Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress
Abstract
:1. Introduction
2. Protein S-Glutathionylation
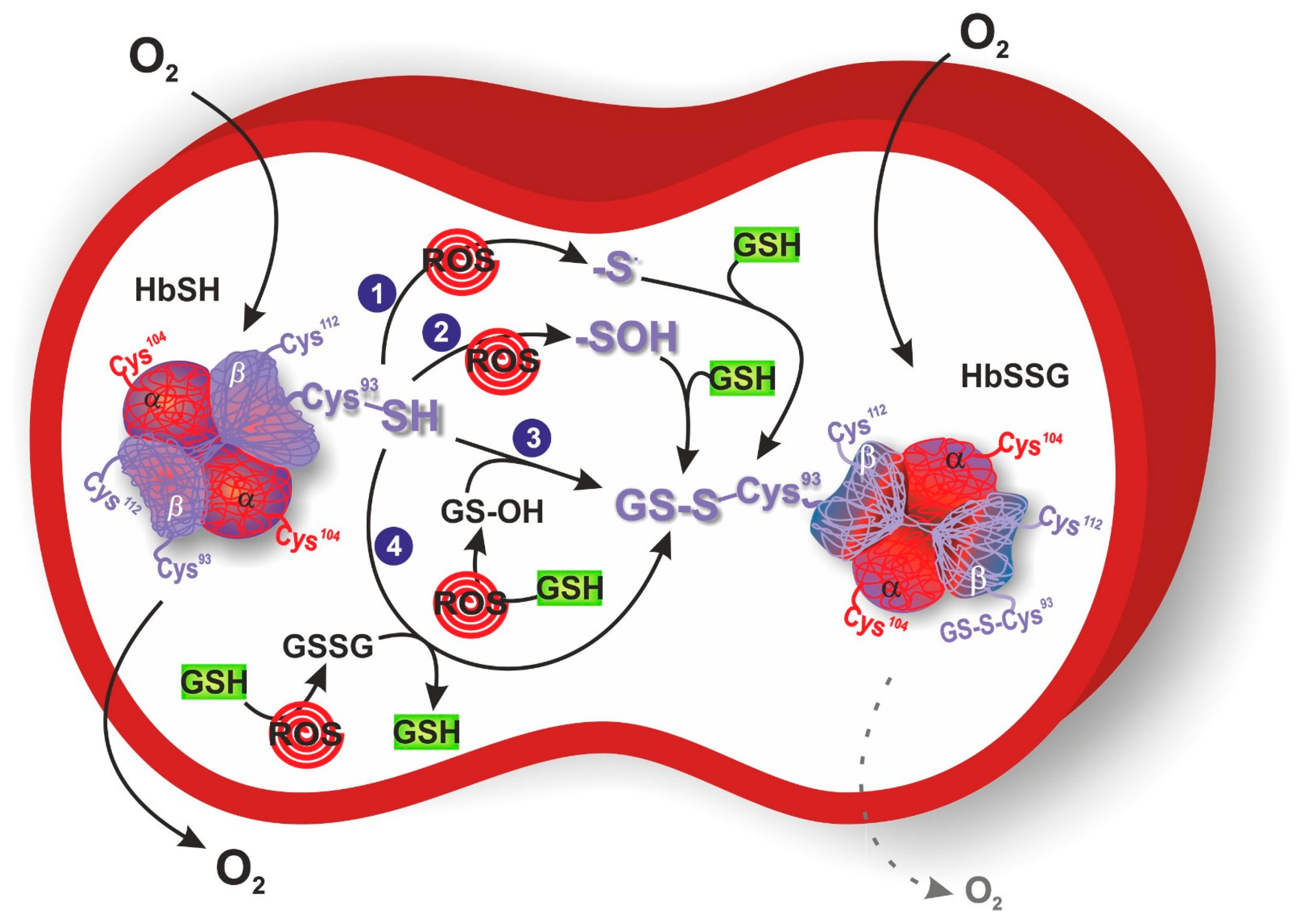
3. S-Glutathionylation of Hemoglobin
4. Glutathionyl Hemoglobin and Methemoglobin
5. Methods for the Detection of Glutathionyl Hemoglobin
6. Glutathionyl Hemoglobin and Diseases
| Disease/Treatment | Values | Reference |
|---|---|---|
| HD | HbSSG β (%) | |
| Normal n = 20 | 3.7 ± 0.3 | |
| HD n = 10 | 18.6 ± 0.9 a* | Naito et al. (1999) [72] |
| HD n = 10 | 20.8 ± 0.9 b* | |
| Normal n = 20 | 3.0 ± 1.6 | |
| HD n = 30 | 8.0 ± 3.6 * | |
| HD n = 12 | 8.7 ± 3.2 a* | Takayama et al. (2001) [27] |
| HD n = 12 | 8.7 ± 2.8 b* | |
| CAPD | HbSSG β (%) | |
| Normal n = 20 | 3.0 ± 1.6 | Takayama et al. (2001) [27] |
| CAPD n = 10 | 5.9 ± 2.7 * | |
| DM | HbSSG β (%) | |
| Normal n = 20 | 3.7 ± 0.3 | |
| DM n = 10 | 10.2 ± 0.8 c* | Naito et al. (2000) [73] |
| DM n = 10 | 4.1 ± 0.4 d# | |
| Normal n = 20 | 3.7 ± 0.3 | |
| DM n = 37 | 7.9 ± 0.5 * | Niwa et al. (2000) [62] |
| HbSSG A1d3 (%) | ||
| Normal n = 9 | 1.2 ± 0.1 | Al-Abed et al. (2001) [74] |
| DM n = 20 | 2.3 ± 0.3 * | |
| HLD | HbSSG β (%) | |
| Normal n = 20 | 3.7 ± 0.3 | Niwa et al. (2000) [62] |
| HLD n = 17 | 8.1 ± 0.8 * | |
| FRDA | HbSSG β (%) | |
| Normal n = 20 | 8.0 ± 1.8 | Piemonte et al. (2001) [25] |
| FRDA n = 14 | 15.0 ± 1.5 * | |
| DS | HbSSG β (%) | |
| Normal n = 64 | 2.65 ± 1.1 | Pastore et al. (2003) [78] |
| DS n = 46 | 1.47 ± 0.6 * | |
| IDA | HbSSG β (%) | |
| Normal n = 15 | 7.7 ± 3.7 | Shet et al. (2012) [80] |
| IDA n = 23 | 16.9 ± 9.6 * | |
| MDD | HbSSG β (%) | |
| Normal n = 17 | 5.73 | Mathew et al. (2019) [82] |
| MDD n = 26 | 8.34 * | |
| MDD n = 11 | 8.07 e | |
| MDD n = 11 | 7.68 f | Mathew et al. (2019) [82] |
| CS | HbSSG β (%) g | |
| Nonsmokers n = 354 | 5.6 | Muscat et al. (2004) [84] |
| Smokers n = 97 | 8.1 * | |
| HbSSG α (%) h | ||
| Nonsmokers n = 20 | 2.24 ± 0.91 | Chen et al. (2014) [85] |
| Smokers n = 20 | 3.61 ± 1.41 * | |
| HbSSG β (%) i | ||
| Nonsmokers n = 20 | 3.79 ± 1.42 | Chen et al. (2014) [85] |
| Smokers n = 20 | 6.69 ± 2.33 * | |
| HbSSG β (%) l | ||
| Nonsmokers n = 20 | 0.54 ± 0.68 | Chen et al. (2014) [85] |
| Smokers n = 20 | 0.56 ± 0.39 |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Engin, A. Non-Alcoholic Fatty Liver Disease. Adv. Exp. Med. Biol. 2017, 960, 443–467. [Google Scholar] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Kim, H.; Choi, H.J.; Lee, S.; Kim, K. Protein Glutathionylation in the Pathogenesis of Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2017, 2017, 2818565. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Galvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021, 18, 251–275. [Google Scholar] [CrossRef]
- Wronka, M.; Krzeminska, J.; Mlynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Krankel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Epidemiology of rheumatoid arthritis: Genetic and environmental influences. Expert. Rev. Clin. Immunol. 2022, 18, 923–931. [Google Scholar] [CrossRef]
- Niwa, T. Protein glutathionylation and oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 855, 59–65. [Google Scholar] [CrossRef]
- Smith, F.M.; Kosman, D.J. Molecular Defects in Friedreich’s Ataxia: Convergence of Oxidative Stress and Cytoskeletal Abnormalities. Front. Mol. Biosci. 2020, 7, 569293. [Google Scholar] [CrossRef]
- Newman, S.F.; Sultana, R.; Perluigi, M.; Coccia, R.; Cai, J.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Butterfield, D.A. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J. Neurosci. Res. 2007, 85, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.; Parakh, S.; Atkin, J.D. The role of s-nitrosylation and s-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int. J. Cell Biol. 2013, 2013, 797914. [Google Scholar] [CrossRef]
- Sabens Liedhegner, E.A.; Gao, X.H.; Mieyal, J.J. Mechanisms of altered redox regulation in neurodegenerative diseases--focus on S--glutathionylation. Antioxid. Redox Signal 2012, 16, 543–566. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A. Red cell membrane damage. J. Heart Valve Dis. 1998, 7, 387–395. [Google Scholar] [PubMed]
- Piemonte, F.; Pastore, A.; Tozzi, G.; Tagliacozzi, D.; Santorelli, F.M.; Carrozzo, R.; Casali, C.; Damiano, M.; Federici, G.; Bertini, E. Glutathione in blood of patients with Friedreich’s ataxia. Eur. J. Clin. Investig. 2001, 31, 1007–1011. [Google Scholar] [CrossRef]
- Sampathkumar, R.; Balasubramanyam, M.; Sudarslal, S.; Rema, M.; Mohan, V.; Balaram, P. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin. Biochem. 2005, 38, 892–899. [Google Scholar] [CrossRef]
- Takayama, F.; Tsutsui, S.; Horie, M.; Shimokata, K.; Niwa, T. Glutathionyl hemoglobin in uremic patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis. Kidney Int. Suppl. 2001, 78, S155–S158. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Gill, R.; Young, A. Chapter 13—Protein S-glutathionylation and the regulation of cellular functions. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2020; pp. 217–247. [Google Scholar] [CrossRef]
- Shelton, M.D.; Chock, P.B.; Mieyal, J.J. Glutaredoxin: Role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox Signal 2005, 7, 348–366. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Musaogullari, A.; Chai, Y.C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Aldini, G.; Carini, M.; Rossi, R.; Dalle-Donne, I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxid. Redox Signal 2005, 7, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Colombo, R.; Dalle-Donne, I. S-glutathionylation: From redox regulation of protein functions to human diseases. J. Cell Mol. Med. 2004, 8, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal 2008, 10, 1941–1988. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal 2020, 32, 677–700. [Google Scholar] [CrossRef]
- Manevich, Y.; Feinstein, S.I.; Fisher, A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. USA 2004, 101, 3780–3785. [Google Scholar] [CrossRef]
- Mannervik, B.; Axelsson, K. Role of cytoplasmic thioltransferase in cellular regulation by thiol-disulphide interchange. Biochem. J. 1980, 190, 125–130. [Google Scholar] [CrossRef]
- Thornalley, P.J. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef]
- Nagy, P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Haendeler, J. Thioredoxin-1 and posttranslational modifications. Antioxid. Redox Signal 2006, 8, 1723–1728. [Google Scholar] [CrossRef]
- Starke, D.W.; Chock, P.B.; Mieyal, J.J. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J. Biol. Chem. 2003, 278, 14607–14613. [Google Scholar] [CrossRef]
- Jessop, C.E.; Chakravarthi, S.; Watkins, R.H.; Bulleid, N.J. Oxidative protein folding in the mammalian endoplasmic reticulum. Biochem. Soc. Trans. 2004, 32, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Bhatnagar, A. Protein S-glutathiolation: Redox-sensitive regulation of protein function. J. Mol. Cell Cardiol. 2012, 52, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Willmore, W.G. S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2014, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Muralidharan, M.; Srivastava, D.; Das, R.; Bhat, V.; Mandal, A.K. Assessment of Cysteine Reactivity of Human Hemoglobin at Its Residue Level: A Mass Spectrometry-Based Approach. Hemoglobin 2017, 41, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.; Muralidharan, M.; Pinto, J.; Srinivasan, K.; Mandal, A.K. Structural perturbation of human hemoglobin on glutathionylation probed by hydrogen-deuterium exchange and MALDI mass spectrometry. Bioconjug Chem. 2011, 22, 785–793. [Google Scholar] [CrossRef]
- Rubino, F.M. The Redox Potential of the beta-(93)-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules 2021, 26, 2528. [Google Scholar] [CrossRef]
- Craescu, C.T.; Poyart, C.; Schaeffer, C.; Garel, M.C.; Kister, J.; Beuzard, Y. Covalent binding of glutathione to hemoglobin. II. Functional consequences and structural changes reflected in NMR spectra. J. Biol. Chem. 1986, 261, 14710–14716. [Google Scholar] [CrossRef]
- Kuleshova, I.D.; Zaripov, P.I.; Poluektov, Y.M.; Anashkina, A.A.; Kaluzhny, D.N.; Parshina, E.Y.; Maksimov, G.V.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. Int. J. Mol. Sci. 2023, 24, 13557. [Google Scholar] [CrossRef]
- Muralidharan, M.; Mitra, A.; Maity, D.; Pal, D.; Mandal, A.K. Structural analysis of glutathionyl hemoglobin using native mass spectrometry. J. Struct. Biol. 2019, 208, 107386. [Google Scholar] [CrossRef]
- Mitra, G.; Muralidharan, M.; Narayanan, S.; Pinto, J.; Srinivasan, K.; Mandal, A.K. Glutathionylation induced structural changes in oxy human hemoglobin analyzed by backbone amide hydrogen/deuterium exchange and MALDI-mass spectrometry. Bioconjug Chem. 2012, 23, 2344–2353. [Google Scholar] [CrossRef]
- Murakami, K.; Mawatari, S. Oxidation of hemoglobin to methemoglobin in intact erythrocyte by a hydroperoxide induces formation of glutathionyl hemoglobin and binding of alpha-hemoglobin to membrane. Arch. Biochem. Biophys. 2003, 417, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Skold, A.; Cosco, D.L.; Klein, R. Methemoglobinemia: Pathogenesis, diagnosis, and management. South. Med. J. 2011, 104, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Lurie, A.A. Concise review: Methemoglobinemia. Am. J. Hematol. 1993, 42, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999, 34, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Curry, S. Methemoglobinemia. Ann. Emerg. Med. 1982, 11, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Murakami, K. Different types of glutathionylation of hemoglobin can exist in intact erythrocytes. Arch. Biochem. Biophys. 2004, 421, 108–114. [Google Scholar] [CrossRef]
- Rossi, R.; Cardaioli, E.; Scaloni, A.; Amiconi, G.; Di Simplicio, P. Thiol groups in proteins as endogenous reductants to determine glutathione-protein mixed disulphides in biological systems. Biochim. Biophys. Acta 1995, 1243, 230–238. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Petralia, S.; Giampaoletti, S.; Milzani, A.; Rossi, R. Protein glutathionylation in erythrocytes. Clin. Chem. 2003, 49, 327–330. [Google Scholar] [CrossRef]
- Meredith, M.J. Analysis of protein-glutathione mixed disulfides by high performance liquid chromatography. Anal. Biochem. 1983, 131, 504–509. [Google Scholar] [CrossRef]
- Paroni, R.; De Vecchi, E.; Cighetti, G.; Arcelloni, C.; Fermo, I.; Grossi, A.; Bonini, P. HPLC with o-phthalaldehyde precolumn derivatization to measure total, oxidized, and protein-bound glutathione in blood, plasma, and tissue. Clin. Chem. 1995, 41, 448–454. [Google Scholar] [CrossRef]
- Niwa, T.; Naito, C.; Mawjood, A.H.; Imai, K. Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin. Chem. 2000, 46, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Urbani, A.; Massoud, R.; di Ilio, C.; Sacchetta, P.; Bernardini, S.; Cortese, C.; Federici, G. A quantitative method for the analysis of glycated and glutathionylated hemoglobin by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Anal. Biochem. 2005, 336, 279–288. [Google Scholar] [CrossRef]
- Rubino, F.M.; Ottolenghi, S.; Brizzolari, A.; Maioli, C.; Samaja, M.; Paroni, R. Enhanced-Precision Measurement of Glutathionyl Hemoglobin by MALDI-ToF MS. Molecules 2023, 28, 497. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Boriero, D.; Carcereri de Prati, A.; Mariotto, S. Immunoprecipitation methods to identify S-glutathionylation in target proteins. MethodsX 2019, 6, 1992–1998. [Google Scholar] [CrossRef]
- Hill, B.G.; Ramana, K.V.; Cai, J.; Bhatnagar, A.; Srivastava, S.K. Measurement and identification of S-glutathiolated proteins. Methods Enzym. 2010, 473, 179–197. [Google Scholar]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. Measurement of S-glutathionylated proteins by HPLC. Amino Acids 2022, 54, 675–686. [Google Scholar] [CrossRef]
- Garel, M.C.; Beuzard, Y.; Thillet, J.; Domenget, C.; Martin, J.; Galacteros, F.; Rosa, J. Binding of 21 thiol reagents to human hemoglobin in solution and in intact cells. Eur. J. Biochem. 1982, 123, 513–519. [Google Scholar] [CrossRef]
- Pastore, A.; Mozzi, A.F.; Tozzi, G.; Gaeta, L.M.; Federici, G.; Bertini, E.; Lo Russo, A.; Mannucci, L.; Piemonte, F. Determination of glutathionyl-hemoglobin in human erythrocytes by cation-exchange high-performance liquid chromatography. Anal. Biochem. 2003, 312, 85–90. [Google Scholar] [CrossRef]
- Bursell, S.E.; King, G.L. The potential use of glutathionyl hemoglobin as a clinical marker of oxidative stress. Clin. Chem. 2000, 46, 145–146. [Google Scholar] [CrossRef]
- Mandal, A.K.; Woodi, M.; Sood, V.; Krishnaswamy, P.R.; Rao, A.; Ballal, S.; Balaram, P. Quantitation and characterization of glutathionyl haemoglobin as an oxidative stress marker in chronic renal failure by mass spectrometry. Clin. Biochem. 2007, 40, 986–994. [Google Scholar] [CrossRef]
- Naito, C.; Kajita, M.; Niwa, T. Determination of glutathionyl hemoglobin in hemodialysis patients using electrospray ionization liquid chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Naito, C.; Niwa, T. Analysis of glutathionyl hemoglobin levels in diabetic patients by electrospray ionization liquid chromatography-mass spectrometry: Effect of vitamin E administration. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; VanPatten, S.; Li, H.; Lawson, J.A.; FitzGerald, G.A.; Manogue, K.R.; Bucala, R. Characterization of a novel hemoglobin-glutathione adduct that is elevated in diabetic patients. Mol. Med. 2001, 7, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 1994, 16, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, M. Molecular pathogenesis of Friedreich ataxia. Arch. Neurol. 1999, 56, 1201–1208. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Clements, D.; MacLeod, K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic. Biol. Med. 1998, 25, 1044–1048. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Giannotti, A.; Bertini, E.; Federici, G.; Digilio, M.C.; Piemonte, F. Glutathione metabolism and antioxidant enzymes in children with Down syndrome. J. Pediatr. 2003, 142, 583–585. [Google Scholar] [CrossRef]
- Yoo, J.H.; Maeng, H.Y.; Sun, Y.K.; Kim, Y.A.; Park, D.W.; Park, T.S.; Lee, S.T.; Choi, J.R. Oxidative status in iron-deficiency anemia. J. Clin. Lab. Anal. 2009, 23, 319–323. [Google Scholar] [CrossRef]
- Shet, A.S.; Pinto, S.M.; Mitra, G.; Mandal, A.K. Glutathionyl hemoglobin is elevated in iron deficiency anemia. Acta Haematol. 2012, 127, 26–30. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M.; Gautam, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian. J. Psychiatry 2012, 54, 244–247. [Google Scholar] [CrossRef]
- Mathew, B.; Srinivasan, K.; Johnson, P.; Thomas, T.; Mandal, A.K. Elevated levels of glutathionyl haemoglobin as an oxidative stress marker in patients with major depressive disorder. Indian. J. Med. Res. 2019, 149, 497–502. [Google Scholar] [PubMed]
- Pryor, W.A. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Env. Health Perspect. 1997, 105 (Suppl. 4), 875–882. [Google Scholar]
- Muscat, J.E.; Kleinman, W.; Colosimo, S.; Muir, A.; Lazarus, P.; Park, J.; Richie, J.P., Jr. Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic. Biol. Med. 2004, 36, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, W.P.; Chiu, S.D.; Fan, C.H. Multistage mass spectrometric analysis of human hemoglobin glutathionylation: Correlation with cigarette smoking. Chem. Res. Toxicol. 2014, 27, 864–872. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scirè, A.; Casari, G.; Romaldi, B.; de Bari, L.; Antognelli, C.; Armeni, T. Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress. Antioxidants 2023, 12, 1976. https://doi.org/10.3390/antiox12111976
Scirè A, Casari G, Romaldi B, de Bari L, Antognelli C, Armeni T. Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress. Antioxidants. 2023; 12(11):1976. https://doi.org/10.3390/antiox12111976
Chicago/Turabian StyleScirè, Andrea, Giulia Casari, Brenda Romaldi, Lidia de Bari, Cinzia Antognelli, and Tatiana Armeni. 2023. "Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress" Antioxidants 12, no. 11: 1976. https://doi.org/10.3390/antiox12111976
APA StyleScirè, A., Casari, G., Romaldi, B., de Bari, L., Antognelli, C., & Armeni, T. (2023). Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress. Antioxidants, 12(11), 1976. https://doi.org/10.3390/antiox12111976










