N-Acetylcysteine and Atherosclerosis: Promises and Challenges
Abstract
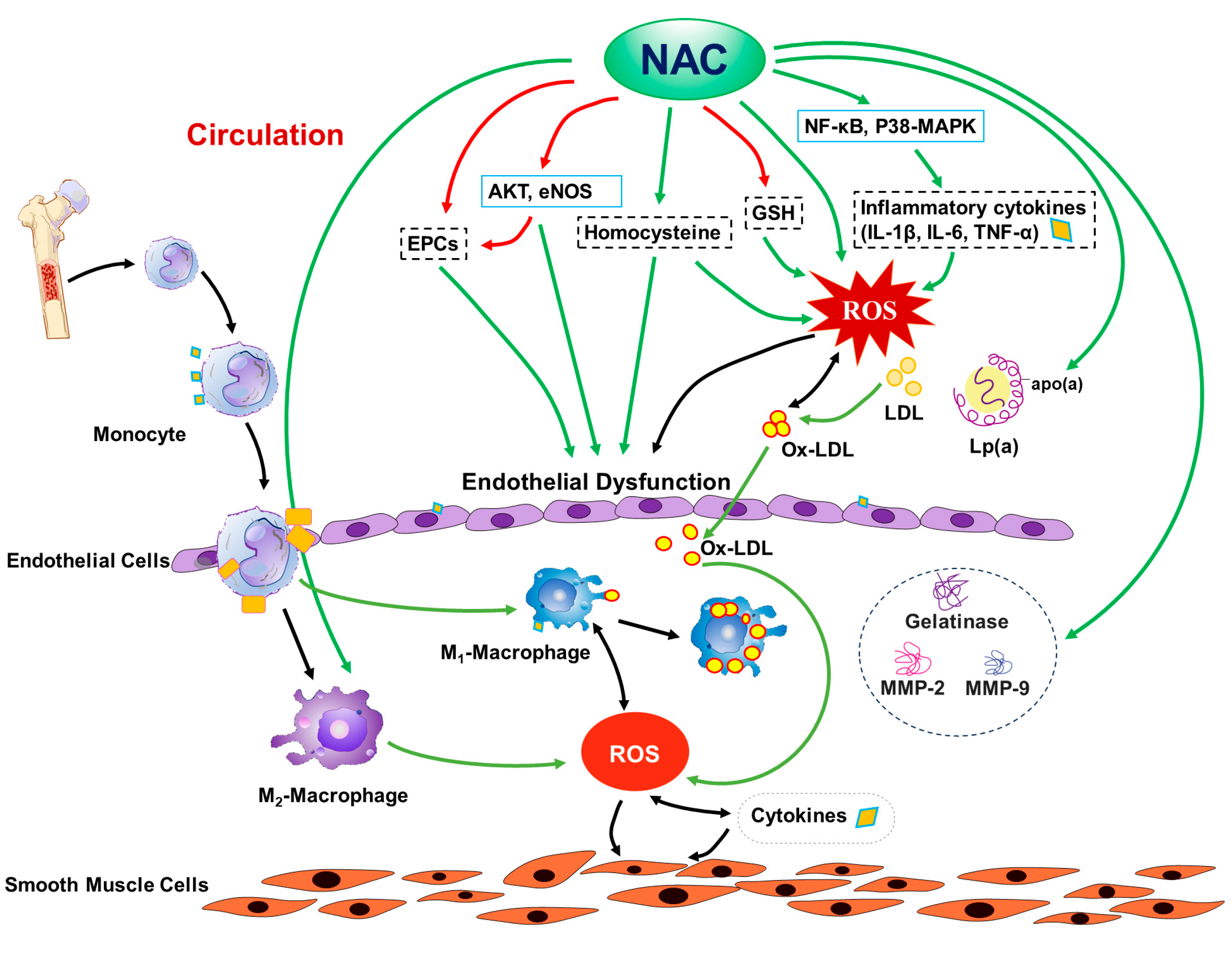
1. Introduction
2. Overview of NAC and Cardiovascular Diseases
3. NAC and ROS
4. NAC, Inflammation, and Macrophages
5. NAC and Atherosclerosis and CAD
6. NAC and Lipid Metabolism
7. NAC and Homocysteine
8. Effects of NAC on Endothelial Cells
9. Mechanisms for the Actions of NAC on Atherosclerosis
10. Other Effects of NAC and Mechanisms
11. Tolerability and Potential Toxicity and Side Effects of NAC
12. Other Antioxidants and Atherosclerosis
13. Unanswered Questions on NAC and Atherosclerosis and Challenges for Clinical Studies in Patients with Atherosclerosis
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2008, CD007176. [Google Scholar] [CrossRef]
- Tenorio, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Sochman, J. N-acetylcysteine in acute cardiology: 10 years later: What do we know and what would we like to know?! J. Am. Coll. Cardiol. 2002, 39, 1422–1428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wittstock, A.; Burkert, M.; Zidek, W.; Tepel, M.; Scholze, A. N-acetylcysteine improves arterial vascular reactivity in patients with chronic kidney disease. Nephron Clin. Pract. 2009, 112, c184–c189. [Google Scholar] [CrossRef] [PubMed]
- Pereira Filho Nde, A.; Pereira Filho Ade, A.; Soares, F.P.; Coutinho, L.M. Effect of N-acetylcysteine on vasospasm in subarachnoid hemorrhage. Arq. Neuro-Psiquiatr. 2010, 68, 918–922. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Klein, U.; Palutke, M.; Schirrmeister, W. Reduction of ischemia-reperfusion syndrome after abdominal aortic aneurysmectomy by N-acetylcysteine but not mannitol. Acta Anaesthesiol. Scand. 1996, 40, 657–664. [Google Scholar] [CrossRef]
- Sambo, P.; Amico, D.; Giacomelli, R.; Matucci-Cerinic, M.; Salsano, F.; Valentini, G.; Gabrielli, A. Intravenous N-acetylcysteine for treatment of Raynaud’s phenomenon secondary to systemic sclerosis: A pilot study. J. Rheumatol. 2001, 28, 2257–2262. [Google Scholar] [PubMed]
- Rosato, E.; Borghese, F.; Pisarri, S.; Salsano, F. The treatment with N-acetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: A prospective observational study of 50 patients. Clin. Rheumatol. 2009, 28, 1379–1384. [Google Scholar] [CrossRef]
- Salsano, F.; Letizia, C.; Proietti, M.; Rossi, C.; Proietti, A.R.; Rosato, E.; Pisarri, S. Significant changes of peripheral perfusion and plasma adrenomedullin levels in N-acetylcysteine long term treatment of patients with sclerodermic Raynauds phenomenon. Int. J. Immunopathol. Pharmacol. 2005, 18, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.J.; Mariz, H.A.; Andrade, L.E.; Kayser, C. Oral N-acetylcysteine in the treatment of Raynaud’s phenomenon secondary to systemic sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Rev. Bras. Reumatol. 2014, 54, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]
- Tepel, M.; van der Giet, M.; Statz, M.; Jankowski, J.; Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 2003, 107, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, X.Z.; Shen, M.Z.; Xing, C.Y.; Ma, J.; Duan, Y.Y.; Yuan, L.J. N-Acetyl Cysteine improves the diabetic cardiac function: Possible role of fibrosis inhibition. BMC Cardiovasc. Disord. 2015, 15, 84. [Google Scholar] [CrossRef]
- Phaelante, A.; Rohde, L.E.; Lopes, A.; Olsen, V.; Tobar, S.A.; Cohen, C.; Martinelli, N.; Biolo, A.; Dal-Pizzol, F.; Clausell, N.; et al. N-acetylcysteine Plus Deferoxamine Improves Cardiac Function in Wistar Rats After Non-reperfused Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2015, 8, 328–337. [Google Scholar] [CrossRef]
- Costa, C.R.M.; Seara, F.A.C.; Peixoto, M.S.; Ramos, I.P.; Barbosa, R.A.Q.; Carvalho, A.B.; Fortunato, R.S.; Silveira, A.L.B.; Olivares, E.L. Progression of heart failure is attenuated by antioxidant therapy with N-acetylcysteine in myocardial infarcted female rats. Mol. Biol. Rep. 2020, 47, 8645–8656. [Google Scholar] [CrossRef]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T.; et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef]
- Sucu, N.; Cinel, I.; Unlu, A.; Aytacoglu, B.; Tamer, L.; Kocak, Z.; Karaca, K.; Gul, A.; Dikmengil, M.; Atik, U.; et al. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg. Today 2004, 34, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Tossios, P.; Bloch, W.; Huebner, A.; Raji, M.R.; Dodos, F.; Klass, O.; Suedkamp, M.; Kasper, S.M.; Hellmich, M.; Mehlhorn, U. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: Results of a randomized, double-blind, placebo-controlled clinical trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.E.G.; El Dib, R.; Braz, L.G.; Escudero, J.; Hayes, J.; Johnston, B.C. N-acetylcysteine use among patients undergoing cardiac surgery: A systematic review and meta-analysis of randomized trials. PLoS ONE 2019, 14, e0213862. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Habibi, M.R.; Hasanzadeh Kiabi, F.; Alipour, A.; Habibi, V.; Azizi, S.; Emami Zeydi, A.; Sohrabi, F.B. The effect of intravenous N-acetylcysteine on prevention of atrial fibrillation after coronary artery bypass graft surgery: A double-blind, randomised, placebo-controlled trial. Kardiol. Pol. 2018, 76, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Akcay, S.; Yucel, H.; Icli, A.; Ceyhan, B.M.; Sutcu, R.; Uysal, B.A.; Varol, E.; et al. Oxidative status, inflammation, and postoperative atrial fibrillation with metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine treatment. Clin. Cardiol. 2014, 37, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin, M.; Icli, A.; Yucel, H.; Akcay, S.; Peker, O.; Erdogan, D.; Varol, E.; Dogan, A.; Okutan, H. Metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine on post-operative atrial fibrillation: A randomized, double-blind, placebo-controlled study. Eur. Heart J. 2013, 34, 597–604. [Google Scholar] [CrossRef]
- Ozaydin, M.; Erdogan, D.; Yucel, H.; Peker, O.; Icli, A.; Akcay, S.; Etli, M.; Ceyhan, B.M.; Sutcu, R.; Varol, E.; et al. N-acetyl cysteine for the conversion of atrial fibrillation into sinus rhythm after cardiac surgery: A prospective, randomized, double-blind, placebo-controlled pilot study. Int. J. Cardiol. 2013, 165, 580–583. [Google Scholar] [CrossRef]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Kapan, S.; Turker, Y.; Varol, E.; Ozguner, F.; Dogan, A.; Ibrisim, E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: A prospective, randomized, placebo-controlled pilot study. Eur. Heart J. 2008, 29, 625–631. [Google Scholar] [CrossRef]
- Kazemi, B.; Akbarzadeh, F.; Safaei, N.; Yaghoubi, A.; Shadvar, K.; Ghasemi, K. Prophylactic high-dose oral-N-acetylcysteine does not prevent atrial fibrillation after heart surgery: A prospective double blind placebo-controlled randomized clinical trial. Pacing Clin. Electrophysiol. PACE 2013, 36, 1211–1219. [Google Scholar] [CrossRef]
- Mehra, A.; Shotan, A.; Ostrzega, E.; Hsueh, W.; Vasquez-Johnson, J.; Elkayam, U. Potentiation of isosorbide dinitrate effects with N-acetylcysteine in patients with chronic heart failure. Circulation 1994, 89, 2595–2600. [Google Scholar] [CrossRef]
- Dresdale, A.R.; Barr, L.H.; Bonow, R.O.; Mathisen, D.J.; Myers, C.E.; Schwartz, D.E.; d’Angelo, T.; Rosenberg, S.A. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am. J. Clin. Oncol. 1982, 5, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Unverferth, D.V.; Jagadeesh, J.M.; Unverferth, B.J.; Magorien, R.D.; Leier, C.V.; Balcerzak, S.P. Attempt to prevent doxorubicin-induced acute human myocardial morphologic damage with acetylcysteine. J. Natl. Cancer Inst. 1983, 71, 917–920. [Google Scholar] [PubMed]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine With Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Yesilbursa, D.; Serdar, A.; Senturk, T.; Serdar, Z.; Sag, S.; Cordan, J. Effect of N-acetylcysteine on oxidative stress and ventricular function in patients with myocardial infarction. Heart Vessel. 2006, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Koramaz, I.; Pulathan, Z.; Usta, S.; Karahan, S.C.; Alver, A.; Yaris, E.; Kalyoncu, N.I.; Ozcan, F. Cardioprotective effect of cold-blood cardioplegia enriched with N-acetylcysteine during coronary artery bypass grafting. Ann. Thorac. Surg. 2006, 81, 613–618. [Google Scholar] [CrossRef]
- Vento, A.E.; Nemlander, A.; Aittomaki, J.; Salo, J.; Karhunen, J.; Ramo, O.J. N-acetylcysteine as an additive to crystalloid cardioplegia increased oxidative stress capacity in CABG patients. Scand. Cardiovasc. J. SCJ 2003, 37, 349–355. [Google Scholar] [CrossRef]
- Horowitz, J.D.; Henry, C.A.; Syrjanen, M.L.; Louis, W.J.; Fish, R.D.; Smith, T.W.; Antman, E.M. Combined use of nitroglycerin and N-acetylcysteine in the management of unstable angina pectoris. Circulation 1988, 77, 787–794. [Google Scholar] [CrossRef]
- Roseguini, B.T.; Silva, L.M.; Polotow, T.G.; Barros, M.P.; Souccar, C.; Han, S.W. Effects of N-acetylcysteine on skeletal muscle structure and function in a mouse model of peripheral arterial insufficiency. J. Vasc. Surg. 2015, 61, 777–786. [Google Scholar] [CrossRef]
- Lejay, A.; Charles, A.L.; Georg, I.; Goupilleau, F.; Delay, C.; Talha, S.; Thaveau, F.; Chakfe, N.; Geny, B. Critical Limb Ischaemia Exacerbates Mitochondrial Dysfunction in ApoE-/- Mice Compared with ApoE+/+ Mice, but N-acetyl Cysteine still Confers Protection. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, L.; Xiao, Y.; Li, X.; Zhang, J.; Xie, X.; Tian, J.; Sen, C.K.; He, X.; Hao, H.; et al. N-acetylcysteine differentially regulates the populations of bone marrow and circulating endothelial progenitor cells in mice with limb ischemia. Eur. J. Pharmacol. 2020, 881, 173233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hao, H.; Xu, H.; Fichman, Y.; Cui, Y.; Yang, C.; Wang, M.; Mittler, R.; Hill, M.A.; Cowan, P.J.; et al. Combination of Antioxidant Enzyme Overexpression and N-Acetylcysteine Treatment Enhances the Survival of Bone Marrow Mesenchymal Stromal Cells in Ischemic Limb in Mice With Type 2 Diabetes. J. Am. Heart Assoc. 2021, 10, e023491. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Li, S.H.; Szmitko, P.E.; Fedak, P.W.; Verma, S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Narasimhulu, C.A.; Liu, L.; Li, X.; Xiao, Y.; Zhang, J.; Xie, X.; Hao, H.; Liu, J.Z.; He, G.; et al. Oxidized low-density lipoprotein alters endothelial progenitor cell populations. Front. Biosci. 2015, 20, 975–988. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, H.; Jiang, Y.; Zhuo, X.; Hu, K.; Si, Z.; Chen, Y.; Liu, Q.; Gong, X.; Sun, H.; et al. N-acetyl cysteine prevents ambient fine particulate matter-potentiated atherosclerosis via inhibition of reactive oxygen species-induced oxidized low density lipoprotein elevation and decreased circulating endothelial progenitor cell. Mol. Med. Rep. 2022, 26, 236. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 2002, 86, 123–130. [Google Scholar] [CrossRef]
- Cailleret, M.; Amadou, A.; Andrieu-Abadie, N.; Nawrocki, A.; Adamy, C.; Ait-Mamar, B.; Rocaries, F.; Best-Belpomme, M.; Levade, T.; Pavoine, C.; et al. N-acetylcysteine prevents the deleterious effect of tumor necrosis factor-(alpha) on calcium transients and contraction in adult rat cardiomyocytes. Circulation 2004, 109, 406–411. [Google Scholar] [CrossRef]
- Zhang, D.X.; Gutterman, D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2023–H2031. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Asanuma, K.; Godin, D.; Meng, X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: New target for antioxidant therapy? Circulation 1998, 97, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.P.; Yin, C.S.; Cui, J.H.; Li, Z.X.; Wang, L.; Wang, Y.W.; Li, Y.L. Inhibitory effect of N-acetylcysteine upon atherosclerotic processes in rabbit carotid. Zhonghua Yi Xue Za Zhi 2009, 89, 1850–1853. [Google Scholar] [PubMed]
- Sung, H.J.; Kim, J.; Kim, Y.; Jang, S.W.; Ko, J. N-acetyl cysteine suppresses the foam cell formation that is induced by oxidized low density lipoprotein via regulation of gene expression. Mol. Biol. Rep. 2012, 39, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, O.; Szumilak, D.; Nguyen-Khoa, T.; Ruellan, N.; Phan, O.; Lacour, B.; Descamps-Latscha, B.; Drueke, T.B.; Massy, Z.A. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005, 67, 2288–2294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, K.; Murayama, T.; Yokode, M.; Kita, T.; Uzui, H.; Ueda, T.; Lee, J.D.; Kishimoto, C. N-acetylcysteine reduces the severity of atherosclerosis in apolipoprotein E-deficient mice by reducing superoxide production. Circ. J. 2009, 73, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, L.; Zhou, S.; Zhu, M.; Wang, B. Nacetylcysteine inhibits atherosclerosis by correcting glutathionedependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice. Mol. Med. Rep. 2021, 23, 201. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, Y.; Jiang, M.; Liu, X.; Cui, Y.; Hao, H.; Flaker, G.C.; Liu, Q.; Zhou, S.; Liu, Z. N-acetylcysteine attenuates atherosclerosis progression in aging LDL receptor deficient mice with preserved M2 macrophages and increased CD146. Atherosclerosis 2022, 357, 41–50. [Google Scholar] [CrossRef]
- Cui, Y.; Narasimhulu, C.A.; Liu, L.; Zhang, Q.; Liu, P.Z.; Li, X.; Xiao, Y.; Zhang, J.; Hao, H.; Xie, X.; et al. N-acetylcysteine inhibits in vivo oxidation of native low-density lipoprotein. Sci. Rep. 2015, 5, 16339. [Google Scholar] [CrossRef]
- Wagberg, M.; Jansson, A.H.; Westerlund, C.; Ostlund-Lindqvist, A.M.; Sarnstrand, B.; Bergstrand, H.; Pettersson, K. N,N′-diacetyl-L-cystine (DiNAC), the disulphide dimer of N-acetylcysteine, inhibits atherosclerosis in WHHL rabbits: Evidence for immunomodulatory agents as a new approach to prevent atherosclerosis. J. Pharmacol. Exp. Ther. 2001, 299, 76–82. [Google Scholar] [PubMed]
- Krieger, M.H.; Santos, K.F.; Shishido, S.M.; Wanschel, A.C.; Estrela, H.F.; Santos, L.; De Oliveira, M.G.; Franchini, K.G.; Spadari-Bratfisch, R.C.; Laurindo, F.R. Antiatherogenic effects of S-nitroso-N-acetylcysteine in hypercholesterolemic LDL receptor knockout mice. Nitric Oxide Biol. Chem. 2006, 14, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Siasos, G.; Mastrogeorgiou, M.; Spartalis, E.; Kaminiotis, V.V.; Mylonas, K.S.; Kapelouzou, A.; Kontogiannis, C.; Doulamis, I.P.; Toutouzas, K.; et al. The effect of per os colchicine administration in combination with fenofibrate and N-acetylcysteine on triglyceride levels and the development of atherosclerotic lesions in cholesterol-fed rabbits. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7765–7776. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Parthasarathy, S.; Hao, H.; Luo, M.; Ahmed, S.; Zhu, J.; Luo, S.; Kuppusamy, P.; Sen, C.K.; Verfaillie, C.M.; et al. Reactive oxygen species mediate oxidized low-density lipoprotein-induced inhibition of oct-4 expression and endothelial differentiation of bone marrow stem cells. Antioxid. Redox Signal. 2010, 13, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.; Si, Z.; Bu, H.; Narasimhulu, C.A.; Song, X.; Cui, M.Y.; Liu, H.; Lu, T.; He, G.; et al. Probucol Protects Endothelial Progenitor Cells Against Oxidized Low-Density Lipoprotein via Suppression of Reactive Oxygen Species Formation In Vivo. Cell. Physiol. Biochem. 2016, 39, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rattan, A.K.; Arad, Y. Temporal and kinetic determinants of the inhibition of LDL oxidation by N-acetylcysteine (NAC). Atherosclerosis 1998, 138, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Libby, P.; Galis, Z.S. Cytokines regulate genes involved in atherogenesis. Ann. N. Y. Acad. Sci. 1995, 748, 158–168; discussion 168–170. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kuhn, H.; Hennig, B.; Neish, A.S.; Toborek, M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell. Cardiol. 2001, 33, 83–94. [Google Scholar] [CrossRef]
- Jain, S.K.; Kannan, K.; Lim, G.; Matthews-Greer, J.; McVie, R.; Bocchini, J.A., Jr. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care 2003, 26, 2139–2143. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, K.; Iwase, M.; Ohdo, S.; Ieiri, I.; Matsuyama, N.; Takata, Y.; Kitazono, T. Telmisartan and N-acetylcysteine suppress group V secretory phospholipase A2 expression in TNFalpha-stimulated human endothelial cells and reduce associated atherogenicity. J. Cardiovasc. Pharmacol. 2012, 60, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Takahata, H.; Kitagawa, N.; Kitange, G.; Kaminogo, M.; Shibata, S. N-acetylcysteine inhibited nuclear factor-kappaB expression and the intimal hyperplasia in rat carotid arterial injury. Neurol. Res. 2001, 23, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.M.; Ward, L.J.; Forssell, C.; Siraj, N.; Li, W. Carotid Atheroma From Men Has Significantly Higher Levels of Inflammation and Iron Metabolism Enabled by Macrophages. Stroke 2018, 49, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Li, Q.; Evcimen, N.D.; Rask-Madsen, C.; Maeda, Y.; Maddaloni, E.; Yokomizo, H.; Shinjo, T.; St-Louis, R.; Fu, J.; et al. Exogenous Insulin Infusion Can Decrease Atherosclerosis in Diabetic Rodents by Improving Lipids, Inflammation, and Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Dunning, J.; Kok, W.L.; Benam, K.H.; Benlahrech, A.; Repapi, E.; Martinez, F.O.; Drumright, L.; Powell, T.J.; Bennett, M.; et al. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight. 2017, 2, e91868. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflug. Arch. 2017, 469, 485–499. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Marchetti, G.; Lodola, E.; Licciardello, L.; Colombo, A. Use of N-acetylcysteine in the management of coronary artery diseases. Cardiologia 1999, 44, 633–637. [Google Scholar]
- Talasaz, A.H.; Khalili, H.; Fahimi, F.; Mojtaba, S. Potential role of N-acetylcysteine in cardiovascular disorders. Therapy 2011, 8, 237–245. [Google Scholar] [CrossRef]
- Gu, W.J.; Wu, Z.J.; Wang, P.F.; Aung, L.H.; Yin, R.X. N-Acetylcysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: A meta-analysis of eight randomized controlled trials. BMC Cardiovasc. Disord. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.J.; Nguyen, T.H.; Stansborough, J.; Surikow, S.; Mahadavan, G.; Worthley, M.; Horowitz, J. The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and design of a randomised controlled trial of sequential N-Acetylcysteine and ramipril for the management of Takotsubo Syndrome. Contemp. Clin. Trials 2020, 90, 105894. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; Santos, M.V.; Lima, A.; Maia, A.L.; Wajner, S.M. N-Acetylcysteine Prevents Low T3 Syndrome and Attenuates Cardiac Dysfunction in a Male Rat Model of Myocardial Infarction. Endocrinology 2017, 158, 1502–1510. [Google Scholar] [CrossRef][Green Version]
- Senturk, T.; Cavun, S.; Avci, B.; Yermezler, A.; Serdar, Z.; Savci, V. Effective inhibition of cardiomyocyte apoptosis through the combination of trimetazidine and N-acetylcysteine in a rat model of myocardial ischemia and reperfusion injury. Atherosclerosis 2014, 237, 760–766. [Google Scholar] [CrossRef]
- Meyer, M.; Bell, S.P.; Chen, Z.; Nyotowidjojo, I.; Lachapelle, R.R.; Christian, T.F.; Gibson, P.C.; Keating, F.F.; Dauerman, H.L.; LeWinter, M.M. High dose intracoronary N-acetylcysteine in a porcine model of ST-elevation myocardial infarction. J. Thromb. Thrombolysis 2013, 36, 433–441. [Google Scholar] [CrossRef]
- Lu, H.; Daugherty, A. Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 485–491. [Google Scholar] [CrossRef]
- Calzadilla, P.; Gomez-Serrano, M.; Garcia-Santos, E.; Schiappacasse, A.; Abalde, Y.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-Acetylcysteine affects obesity-related protein expression in 3T3-L1 adipocytes. Redox Rep. 2013, 18, 210–218. [Google Scholar] [CrossRef]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M.C.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine inhibits lipid accumulation in mouse embryonic adipocytes. Redox Biol. 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Scanu, A.M.; Fless, G.M. Lipoprotein(a). Heterogeneity and biological relevance. J. Clin. Investig. 1990, 85, 1709–1715. [Google Scholar] [CrossRef]
- Kroon, A.A.; Demacker, P.N.; Stalenhoef, A.F. N-acetylcysteine and serum concentrations of lipoprotein(a). J. Intern. Med. 1991, 230, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, O.; Fager, G.; Andersson, A.; Lundstam, U.; Masson, P.; Hultberg, B. N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 1996, 119, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev. Clin. Pharmacol. 2015, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Shemin, D.; Yoburn, D.; Fisher, D.H.; Nadeau, M.R.; Selhub, J. Lack of effect of oral N-acetylcysteine on the acute dialysis-related lowering of total plasma homocysteine in hemodialysis patients. Atherosclerosis 1996, 120, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Panini, R.; Pasini, M.C.; Scarpetta, G.; Salvioli, G. N -Acetyl-cysteine reduces homocysteine plasma levels after single intravenous administration by increasing thiols urinary excretion. Pharmacol. Res. 1999, 40, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, W.; Sauer, R.; Bonaterra, G.; Dugi, K.A.; Edler, L.; Kinscherf, R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am. J. Clin. Nutr. 2015, 102, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Miner, S.E.; Cole, D.E.; Evrovski, J.; Forrest, Q.; Hutchison, S.J.; Holmes, K.; Ross, H.J. N-acetylcysteine neither lowers plasma homocysteine concentrations nor improves brachial artery endothelial function in cardiac transplant recipients. Can. J. Cardiol. 2002, 18, 503–507. [Google Scholar]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Toborek, M.; Barger, S.W.; Mattson, M.P.; McClain, C.J.; Hennig, B. Role of glutathione redox cycle in TNF-alpha-mediated endothelial cell dysfunction. Atherosclerosis 1995, 117, 179–188. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, J.M.; Han, N.J.; Kim, Y.J.; Chang, J.W.; Park, S.K. Mycophenolic acid attenuates tumor necrosis factor-alpha-induced endothelin-1 production in human aortic endothelial cells. Atherosclerosis 2010, 211, 48–54. [Google Scholar] [CrossRef]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Roddy, M.A.; Boles, K.; Stamler, J.S. N-acetylcysteine does not influence the activity of endothelium-derived relaxing factor in vivo. Hypertension 1997, 29, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.P.; Prasad, A.; Quyyumi, A.A. N-acetylcysteine improves coronary and peripheral vascular function. J. Am. Coll. Cardiol. 2001, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezerina, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H(2)S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Yu, Z.; Taniguchi, M.; Picotin, R.; Oyama, N.; Stellwagen, D.; Ono, C.; Kikuchi, Y.; Matsui, K.; Nakanishi, M.; et al. N-Acetylcysteine Suppresses Microglial Inflammation and Induces Mortality Dose-Dependently via Tumor Necrosis Factor-alpha Signaling. Int. J. Mol. Sci. 2023, 24, 3798. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, Q.; Liu, Z. Ambient particulate matter exposure and cardiovascular diseases: A focus on progenitor and stem cells. J. Cell. Mol. Med. 2016, 20, 782–793. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef]
- Tzeng, H.P.; Yang, R.S.; Ueng, T.H.; Liu, S.H. Upregulation of cyclooxygenase-2 by motorcycle exhaust particulate-induced reactive oxygen species enhances rat vascular smooth muscle cell proliferation. Chem. Res. Toxicol. 2007, 20, 1170–1176. [Google Scholar] [CrossRef]
- Du, Y.; Navab, M.; Shen, M.; Hill, J.; Pakbin, P.; Sioutas, C.; Hsiai, T.K.; Li, R. Ambient ultrafine particles reduce endothelial nitric oxide production via S-glutathionylation of eNOS. Biochem. Biophys. Res. Commun. 2013, 436, 462–466. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Zhu, Q.; Cui, Y.; Hao, H.; Wang, M.; Cowan, P.J.; Korthuis, R.J.; Li, G.; Sun, Q.; et al. Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. Int. J. Mol. Sci. 2021, 22, 7200. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jia, F.; He, J.; Xie, X.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, D.Z.; Cowan, P.J.; et al. Ambient Fine Particulate Matter Suppresses In Vivo Proliferation of Bone Marrow Stem Cells through Reactive Oxygen Species Formation. PLoS ONE 2015, 10, e0127309. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell. Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Lizarrondo, S.; Gakuba, C.; Herbig, B.A.; Repesse, Y.; Ali, C.; Denis, C.V.; Lenting, P.J.; Touze, E.; Diamond, S.L.; Vivien, D.; et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation 2017, 136, 646–660. [Google Scholar] [CrossRef]
- Chatziathanasiou, G.N.; Nikas, D.N.; Katsouras, C.S.; Kazakos, N.D.; Bouba, V.; Vougiouklakis, T.; Naka, K.K.; Michalis, L.K. Combined intravenous treatment with ascorbic acid and desferrioxamine to reduce myocardial reperfusion injury in an experimental model resembling the clinical setting of primary PCI. Hell. J. Cardiol. 2012, 53, 195–204. [Google Scholar]
- Parra-Flores, P.; Riquelme, J.A.; Valenzuela-Bustamante, P.; Leiva-Navarrete, S.; Vivar, R.; Cayupi-Vivanco, J.; Castro, E.; Espinoza-Perez, C.; Ruz-Cortes, F.; Pedrozo, Z.; et al. The Association of Ascorbic Acid, Deferoxamine and N-Acetylcysteine Improves Cardiac Fibroblast Viability and Cellular Function Associated with Tissue Repair Damaged by Simulated Ischemia/Reperfusion. Antioxidants 2019, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Mushtaq, I.; Maryam, S.; Farhan, A.; Saba, K.; Jan, M.I.; Sultan, A.; Anees, M.; Duygu, B.; Hamera, S.; et al. Interplay of N acetyl cysteine and melatonin in regulating oxidative stress-induced cardiac hypertrophic factors and microRNAs. Arch. Biochem. Biophys. 2019, 661, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, K.; Bergstrand, H. The antiatherogenic effect of DiNAC: Experimental findings supporting immunomodulation as a new treatment for atherosclerosis related diseases. Cardiovasc. Drug Rev. 2003, 21, 119–132. [Google Scholar] [CrossRef]
- Doggrell, S.A. Immunomodulation with DiNAC—A new approach to the treatment of atherosclerosis? Expert Opin. Investig. Drugs 2002, 11, 717–720. [Google Scholar]
- Pettersson, K.S.; Eliasson, U.B.; Abrahamsson, T.; Wagberg, M.; Carrier, M.; Kengatharan, K.M. N,N-diacetyl-L-cystine improves endothelial function in atherosclerotic Watanabe heritable hyperlipidaemic rabbits. Basic Clin. Pharmacol. Toxicol. 2007, 100, 36–42. [Google Scholar] [CrossRef]
- Pettersson, K.; Kjerrulf, M.; Jungersten, L.; Johansson, K.; Langstrom, G.; Kalies, I.; Lenkei, R.; Walldius, G.; Lind, L. The new oral immunomodulating drug DiNAC induces brachial artery vasodilatation at rest and during hyperemia in hypercholesterolemic subjects, likely by a nitric oxide-dependent mechanism. Atherosclerosis 2008, 196, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mazo, D.F.; de Oliveira, M.G.; Pereira, I.V.; Cogliati, B.; Stefano, J.T.; de Souza, G.F.; Rabelo, F.; Lima, F.R.; Ferreira Alves, V.A.; Carrilho, F.J.; et al. S-nitroso-N-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des. Dev. Ther. 2013, 7, 553–563. [Google Scholar] [CrossRef]
- Salamon, S.; Kramar, B.; Marolt, T.P.; Poljsak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Larczynski, W.; Neuwelt, A.; Aleksandrowicz, E.; Lysiak-Szydlowska, W.; Rutkowski, B. The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: A placebo-controlled, randomized, cross-over study. Med. Sci. Monit. 2010, 16, PI13–PI18. [Google Scholar] [PubMed]
- Mahmoudi, G.A.; Astaraki, P.; Mohtashami, A.Z.; Ahadi, M. N-acetylcysteine overdose after acetaminophen poisoning. Int. Med. Case Rep. J. 2015, 8, 65–69. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, 14D–19D. [Google Scholar] [CrossRef]
- De Rosa, S.; Cirillo, P.; Paglia, A.; Sasso, L.; Di Palma, V.; Chiariello, M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: Does the actual knowledge justify a clinical approach? Curr. Vasc. Pharmacol. 2010, 8, 259–275. [Google Scholar] [CrossRef]
- Kadri, A.; Sjahrir, H.; Juwita Sembiring, R.; Ichwan, M. Combination of vitamin A and D supplementation for ischemic stroke: Effects on interleukin-1ss and clinical outcome. Med. Glas. 2020, 17, 425–432. [Google Scholar] [CrossRef]
- Mottaghi, A.; Ebrahimof, S.; Angoorani, P.; Saboor-Yaraghi, A.A. Vitamin A supplementation reduces IL-17 and RORc gene expression in atherosclerotic patients. Scand. J. Immunol. 2014, 80, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, A.; Salehi, E.; Keshvarz, A.; Sezavar, H.; Saboor-Yaraghi, A.A. The influence of vitamin A supplementation on Foxp3 and TGF-beta gene expression in atherosclerotic patients. J. Nutr. Nutr. 2012, 5, 314–326. [Google Scholar] [CrossRef]
- Redlich, C.A.; Chung, J.S.; Cullen, M.R.; Blaner, W.S.; Van Bennekum, A.M.; Berglund, L. Effect of long-term beta-carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the Carotene and Retinol Efficacy Trial (CARET). Atherosclerosis 1999, 143, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Innocenti, M.; Frascerra, S.; Nannipieri, M.; Lippi, A.; Rindi, P.; Ferrannini, E. Effects of hemodialysis and vitamin E supplementation on low-density lipoprotein oxidizability in end-stage renal failure. J. Nephrol. 2013, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Daud, Z.A.; Tubie, B.; Sheyman, M.; Osia, R.; Adams, J.; Tubie, S.; Khosla, P. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc. Health Risk Manag. 2013, 9, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Corina, A.; Rangel-Zuniga, O.A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Quintana-Navarro, G.; Yubero-Serrano, E.M.; Lopez-Moreno, J.; Delgado-Lista, J.; Tinahones, F.; Ordovas, J.M.; et al. Low Intake of Vitamin E Accelerates Cellular Aging in Patients With Established Cardiovascular Disease: The CORDIOPREV Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Atzler, D.; Doring, Y.; Duchene, J.; Steffens, S.; Weber, C. Immunotherapy for cardiovascular disease. Eur. Heart J. 2019, 40, 3937–3946. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Schwarz, L.; Luscher, T.F.; Camici, G.G. Inflammation and cardiovascular diseases: Lessons from seminal clinical trials. Cardiovasc. Res. 2021, 117, 411–422. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Orellana-Urzua, S.; Briones-Valdivieso, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Role of Natural Antioxidants in Countering Reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Badacz, R.; Przewlocki, T.; Legutko, J.; Zmudka, K.; Kablak-Ziembicka, A. microRNAs Associated with Carotid Plaque Development and Vulnerability: The Clinician’s Perspective. Int. J. Mol. Sci. 2022, 23, 5645. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Aminizadeh, N.; Bodhale, N.; Sarkar, A.; Jafarzadeh, S.; Sharifi, I.; Saha, B. Bidirectional cytokine-microRNA control: A novel immunoregulatory framework in leishmaniasis. PLoS Pathog. 2022, 18, e1010696. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. The Interplay among miRNAs, Major Cytokines, and Cancer-Related Inflammation. Mol. Ther. Nucleic Acids 2020, 20, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Albeltagy, R.S.; Mumtaz, F.; Abdel Moneim, A.E.; El-Habit, O.H. N-Acetylcysteine Reduces miR-146a and NF-kappaB p65 Inflammatory Signaling Following Cadmium Hepatotoxicity in Rats. Biol. Trace Elem. Res. 2021, 199, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Mathe, E.; Nguyen, G.H.; Funamizu, N.; He, P.; Moake, M.; Croce, C.M.; Hussain, S.P. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int. J. Cancer 2012, 131, 760–765. [Google Scholar] [CrossRef]
| Patient Information | Intervention | Outcome | Ref. |
|---|---|---|---|
| Pts (6 M and 16 F, YOA: 18–75) with RP secondary to SSc | NAC i.v. starting with a 2 h loading dose of 150 mg/kg, then 15 mg/kg/h for 5 days | Both frequency and severity of RP attacks, active ulcers, and old challenge test mean recovery time decreased | [11] |
| Pts (7 M and 43 F; YOA: 35–67) with RP secondary to SSc | NAC i.v. 15 mg/kg/h for 5 h in every 14 days for about 3 years | Reduction of DU, RP attacks, and RP DU ulcer visual analog scale | [12] |
| Pts (4 M and 22 F, YOA: 25–68) with RP secondary to SSc | NAC i.v. 15 mg/kg/h for 5 h, every 2 weeks for 2 years | Increased global hands perfusion and decreased plasma adrenomedullin concentrations, frequency and severity of RP attacks | [13] |
| Pts (42 M, YOA: 32–58) with RP secondary to SSc | NAC oral 600 mg tid for 4 weeks | Decreased DU but no vasodilator effect on hands’ microcirculation | [14] |
| Pts (20 M and 4 F, YOA: 56–78) with stage 5 CKD during hemolysis | NAC i.v. 5 g in 5% glucose in a final volume of 50 mL during one hemodialysis session | Improved arterial vascular reactivity during reactive hyperemia with decreased reflective index | [8] |
| Pts (total 36, YOA: 56–76, without or with only minor signs of preoperative ischemia of the lower body) undergoing elective infrarenal AAA | NAC i.v. 150 mg/kg b.m. 30 min before infrarenal aortic clamping | Prevented elevation of plasma lipid peroxide, thromboxane, and prostacyclin levels after declamping with increased plasma GSH concentration for over 12 h | [10] |
| Patient Information | Intervention | Outcome | Ref. |
|---|---|---|---|
| Pts (total 150 M and F, YOA: 35–75) with elective CABG surgery using CPB | NAC i.v. 50 mg/kg for 30 min on days 1 and 2 after surgery | Reduced inflammation and incidence of POAF after CABG | [25] |
| Pts (231 M and 28 F, YOA: 53–73) with CABG or combined CABG and valve surgery | Carvedilol plus NAC i.v. 50 mg/kg for 1 h before surgery and at the same dose for 48 h after the procedure | Reduced oxidative stress and inflammation which were associated with POAF | [26] |
| Pts (231 M and 80 F, YOA: 54–72) with CABG or combined CABG and valve surgery | Carvedilol plus NAC i.v. 50 mg/kg/day for 1 h before surgery and at the same dose for 48 h after the procedure | Decreased POAF incidence and duration of hospitalization | [27] |
| Pts (44 M and 31 F, YOA: 56–76) with AF with CABG or valve surgery, or both | Amiodarone plus NAC i.v. 100 mg/kg 30 min and 25 mg/kg for 48 h | NAC plus amiodarone might facilitate converting POAF to SR, decrease the time to conversion, and lower the requirement of EC | [28] |
| Pts (91 M and 24 F, YOA: 25–78) with CABG or valve surgery, or both | NAC i.v. 50 mg/kg/day for 1 h before surgery and at the same dose for 48 h after the procedure | Decreased the incidence of postoperative AF | [29] |
| Pts (180 M and 60 F, YOA: 40–70) with CABG, with or without valve surgery | NAC orally 1200 mg bid starting 48 h before and up to 72 h after surgery | Had no significant effect on the incidence of POAF, in-hospital stay, and postoperative morbidity or mortality | [30] |
| Patient Information | Intervention | Outcome | Ref. |
|---|---|---|---|
| Pts (47 M and 28 F, YOA: 50–78) with STEMI | NAC i.v. 29 g with NTG i.v. 7.2 mg over 2 days | Reduced infarct size in patients with STEMI undergoing PCI | [34] |
| Pts (total 28 M and F, YOA: ≤ 75) with AMI | NAC i.v. 15 g for over 24 h combined with NTG and streptokinase | Appeared to be safe for the treatment of evolving AMI and was associated with significantly less oxidative stress, a trend toward more rapid reperfusion, and better preservation of LV function | [35] |
| Pts (3 M and 19 F, YOA: 42–66) with AMI | NAC i.v. 15 g NAC for over 24 h combined with streptokinase | Diminished oxidative stress and improved LV function | [36] |
| Pts (19 M and 11 F, YOA: 55–61) with LVEF > 40% undergoing CABG | NAC i.v. 50 mg/kg b.w. with cold-blood cardioplegia | Minimized myocardial injury in the early hours after and during cardiac surgery | [37] |
| Pts (35 M, YOA: 59–63) with normal myocardial function undergone CABG | NAC i.v. 0.04 mol/L with Plegisol | Increased tissue capacity against oxidative stress and decreased inflammatory response | [38] |
| Pts (32 M and 14 F, YOA: 40–73) with severe unstable angina pectoris unresponsive to conventional treatment | NAC i.v. 5 g over 15 min after NTG and repeated every 6 h for 24 h. | Lowered incidence of AMI but increased symptomatic hypotension | [39] |
| Animal Model | Intervention | Outcomes | Ref. |
|---|---|---|---|
| Aging mice (M, LDLR KO) | With ND or HFD for 24 mon, with NAC (1 mg/mL in drinking water) treatment for 3 or 6 mon. | Early and sufficient NAC treatment reduces inflammation and slows atherosclerosis progression in aging LDLR−/− mice without HFD, maintaining M2 level with increased CD146. | [59] |
| Mice (M, ApoE KO) | With HFD and NAC (i.p. 20 mg/kg/day, 3 times a week for 8 weeks) | NAC may suppress atherosclerosis via reducing superoxide production. | [57] |
| Diabetic ApoE KO mice (M) | treptozotocin (i.p. for 5 days) and NAC (2 mmol/L in drinking water for 12 weeks) | NAC attenuates atherosclerosis in diabetic ApoE KO mice; correcting glutathione-dependent methylglyoxal elimination; reducing oxidative stress and restoring p-Akt/p-eNOS pathways. | [58] |
| Mice (F, ApoE KO) with chronic renal failure | NAC (200 mg/kg daily by mouth) or placebo for 8 weeks. | NAC can slow atheroma growth in uremia-related atherosclerosis in mice, likely by lowering oxidative stress. | [56] |
| Mice (M, WT, and LDLR KO) | WT: human native LDL (50 μg) or ox-LDL i.v. for 3 days with NAC pretreatment for 3 days (1 mg/mL in drinking water); LDLR KO: ND or HFD for 4 months with NAC (same dose) for 2 months. | NAC attenuates native LDL oxidation to ox-LDL and ROS generation from ox-LDL; NAC decreases atherosclerotic plaque formation in hyperlipidemic LDLR KO mice. | [60] |
| Mice (M, LDLR KO) | NAC (1 mg/mL in drinking water) with ND or HFD, plus PM exposure for either 1 week or 6 months | NAC prevents PM-induced atherosclerosis in association with reductions of ROS formation, ox-LDL, and inflammatory cytokines. | [46] |
| Rabbits (M, LDLR KO) | At 10 weeks of age, DiNAC (0.25 and 25 μmol/L in drinking water for 12 weeks. | DiNAC decreases atherosclerosis, possibly via immune regulations and antioxidant properties. | [61] |
| Mice (M, LDLR KO) | On ND or HFD, SNAC (0.51 μmol /kg i.p. for 15 days) | SNAC attenuates plaque development and improves endothelial cell function | [62] |
| New Zealand white rabbits (M) | Group1: HFD + colchicine (2 mg/kg/day) + fenofibrate (250 mg/kg/day; group 2: HFD + colchicine (2 mg/kg/day) plus NAC (15 mg/kg /day) | Colchicine reduces atherosclerosis, especially when combined with NAC. Colchicine blocks NLRP3 inflammasome, while NAC attenuates IL-6 signaling, reducing inflammation. | [63] |
| Patient Information | Intervention | Outcome | Ref. |
|---|---|---|---|
| Pts (76 M and 58 F, YOA: 46–78) with end-stage renal failure | NAC orally 600 mg bid for 2 years | Reduced composite cardiovascular end points | [16] |
| Pts (29 M and 11 F, YOA: 58–70) with stable angina pectoris who underwent CABG | NAC i.v. 50 mg/kg/day for 3 days | Decreased pump-induced oxidoinflammatory response during CPB | [21] |
| Pts (31 M and 9 F, YOA: 57–75) with elective or urgent CABG | NAC i.v. 100 mg/kg into cardiopulmonary bypass prime followed by infusion at 20 mg/kg/h | Attenuated myocardial oxidative stress in the hearts of patients subjected to cardiopulmonary bypass and cardioplegic arrest | [22] |
| Pts (12 M and 2 F, YOA: 20–67) with severe chronic CHF | NAC i.v. 100 mg/kg body w.t. for over 30 min with 40–120 mg ISDN orally | Activated and potentiated the action of organic nitrates, improved CHF | [31] |
| Pts (14 M and 5 F, YOA: 12–63) with disease-free soft tissue sarcoma and doxorubicin-induced cardiomyopathy | NAC orally 5.5 g/m2 daily for 30 days | Had no effect in reversing longstanding doxorubicin-induced cardiomyopathy | [32] |
| Pts (YOA: >18) with a suspected, or confirmed diagnosis of Takotsubo Syndrome | NAC i.v. 10 g over 24 h | Will evaluate a therapeutic option in acute attacks of Takotsubo Syndrome | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhu, Q.; Hao, H.; Flaker, G.C.; Liu, Z. N-Acetylcysteine and Atherosclerosis: Promises and Challenges. Antioxidants 2023, 12, 2073. https://doi.org/10.3390/antiox12122073
Cui Y, Zhu Q, Hao H, Flaker GC, Liu Z. N-Acetylcysteine and Atherosclerosis: Promises and Challenges. Antioxidants. 2023; 12(12):2073. https://doi.org/10.3390/antiox12122073
Chicago/Turabian StyleCui, Yuqi, Qiang Zhu, Hong Hao, Gregory C. Flaker, and Zhenguo Liu. 2023. "N-Acetylcysteine and Atherosclerosis: Promises and Challenges" Antioxidants 12, no. 12: 2073. https://doi.org/10.3390/antiox12122073
APA StyleCui, Y., Zhu, Q., Hao, H., Flaker, G. C., & Liu, Z. (2023). N-Acetylcysteine and Atherosclerosis: Promises and Challenges. Antioxidants, 12(12), 2073. https://doi.org/10.3390/antiox12122073







