoptosis through the regulation of TLR4/NF-κB signaling pathways. J. Nutr. Biochem. 2023, 111, 109190. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, J.; Huang, W.; Yan, J.; Shan, A.; Gao, X. Curcumin mitigates deoxynivalenol-induced intestinal epithelial barrier disruption by regulating Nrf2/p53 and NF-κB/MLCK signaling in mice. Food Chem. Toxicol. 2022, 167, 113281. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.Z.; Shen, Y.; Lin, J.; Wang, H.R.; Talukder, M.; Li, J.L. Lycopene Prevents DEHP-Induced Leydig Cell Damage with the Nrf2 Antioxidant Signaling Pathway in Mice. J Agric Food Chem. 2020, 68, 2031–2040. [Google Scholar] [CrossRef]
- Fan, L.; Ge, J.; Zan, Q.; Wang, X.; Wang, S.; Zhang, Y.; Dong, W.; Shuang, S.; Dong, C. Real-time tracking the mitochondrial membrane potential by a mitochondria-lysosomes migration fluorescent probe with NIR-emissive AIE characteristics. Sens. Actuators B Chem. 2021, 327, 128929. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Tian, Y.; Shi, J. Sirtuin 3 and mitochondrial permeability transition pore (mPTP): A systematic review. Mitochondrion 2022, 64, 103–111. [Google Scholar] [CrossRef]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Reshmitha, T.R.; Nisha, P. Lycopene mitigates acrylamide and glycidamide induced cellular toxicity via oxidative stress modulation in HepG2 cells. J. Funct. Foods 2021, 80, 104390. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, K.; Ma, W.; Zhan, H.; Sun, Q.; Xie, L.; Zhao, Z. Impaired autophagy and mitochondrial dynamics are involved in Sorafenib-induced cardiomyocyte apoptosis. Toxicology 2022, 481, 153348. [Google Scholar] [CrossRef]
- Guo, D.-F.; Merrill, R.A.; Qian, L.; Hsu, Y.; Zhang, Q.; Lin, Z.; Thedens, D.R.; Usachev, Y.M.; Grumbach, I.; Sheffield, V.C.; et al. The BBSome regulates mitochondria dynamics and function. Mol. Metab. 2023, 67, 101654. [Google Scholar] [CrossRef]
- Noone, J.; O’Gorman, D.J.; Kenny, H.C. OPA1 regulation of mitochondrial dynamics in skeletal and cardiac muscle. Trends Endocrinol. Metab. 2022, 33, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Guo, C.; He, H.; Zuo, Z.; Hu, Y.; Yu, S.; Zhong, Z.; Liu, H.; Zhu, L.; Xu, S.; et al. Effects of deoxynivalenol on mitochondrial dynamics and autophagy in pig spleen lymphocytes. Food Chem. Toxicol. 2020, 140, 111357. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Yang, P.; Yang, Y.; Wang, Y.; Wu, K.; Qi, D.; Wang, S. Deoxynivalenol triggers porcine intestinal tight junction disorder through hijacking SLC5A1 and PGC1α-mediated mitochondrial function. Food Chem. Toxicol. 2022, 163, 112921. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, L.; Li, M.-Z.; Wang, H.-R.; Zhao, Y.; Li, J.-L. Lycopene prevents Di-(2-ethylhexyl) phthalate-induced mitophagy and oxidative stress in mice heart via modulating mitochondrial homeostasis. J. Nutr. Biochem. 2023, 115, 109285. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Chen, F.; Wang, Y.; Wang, X.; Yang, X.; Zhang, C. Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants 2023, 12, 1958. https://doi.org/10.3390/antiox12111958
Cai Z, Chen F, Wang Y, Wang X, Yang X, Zhang C. Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants. 2023; 12(11):1958. https://doi.org/10.3390/antiox12111958
Chicago/Turabian StyleCai, Zihui, Fengjuan Chen, Youshuang Wang, Xuebing Wang, Xu Yang, and Cong Zhang. 2023. "Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol" Antioxidants 12, no. 11: 1958. https://doi.org/10.3390/antiox12111958
APA StyleCai, Z., Chen, F., Wang, Y., Wang, X., Yang, X., & Zhang, C. (2023). Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants, 12(11), 1958. https://doi.org/10.3390/antiox12111958






