Abstract
Piglet weaning is an important stage in production where changes in the environment and diet can cause problems such as intestinal inflammation and diarrhea. Vitamin A is an essential nutrient for human and animal growth and has immunomodulatory and inflammatory effects. A large body of literature has previously reported on the use of vitamin A in piglet production, so our experiment added different concentrations of vitamin A (0, 1100, 2200, 4400, 8800, and 17,600 IU/kg) to weaned piglet diets to study the effects of different doses on growth performance, intestinal barrier, inflammation, and flora in weaned piglets. We selected 4400 IU/kg as the optimum concentration of vitamin A in relation to average daily weight gain, feed intake, feed-to-weight ratio, and diarrhea rate, and subsequently tested the inflammatory factors, immunoglobulin content, antioxidant levels, and intestinal flora of weaned piglets. Results: We observed that the diarrhea rate of weaned piglets was significantly lower after the addition of 4400 IU/kg of vitamin A to the diet (p < 0.05). A control group and a 4400 IU/kg VA group were selected for subsequent experiments. We found that after the addition of vitamin A, the serum CAT level of weaned piglets increased significantly, the expression of Claudin-1 in the jejunum and ileum increased significantly, the expression of Occludin gene in the jejunum increased significantly, the expression of IL-5 and IL-10 in the ileum increased significantly (p < 0.05), and the expression of IL-4, IL-5, and IL-10 in the ileum increased significantly (p < 0.05). Meanwhile, in the colonic flora of vitamin A-added weaned piglets, the relative abundance of Actinobacteria and Erysipelotrichales decreased significantly, while the relative abundance of Bacteroidales increased significantly (p < 0.05). The results of this study indicated that vitamin A at 4400 IU/kg reduces diarrhea in weaned piglets by increasing antioxidant levels, increasing intestinal tight junction protein gene expression, and regulating colonic gut microbiota.
1. Background
Weaning stress always poses a great threat to pig production, and piglets suffer from intestinal inflammation, diarrhea, oxidative stress, and flora imbalance [1,2,3]. The first line of defense against toxins and pathogenic microorganisms in the intestinal lumen, as well as antigenic substances in the feed, is the intestinal epithelial barrier (physical barrier). Cytokines play a critical role in the integrity of the intestinal barrier and are an important part of the regulation of intestinal immune barrier function. Pro-inflammatory cytokines frequently disrupt tight junction (TJ) proteins, whereas anti-inflammatory cytokines protect epithelial barrier function [4]. For animal health, the intestinal microbiota is an important environmental factor. A healthy gut microbiota contributes to the host’s nutrient metabolism, the structural integrity of the gut mucosal barrier, immune regulation, cognitive and behavioral development, and pathogen protection [5]. Previous studies have shown that dietary antibiotics can effectively solve the problems caused by stress in piglets, while the prohibition of antibiotics leads to frequent bacterial diseases, reducing pig performance, greatly increasing the rate of diarrhea, increasing the utilization rate of therapeutic antibiotics, and increasing the production cost of pigs [6]. These studies were concerned with the role of nutrients in weaning piglets.
Vitamin A is a fat-soluble compound that cannot be synthesized in animals and needs to be obtained from feed. Vitamin A, as an organic compound, can be metabolized and transported into retinol, retinoic acid, retinaldehyde, and other substances in the body by related enzymes [7,8,9,10]. Previous studies have shown that vitamin A can improve the growth performance, and antioxidant and immune capacity of animals [11,12,13]. Vitamin A deficiency lowers immunity and causes inflammation, while excessive supplementation can be toxic, causing headaches, bone pain, and brain edema [14]. Zhou et al. found that weaned piglets supplemented with vitamin A significantly improved growth performance, glutathione peroxidase levels, and serum IgA levels [11]. Hu et al. found that vitamin A enhances the activity of immunoglobulin M and glutathione peroxidase in weaner piglets by using starch and gelatin coatings [15]. Moreover, previous research has discovered that vitamin A regulates the interaction of eukaryotic host cells with symbiotic microorganisms as well as the complexity of the microbiome, which in turn regulates vitamin A metabolism in the host [16]. Therefore, the aim of this study was to investigate the effects of dietary supplementation with vitamin A on diarrhea, antioxidant capacity, immunity, and gut microbiota in piglets where no antibiotics were used, and to explore the optimum concentration of vitamin A in the diet of weaned piglets.
2. Methods and Materials
The experimental procedures used in this study were approved by the Animal Care and Use Committee of the Guangdong Academy of Agricultural Sciences (Guangzhou, China) (No. GAASIAS-2020-005).
2.1. Animal Experimental Treatments
Seventy-two piglets (Duroc × Landrace × Yorkshire) at 8.23 ± 0.10 kg average body weight were weaned at the age of 21 days and randomly divided into six groups. Piglets were randomly divided into 6 groups according to body weight, with half male and half female in each group. The six groups were the control group with basic diet and basic diet added with 1100, 2200, 4400, 8800, and 17,600 IU/kg of vitamin A, respectively, each group had 6 replicate pens per group and 2 piglets in each pen. The composition of the basic diet (Table 1) was designed according to the nutritional requirements of the National Research Council (NRC, 2012) for piglets weighing 7 to 11 kg. And no antibiotics were used in the diet. The piglets were free to feed and water throughout the 28-day experimental period. Illness, diarrhea, or any abnormal behavior was observed and recorded (Diarrhea rate% = Number of piglets with diarrhea in the trial period/(number of test heads × test time) × 100%). On days 0, 12, and 28, piglets BW and feed consumed were weighed to determine the average daily feed intake (ADFI) and average daily gain (ADG). The feed conversion ratio (F/G) of weaned piglets was calculated as the ratio of ADFI to ADG.

Table 1.
Basal diet composition and nutrient levels (feeding basis).
2.2. Sample Collection
Each piglet was weighed and recorded on the final day of the experiment after 12 h fasting. Blood samples were taken from the anterior vena cava of each piglet. And then, the piglet was euthanized with Zoletil 50 (Virbac Philippines, 30 mg/kg) injection. After collecting jejunal and ileal bowel segment samples and colon contents in liquid nitrogen, all samples were frozen and stored in a −80 °C ultra-freezer.
2.3. Antioxidant Reagent
The activities of GSH-Px, T-SOD, CAT, and T-AOC were analyzed using commercial test kits (Nanjing Jiancheng, China) according to the manufacturer’s manual and determined by a multifunctional microplate reader (SYNERGY H1, Bio Tek, Winooski, VT, USA). The activity of GSH-PX was analyzed by the colorimetric method of 5,5′-dithio-bis p-nitrobenzoic acid, and the change in absorbance at 412 nm was read. The activity of T-SOD was measured by non-enzymatic NBT test, and the change in absorbance at 450 nm was read. The activity of CAT was determined by ammonium molybdate colorimetry and the change in absorbance was read at 405 nm. T-AOC was measured according to the principle of ABST oxidation into ABST+, and the absorbance change was read at 405 nm.
2.4. ELISA
Immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) in serum were determined by using enzyme-linked immunosorbent assay kits (MLbio, Shanghai, China). The procedures were strictly followed with the instructions from the kits. A microplate reader (SYNERGY H1, Bio Tek, USA) was used to analyze the samples.
2.5. Quantitative Real-Time Polymerase Chain Reaction
Intestine RNA was extracted using Trizol reagent (Aldlab, Shanghai, China) as described by Feng et al. [17]. The concentration and purity of RNA were determined by a nucleic acid protein detector (Ultraviolet spectrophotometer type 1000, Thermo Fisher, Waltham, MA, USA). RNA samples were used to obtain cDNA by using PrimeScript™ RT reagent Kit with gDNA Eraser Perfect Real Time RR047A kit (TaKaRa, USA). And then, cDNA was stored at −20 °C for further use. Real-time quantitative PCR instrument (CFX 96 touch Real-time fluorescence quantitative PCR System, Bio-RAD Laboratories, Hercules, CA, USA) was used to check the relative mRNAs’ expression. The PCR reaction System and reaction conditions were followed according to Lang et al. [18]. The sequences of all primers are listed below (Table 2).

Table 2.
Primers used for real-time quantitative PCR analysis.
2.6. DNA Extraction and Illumina Miseq Sequencing
Microbial DNA was extracted from fecal samples (Omega, USA) in accordance with the manufacturer’s method. The V4–V5 region of bacterial 16S ribosomal RNA gene was amplified by PCR using primers 515F 5′-barcode-GTGCCAGCMGCCGCGG)-3′ and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′. The bar code is a unique sequence of six bases for each sample. PCR reaction was carried out in three 20 μL mixtures containing 4 μL 5 × FastPfu buffer, 2 μL 2.5 mM dNTP, 0.8 μL each primer (5 μM), 0.4 μL FastPfu polymerase, and 10 ng template DNA. According to the manufacturer’s instructions, amplicon was extracted from 2% agarose gel and purified using the AxyPrep DNA Gel Extraction kit (Axygen Biosciences, USA). Illumina MiSeq 250PE sequencing was performed by Shanghai Lingen Biological.
2.7. Statistical Analysis
All data were analyzed by SPSS 22.0 software (IBM Corporation, USA). Growth performance was analyzed by one-way ANOVA, and other experimental results were compared by t-test. Data are represented in tables and graphs as mean and standard error (SEM) (n = 6). p values < 0.05 were considered statistically significant, while p values < 0.10 were used to indicate a trend.
3. Results
3.1. Growth Performance and Diarrhea Rate
Compared to those in the control group, there was no significant difference in body weight, ADFI, ADG, and F:G after weaned piglets treated with different concentrations of vitamin A. However, the vitamin A supplementation group showed a much lower diarrhea rate than that in the control group (Table 3). After the antioxidant level, intestinal tight junction protein, and cytokine expression of all treatments were tested, the 4400 IU/kg group had more positive effects in weaned piglets than other treatments (Figures S1–S3). Therefore, 4400 IU/kg vitamin A supplemental treatment was fully chosen and discussed below.

Table 3.
Effect of diet supplementary Vitamin A on growth performance of weaned piglets.
3.2. Serum Antioxidant
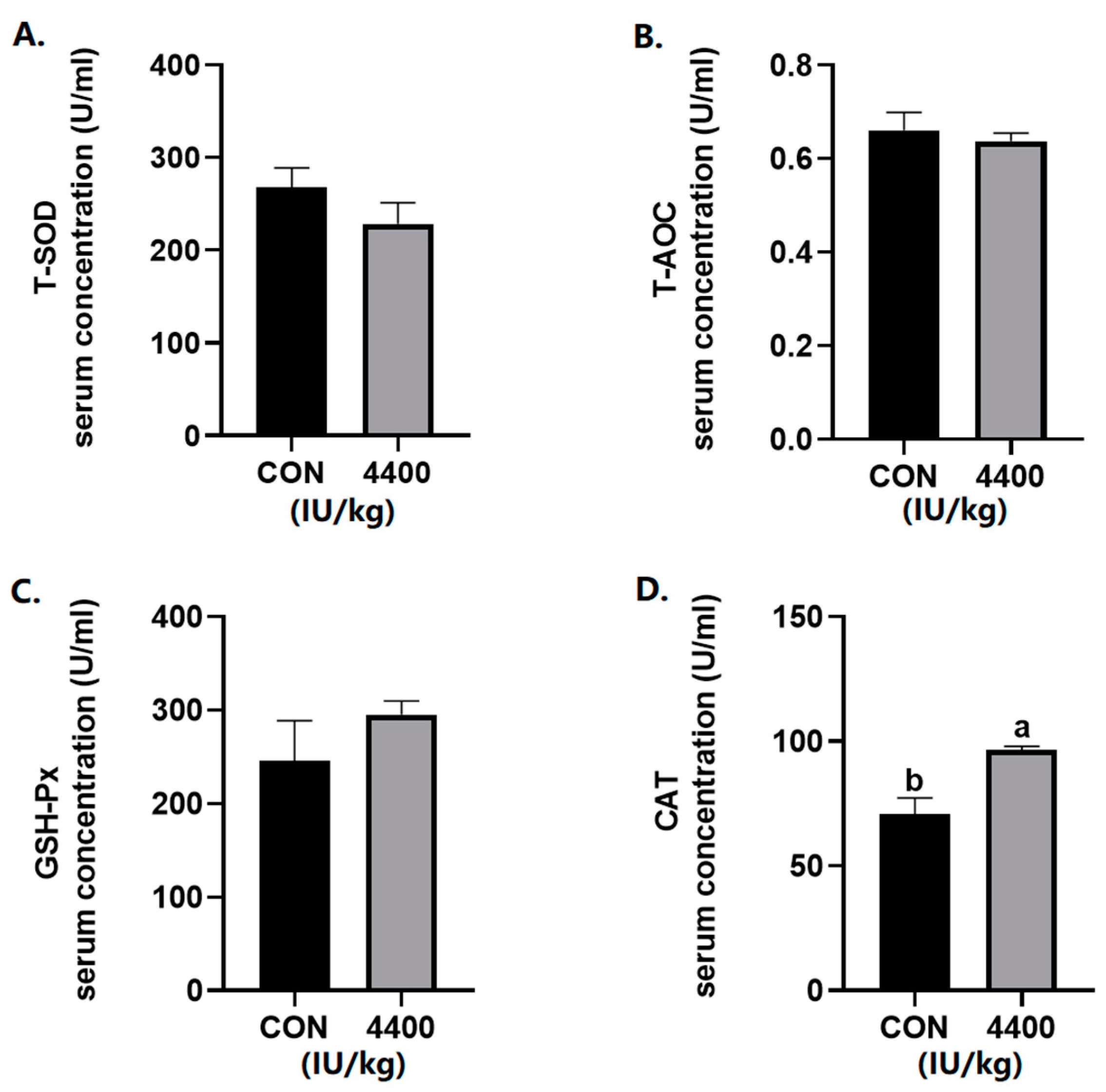
According to the serum antioxidant results (Figure 1), there was no significant change in the levels of T-AOC, T-SOD, and GSH-Px. The serum CAT content was significantly increased after the addition of vitamin A (p < 0.05).
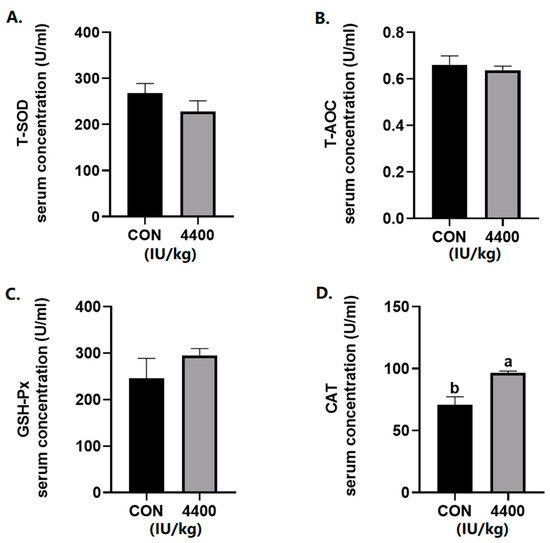
Figure 1.
Effect of vitamin A supplementation on serum antioxidant levels: (A) The concentration of T-SOD in serum. (B) The concentration of T-AOC in serum. (C) The concentration of GSH-Px in serum. (D) The concentration of CAT in serum. a, b showed means ± SEM (n = 6), different letters represented significant differences (p < 0.05). The same as below.
3.3. Serum Immunoglobulin
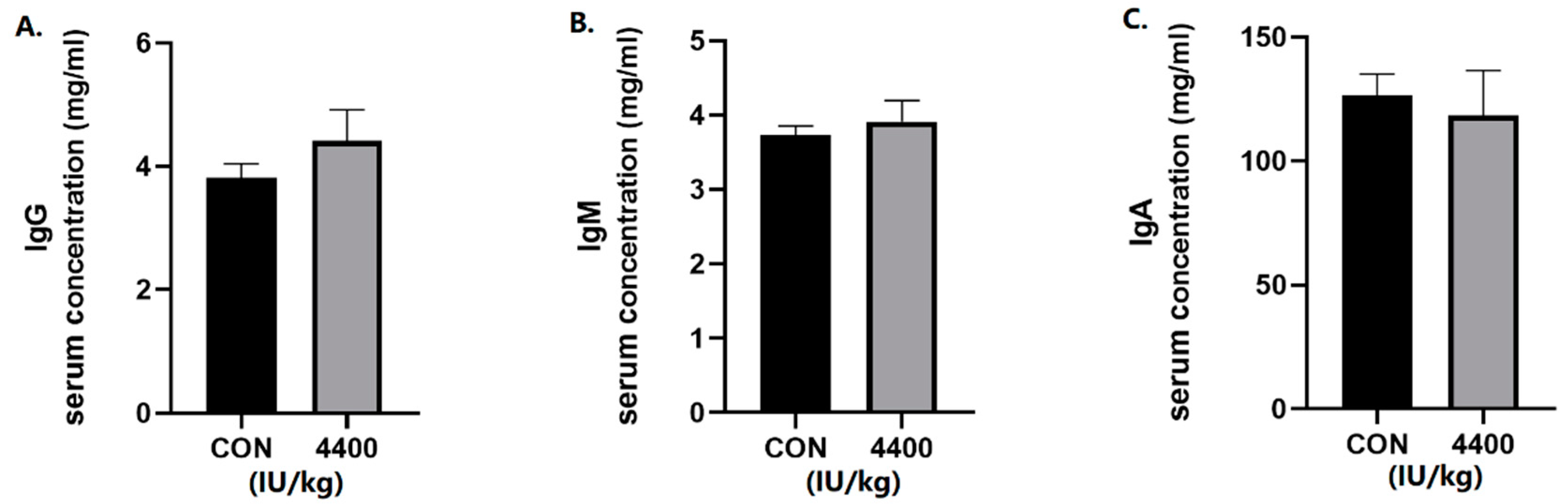
We further tested the serum for immunoglobulins. The concentration of IgG (Figure 2A), IgM (Figure 2B), and IgA (Figure 2C) in serum was not significantly affected by VA in the 4400 IU/kg group compared with the control group.
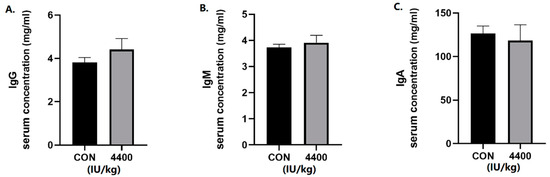
Figure 2.
Effect of vitamin A supplementation on serum immunoglobulin levels: (A) The concentration of IgG in serum. (B) The concentration of IgM in serum. (C)The concentration of IgA in serum.
3.4. Intestinal Tight Junction Protein
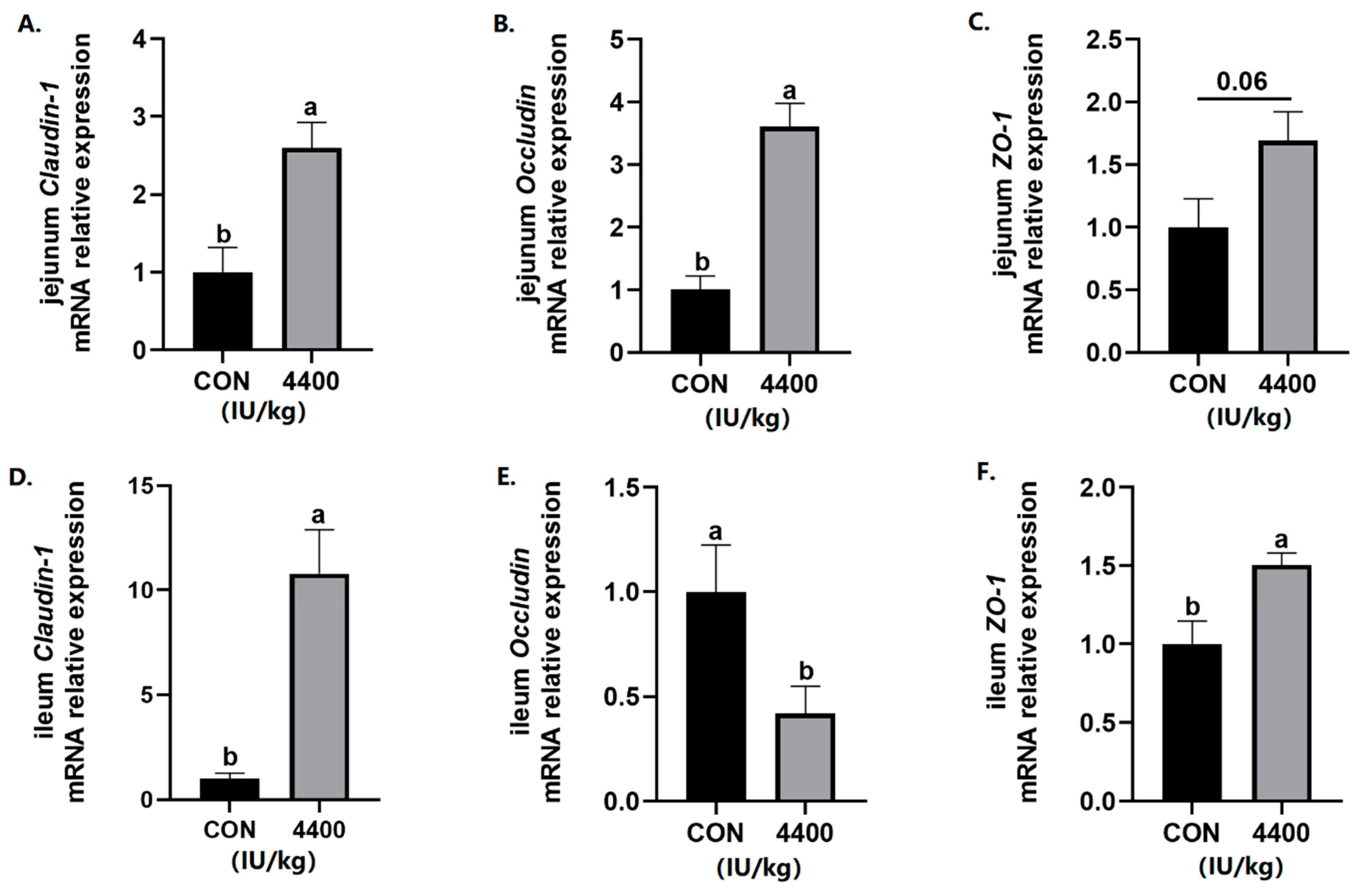
Since intestinal integrity is closely related to the diarrhea rate, intestinal tight junction protein gene expression was further detected. The data showed that compared with the control group, the expression of Claudin-1 and Occludin was significantly improved in the jejunum (p < 0.05), and ZO-1 expression had a trend to increase (p < 0.1) after vitamin A supplementation (Figure 3A–C). In the ileum, both Claudin-1 and ZO-1 expression was higher than the control group (p < 0.05), while the expression of Occludin was significantly decreased in the 4400 IU/kg group (p < 0.05, Figure 3D–F).
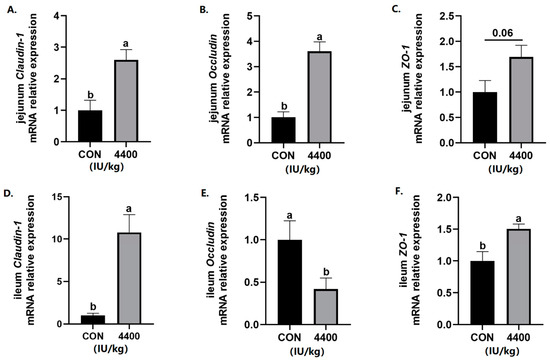
Figure 3.
Effect of vitamin A supplementation on the expression levels of tight junction proteins in the jejunum: (A) Relative expression of Claudin-1 mRNA in the jejunum. (B) Relative expression of Occludin mRNA in the jejunum. (C) Relative expression of ZO-1 mRNA in the jejunum. (D) Relative expression of Claudin-1 mRNA in the ileum. (E) Relative expression of Occludin mRNA in the ileum. (F) Relative expression of ZO-1 mRNA in the ileum. a, b showed means ± SEM (n = 6), different letters represented significant differences (p < 0.05).
3.5. Intestinal Inflammatory Cytokines
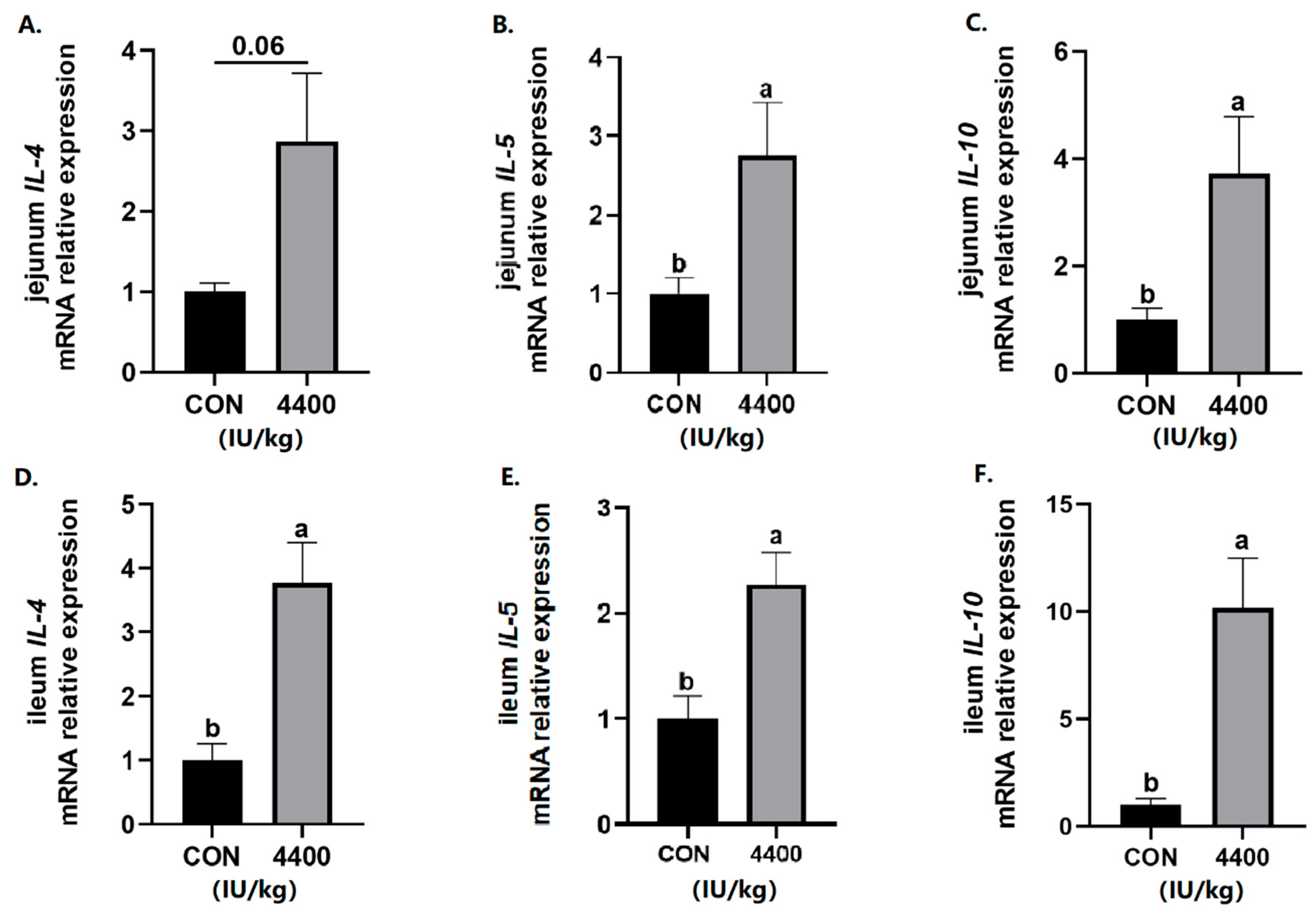
Next, intestinal cytokines expression was tested. In the jejunum, the expression of IL-5 and IL-10 was significantly higher (p < 0.05) and IL-4 level had a rising trend (p < 0.1) after vitamin A supplementation (Figure 4A–C). Compared with the control group, vitamin A treatment showed a significant increase in the expression of IL-4, IL-5, and IL-10 in the ileum (p < 0.05, Figure 4D–F).
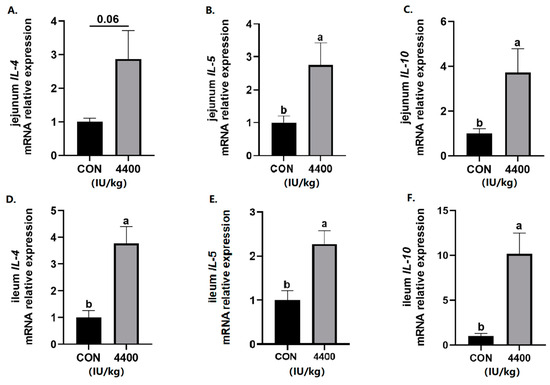
Figure 4.
Effect of vitamin A supplementation on the expression levels of inflammatory cytokines in the jejunum and ileum: (A) Relative expression of IL-4 mRNA in the jejunum. (B) Relative expression of IL-5 mRNA in the jejunum. (C) Relative expression of IL-6 mRNA in the jejunum. (D) Relative expression of IL-4 mRNA in the ileum. (E) Relative expression of IL-5 mRNA in the ileum. (F) Relative expression of IL-6 mRNA in the ileum. a, b showed means ± SEM (n = 6), different letters represented significant differences (p < 0.05).
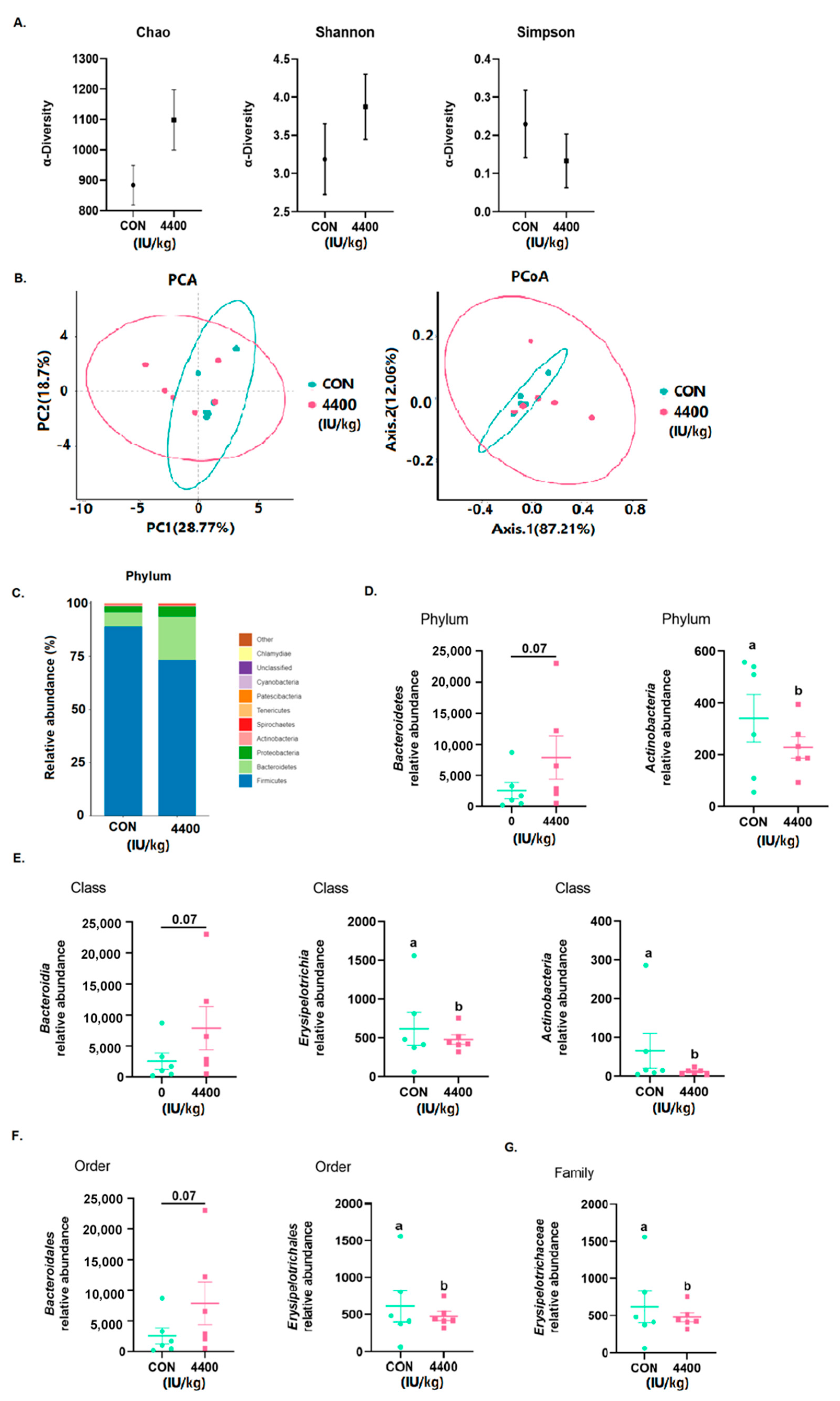
3.6. Intestinal Contents Flora
Using 16S rRNA Illumina MiSeq sequencing, the microbial composition of the cecum contents was determined. According to Figure 5A, no changes were found in Chao, Shannon, and Simpson. Using OTU data, we then examined the beta diversity of the cecum flora. After the addition of vitamin A (n = 6), neither PCA nor PCoA was completely overlapped with the control group (Figure 5B). Firmicutes, Bacteroidetes, and Proteobacteria were the three most abundant microorganisms in both groups (Figure 5C), with Bacteroidetes being significantly more abundant in the 4400 IU/kg group (p < 0.05). Finally, at the phylum (Figure 5D), class (Figure 5E), order (Figure 5F), and family (Figure 5G) levels, we examined the relative abundance of the top ten most abundant groups. Actinobacteria was significantly lower (p < 0.05) at the phylum level, while Bacteroidetes showed an increasing trend. Erysipelotrichia and Actinobacteria were significantly lower (p < 0.05) at the phylum level, while Bacteroidetes showed a rising trend. Erysipelotrichales was significantly lower (p < 0.05) at the order level, while Bacteroidales was increasing. Erysipelotrichaceae was significantly lower (p < 0.05) at the family level (Figure 5G).
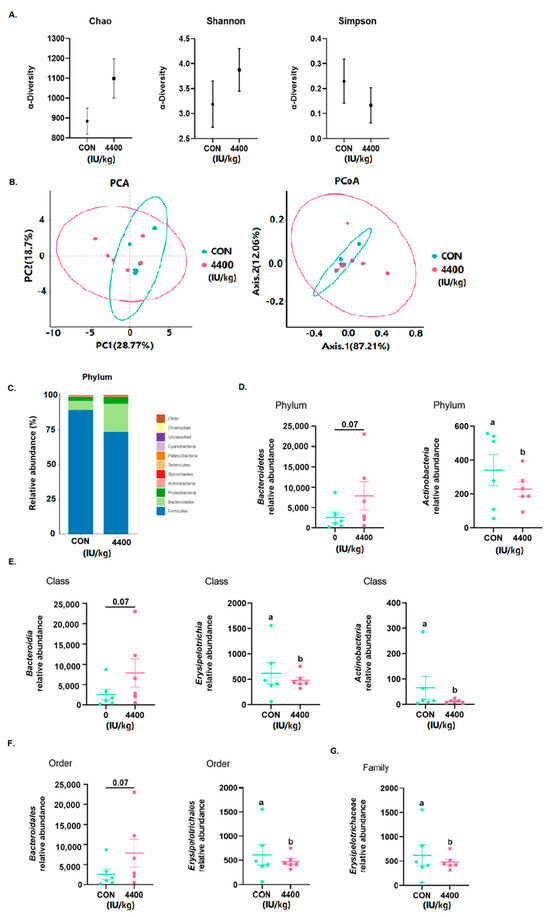
Figure 5.
Effect of vitamin A supplementation on intestinal flora: (A) Alpha diversity of the cecum flora. (B) Beta diversity of the cecum flora. (C) Relative abundance of the top 20 cecal microbiota. (D) Bacteria with significant differences at phylum level. (E) Bacteria with significant differences at class level. (F) Bacteria with significant differences at order level. (G) Bacteria with significant differences at family level. a, b showed means ± SEM (n = 6), different letters represented significant differences (p < 0.05).
4. Discussion
Piglets with underdeveloped intestinal function at weaning are susceptible to factors such as internal and external environment, resulting in loss of appetite, slow growth, and diarrhea. Vitamin A, as an essential microelement for animal growth, plays a positive role in growth and development and enhances immunity. Wang et al. [19] found that the supplementation of 16 mg/kg vitamin A to the diet significantly increased ADG in weaned piglets at 3 weeks of age. Our study found that vitamin A had no significant effect on body weight, feed intake, and feed/gain of weaned piglets, but vitamin A could reduce the diarrhea rate of piglets. This may be a meaningful indicator of weaning stress.
Weaning causes oxidative stress [20]. Oxidative damage is an important mechanism that damages the immune function and health of the body. The antioxidant system of the body has limited ability to clear free radicals, and excessive oxygen free radicals will destroy fat, protein, and nucleic acids in the body. Changes in the activities of oxidase GSH-Px, SOD, and CAT are closely related to the stress response of animals and can be used to reflect the immune state of the body [21]. Serum antioxidant capacity can reflect the resistance of animals to endogenous oxidative damage, and a higher antioxidant capacity has a beneficial effect on alleviating oxidative stress [22]. CAT is an enzyme that catalyzes the decomposition of hydrogen peroxide into water and oxygen, and is one of the important antioxidant enzymes that protect cells from reactive oxygen species. Supplementation of different concentrations of vitamin A in rats decreased the activity of CAT in the lungs, causing oxidative stress [23]. But our results showed that blood CAT levels increased significantly after the addition of vitamin A. In this study, vitamin A enhances the antioxidant capacity of weaned piglets by increasing CAT level.
In addition, vitamin A is also essential in humoral immunity, especially for antibody production [24]. Immunoglobulin is synthesized by plasma cells and lymphocytes. As an antibody, it is abundant in serum, accounting for about 20–25% of the total protein in serum. In addition, studies have shown that the addition of gelatin and vitamin A starch can improve the levels of IgA and IgM in serum [15]. IgM is the primary antibody of the primary immune response and the most common immunoglobulin expressed on the surface of B cells. The most prevalent antibody in blood circulation, IgG binds to a wide range of pathogens, including bacteria, fungi, and viruses, to prevent infection [25]. IgA is the main antibody heterotype produced on the mucosal surface, which can effectively prevent bacterial colonization and transfer in intestinal mucosa [26]. Our results showed that vitamin A did not significantly increase the serum immunoglobulin content of weaned piglets, but there was no downward trend. Our results may be related to the method of vitamin A supplementation.
The principal organ for nutrition digestion and absorption, the intestine also acts as a selective barrier against both endogenous and foreign antigens. Intestinal health, particularly the integrity of the intestinal barrier, is essential for preserving the organism’s healthy state. Tight junctions, which are the primary determinants of intestinal barrier function and close the paracellular space between adjacent epithelial cells, are necessary for intestinal integrity [27,28]. Vitamin A deficiency caused a colon-intestinal barrier in mice, which not only reduced the secretion of immunoglobulin A, but also down-regulated the colon-tight-junction-related protein Occludin and Claudin-1 [29]. Our results showed that the expression of Claudin-1, Occludin, and ZO-1 in the jejunum and the expression of Claudin-1 and ZO-1 in the ileum of weaned piglets increased significantly after vitamin A was added. Vitamin A plays a protective role in the intestinal tract of weaned piglets. E. coli is one of the important causes of diarrhea in piglets. The addition of probiotics can improve the decreased expression levels of Occludin, Claudin-1, and ZO-1 in the intestinal tract infected by E. coli [30]. More confirmation that vitamin A enhances tight junction protein gene expression may be one of the reasons for improving diarrhea in weaned piglets.
Vitamin A has been shown to be associated with inflammation in animals, and intestinal inflammation in piglets is expressed at the genetic level by the interleukin gene [31,32]. Immunity in the organism is a complex process and is often divided into innate and acquired immunity. Vitamin A may be involved in the immune response associated with the intestinal mucosa through the regulation of the cytokines IL-4 [33]. Chen et al. observed that ATRA increased the mRNA of the IL-4 receptor alpha chain, and increased IL-4-induced phosphorylation of the signal transducer and activator of transcription 6 (STAT6) and mRNA expression of chloride channels [34]. IL-5 regulates the acquired immune response and acts on target cells by binding to its specific IL-5 receptor (IL-5R). IL-5 activates JAK1/2-STAT1/2 and Ras/ERK channels to act on inflammation in the body [35]. In addition to this, IL-5 can stimulate the secretion of IgM and IgG by staphylococcal A-activated B cells [36], and recent reports have found that IL-5 can collaborate with IL-21 to induce the proliferation of IgA cells [37]. IL-4 and IL-5 are cytokines produced by TH2 helper T cells and mast cells, basophils, and eosinophils, both markers of the development of TH2 inflammation, along with systemic inflammation [38]. Nutritional regulation of allergic inflammation of mucosal surfaces is of great importance. Vitamin A deficiency decreased the TH2 response and increased the TH1 response [39]. In this study, the addition of vitamin A significantly increased the expression of IL-4, IL-5, and IL-10 in the gut. Vitamin A has a regulatory effect on stress-induced intestinal inflammation in weaned piglets, but its effect on related pathways needs to be confirmed by subsequent experiments.
The gut microbiome is increasingly recognized to play a role in regulating host growth performance, metabolism, diarrhea [40], and inflammatory responses [41]. Dietary nutrients are digested and absorbed in the foregut before undigested components and endogenous substances are fermented by the gut microbiota in the hindgut [42,43]. The establishment of a healthy, stable, and diverse intestinal flora is important for weaning piglets to resist stress and intestinal disease. In the present study, we demonstrated significant differences in the structure and content of intestinal flora between piglets fed vitamin A and control piglets. α diversity can be used as an indicator of the functional resilience of the intestinal microbial ecosystem, including species richness and species diversity [44]. From our experimental results, there was no significant change. PCA and PCoA are methods for downscaling and displaying multidimensional data differences on a two-dimensional coordinate plot. The closer the samples are in the graph, the more similar their community composition is [45]. Our data show that after the addition of vitamin A, the cecum flora of piglets still changed and did not completely overlap. The ratio of thick-walled to Bacteroidetes phylum is a common evaluation indicator that represents the imbalance in intestinal flora composition [46]. Our study discovered an increase in Bacteroidetes, and it was hypothesized that vitamin A altered the composition of weaned piglet cecum flora. Actinobacteria are Gram-positive bacteria with mycelium and substrates that are rich in bioactive secondary metabolites such as enzymes, antibiotics, and antioxidants [47]. Actinobacteria also produce a variety of antimicrobial agents [48]. In this experiment, we found that Actinobacteria at the gateway level increased the abundance of Actinobacteria in the piglet cecum. In one study [49], researchers discovered that Erysipelotrichia of the intestine reduces improving oxidative stress caused by weaning stress in piglets. Similar to our findings, it is hypothesized that vitamin A can reduce weaning-induced oxidative stress by decreasing Erysipelotrichia. Intestinal oxidative stress triggers intestinal microbiota dysbiosis, which is associated with post-weaning diarrhea and intestinal infections. Ellagic acid with antioxidant capacity can modulate the gut microbiota of weanling piglets to alleviate intestinal damage and oxidative stress [50]. We suspect that vitamin A has a similar effect on relieving diarrhea. Chai et al. [51] found from the transcriptome that the gene expression module for vitamin A effects in the small intestine differed in the colon, again perhaps because vitamin A absorption and metabolism occurs mostly in the small intestine. Although the small intestine and colon are contiguous organs with similar developmental origins, there are differences between their biological functions. The specific mechanisms by which vitamin A regulates the intestinal flora in weaned piglets are not fully defined by changes in flora abundance and need to be explored in more depth. In conclusion, 4400 IU/kg vitamin has positive effects on the growth performance, intestinal inflammation, and intestinal flora of weaned piglets.
5. Conclusions
Dietary supplementation with 4400 IU/kg vitamin A reduced the diarrhea rate of weaned piglets. Vitamin A improved weaned piglet catalase activity, improved weaned piglet antioxidant capacity, improved the intestinal barrier in the jejunum and ileum, regulated intestinal inflammation in the jejunum and ileum, and influenced cecum intestinal flora. This study may provide a foundation for the use of vitamin A in antibiotic-free piglet production, but more in vivo and ex vivo experiments are needed to prove this.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12122049/s1, Figure S1: Effect of vitamin A supplementation on serum antioxidant levels; Figure S2: Effect of vitamin A supplementation on the expression levels of tight junction proteins in the jejunum and ileum; Figure S3: Effect of vitamin A supplementation on the expression levels of inflammatory cytokines in the jejunum and ileum.
Author Contributions
S.H. designed the experiments; S.W., B.C. and X.W. conducted the experiments and analyzed the data; S.W. and L.W. wrote the manuscript; S.H., L.W. and Z.J. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the earmarked fund for CARS (CARS-35) and Key Projects of Maoming Laboratory (2022ZD003).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (Guangzhou, China), (No. GAASIAS-2020-005).
Informed Consent Statement
Not applicable.
Data Availability Statement
All experimental data and analytical results are included in this study. The raw 16S rRNA sequences have been deposited in the NCBI SRA under BioProject PRJNA879935. The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviation
| Vitamin A | Vitamin A |
| BW | Body weight |
| ADG | Average daily gain |
| ADFI | Average daily feed intake |
| F:G | Feed-to-gain ratio |
| GSH-Px | Glutathione peroxidase |
| CAT | Catalase |
| T-SOD | Total superoxide dismutase |
| T-AOC | Total antioxidation capability |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IgA | Immunoglobulin A |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-10 | Interleukin 10 |
| ZO-1 | Zonula occludens-1 |
| PCA | Principal component analysis |
| PCoA | Principal coordinates analysis |
References
- Hay, M.; Orgeur, P.; Lévy, F.; Le Dividich, J.; Concordet, D.; Nowak, R.; Schaal, B.; Mormède, P. Neuroendocrine consequences of very early weaning in swine. Physiol. Behav. 2001, 72, 263–269. [Google Scholar] [CrossRef]
- Marion, J.; Biernat, M.; Thomas, F.; Savary, G.; Le Breton, Y.; Zabielski, R.; Le Huërou-Luron, I.; Le Dividich, J. Small intestine growth and morphometry in piglets weaned at 7 days of age. Effects of level of energy intake. Reprod. Nutr. Develpoment 2002, 42, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Hotzel, M.J.; Machado, F.L.; Irgang, R.; Alexandre, F.L. Short-term behavioural effects of weaning age in outdoor-reared piglets. Animal 2010, 4, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Hall, J.A.; Kroehling, L.; Wu, L.; Najar, T.; Nguyen, H.H.; Lin, W.-Y.; Yeung, S.T.; Silva, H.M.; Li, D.; et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory. Cell 2020, 180, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated with Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Husb. Biotechnol. Engl. Ed. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Chen, J.; Bi, M.; Zhang, Y.; Chen, S.; Zhao, Y.; Wang, F.; Qiu, T.; Chen, L.; Li, C.; et al. Interaction between the expression of retinol binding protein 4 and gonadotropin receptors in follicular granulosa cells of pigs. Livest. Sci. 2019, 220, 205–210. [Google Scholar] [CrossRef]
- Wu, J.; Wan, X.; Zhang, H.; Li, W.; Ma, M.; Pan, B.; Liang, X.; Cao, C. Retinoic acid attenuates contrast-induced acute kidney injury in a miniature pig model. Biochem. Biophys. Res. Commun. 2019, 512, 163–169. [Google Scholar] [CrossRef]
- Ayuso, M.; Óvilo, C.; Fernández, A.; Nuñez, Y.; Isabel, B.; Daza, A.; López-Bote, C.J.; Rey, A.I. Effects of dietary vitamin A supplementation or restriction and its timing on retinol and α-tocopherol accumulation and gene expression in heavy pigs. Anim. Feed. Sci. Technol. 2015, 202, 62–74. [Google Scholar] [CrossRef]
- Jiang, W.; Napoli, J.L. Reorganization of cellular retinol-binding protein type 1 and lecithin:retinol acyltransferase during retinyl ester biosynthesis. Biochim. Biophys. Acta 2012, 1820, 859–869. [Google Scholar] [CrossRef]
- Zhou, H.B.; Huang, X.Y.; Bi, Z.; Hu, Y.H.; Wang, F.Q.; Wang, X.X.; Wang, Y.Z.; Lu, Z.Q. Vitamin A with L-ascorbic acid sodium salt improves the growth performance, immune function and antioxidant capacity of weaned pigs. Animal 2021, 15, 100133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Gou, Z.; Chen, F.; Fan, Q.; Lin, X.; Ye, J.; Zhang, C.; Jiang, S. Effects of maternal and dietary vitamin A on growth performance, meat quality, antioxidant status, and immune function of offspring broilers. Poult. Sci. 2020, 99, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Han, J.Y.; Yan, S.M.; Li, Y.; Shi, B.; Zhao, Y. Effects of Vitamin A on Growth Performance, Immunity and Antioxidant Function of Broilers. Chin. J. Anim. Nutr. 2007, 31, 71–82. [Google Scholar]
- Debelo, H.; Novotny, J.A.; Ferruzzi, M.G. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Zhang, Y.; Xiong, H.; Wang, F.; Wang, Y.; Lu, Z. Effects of starch and gelatin encapsulated vitamin A on growth performance, immune status and antioxidant capacity in weaned piglets. Anim. Nutr. 2020, 6, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, L.; Chen, Y.; Xiong, Y.; Wu, Q.; Jiang, Z.; Yi, H. Effects of niacin on intestinal immunity, microbial community and intestinal barrier in weaned piglets during starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef]
- Cun, L.G.; Ghani, M.W.; Yi, Z.; Jiang, W.; Ye, L.; Bin, L.; Birmani, M.W.; An, L.; Xiao, M. Different combinations of GABA, BMP7, and Activin A induced the in vitro differentiation of rat pancreatic ductal stem cells into insulin-secreting islet-like cell clusters. Life Sci. 2021, 267, 118451. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wang, Y.; Wang, L.; Yin, Y.; Yin, L.; Yang, H.; Yin, Y. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J. Anim. Sci. 2020, 98, skaa020. [Google Scholar] [CrossRef]
- Ma, X.A.; Shang, Q.A.; Hu, J.A.; Liu, H.A.; Cb, B.; Px, A. Effects of replacing soybean meal, soy protein concentrate, fermented soybean meal or fish meal with enzyme-treated soybean meal on growth performance, nutrient digestibility, antioxidant capacity, immunity and intestinal morphology in weaned pigs. Livest. Sci. 2019, 225, 39–46. [Google Scholar] [CrossRef]
- Qi, X.Z.; Xue, M.Y.; Yang, S.B.; Zha, J.W.; Wang, G.X.; Ling, F. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish. Immunol. 2017, 70, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Q.; Sun, S.; Huang, B.; Zhang, Y.; Xu, Y.; Zhang, S.; Xiang, H. Probiotics-fermented Massa Medicata Fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis. Appl. Microbiol. Biotechnol. 2018, 102, 10713–10727. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.A.B.; Gelain, D.P.; Oliveira, M.R.; Behr, G.A.; Motta, L.L.; Rocha, R.F.; Klamt, F.; Moreira, J.C. Vitamin A supplementation induces oxidative stress and decreases the immunocontent of catalase and superoxide dismutase in rat lungs. Exp. Lung Res. 2009, 35, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Rudraraju, R.; Sealy, R.; Jones, B.; Hurwitz, J.L. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 2012, 25, 341. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jin, X.; Yuan, B.; Lv, Y.; Yan, G.; Liu, M.; Xie, C.; Liu, J.; Tang, Y.; Gao, H.; et al. G Protein-Coupled Receptor 109A Maintains the Intestinal Integrity and Protects Against ETEC Mucosal Infection by Promoting IgA Secretion. Front. Immunol. 2020, 11, 583652. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Feng, D.; Chen, B.; Zeng, B.; Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wang, L.; Wei, H.; et al. Fecal microbiota from children with vitamin A deficiency impair colonic barrier function in germ-free mice: The possible role of alterative bile acid metabolites. Nutrition 2021, 90, 111274. [Google Scholar] [CrossRef]
- Ren, S.; Chen, A.; Tian, Y.; Bai, Z.; Wang, C. Lactobacillus paracasei from Koumiss Ameliorates Diarrhea in mice via Tight Junctions Modulation. Nutrition 2022, 98, 111584. [Google Scholar] [CrossRef]
- Du, W.; Xu, H.; Mei, X.; Cao, X.; Gong, L.; Wu, Y.; Li, Y.; Yu, D.; Liu, S.; Wang, Y.; et al. Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benef. Microbes 2018, 9, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cheng, L.; Zhu, Y.; Zhao, X.; Zhang, W.; Gao, X.; Xiong, T.; Guo, L. Immune-related effects of compound astragalus polysaccharide and sulfated epimedium polysaccharide on newborn piglets. Anim. Biotechnol. 2021, 34, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Chu, B.; Jiang, L.R. Effects of vitamin A on the immune function of intestinal mucosa lymphocytes in mice. Zhongguo Dang Dai Er Ke Za Zhi 2010, 12, 976–978. [Google Scholar] [PubMed]
- Chen, C.; Smith, A.D.; Cheung, L.; Pham, Q.; Urban, J.J.; Dawson, H.D. Potentiation of IL-4 Signaling by Retinoic Acid in Intestinal Epithelial Cells and Macrophages-Mechanisms and Targets. Front. Immunol. 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Pazdrak, K.; Stafford, S.; Alam, R. The activation of the Jak-STAT 1 signaling pathway by IL-5 in eosinophils. J. Immunol. 1995, 155, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, J.N.; Sanderson, C.J.; Benson, E.M. Human interleukin-5 induces staphylococcal A Cowan 1 strain-activated human B cells to secrete IgM. Eur. J. Immunol. 2010, 23, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Kashiwakura, Y.; Kanno, Y.; Kojima, H.; Kobata, T. IL-21 and IL-5 coordinately induce surface IgA(+) cells. Immunol. Lett. 2020, 224, 21–27. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Jiang, X.; Freytag, T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J. Nutr. 2004, 134, 2660–2666. [Google Scholar] [CrossRef]
- Han, C.; Dai, Y.; Liu, B.; Wang, L.; Wang, J.; Zhang, J. Diversity analysis of intestinal microflora between healthy and diarrheal neonatal piglets from the same litter in different regions. Anaerobe 2019, 55, 136–141. [Google Scholar] [CrossRef]
- Simon, H.; Vartanian, V.; Wong, M.H.; Nakabeppu, Y.; Sharma, P.; Lloyd, R.S.; Sampath, H. OGG1 deficiency alters the intestinal microbiome and increases intestinal inflammation in a mouse model. PLoS ONE 2020, 15, e227501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hou, G.; Jiang, X.; Song, Z.; Fan, Z.; Hou, D.X.; He, X. Different dietary protein sources in low protein diets regulate colonic microbiota and barrier function in a piglet model. Food Funct. 2019, 10, 6417–6428. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Li, M.; Piao, X. Effects of maternal 25-hydroxycholecalciferol during the last week of gestation and lactation on serum parameters, intestinal morphology and microbiota in suckling piglets. Arch. Anim. Nutr. 2020, 74, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Avershina, E.; Frisli, T.; Rudi, K. De novo semi-alignment of 16S rRNA gene sequences for deep phylogenetic characterization of next generation sequencing data. Microbes Environ. 2013, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clement, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Sapkota, A.; Thapa, A.; Budhathoki, A.; Sainju, M.; Shrestha, P.; Aryal, S. Isolation, Characterization, and Screening of Antimicrobial-Producing Actinomycetes from Soil Samples. Int. J. Syst. Evol. Microbiol. 2020, 2020, 2716584. [Google Scholar] [CrossRef]
- Han, H.; Liu, Z.; Yin, J.; Gao, J.; He, L.; Wang, C.; Hou, R.; He, X.; Wang, G.; Li, T.; et al. D-Galactose Induces Chronic Oxidative Stress and Alters Gut Microbiota in Weaned Piglets. Front. Physiol. 2021, 12, 634283. [Google Scholar] [CrossRef]
- Qin, W.; Xu, B.; Chen, Y.; Yang, W.; Xu, Y.; Huang, J.; Duo, T.; Mao, Y.; Zhou, G.; Yan, X.; et al. Dietary ellagic acid supplementation attenuates intestinal damage and oxidative stress by regulating gut microbiota in weanling piglets. Anim. Nutr. 2022, 11, 322–333. [Google Scholar] [CrossRef]
- Chai, Z.; Lyu, Y.; Chen, Q.; Wei, C.H.; Snyder, L.M.; Weaver, V.; Sebastian, A.; Albert, I.; Li, Q.; Cantorna, M.T.; et al. RNAseq studies reveal distinct transcriptional response to vitamin A deficiency in small intestine versus colon, uncovering novel vitamin A-regulated genes. J. Nutr. Biochem. 2021, 98, 108814. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).