The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota
Abstract
:1. Introduction
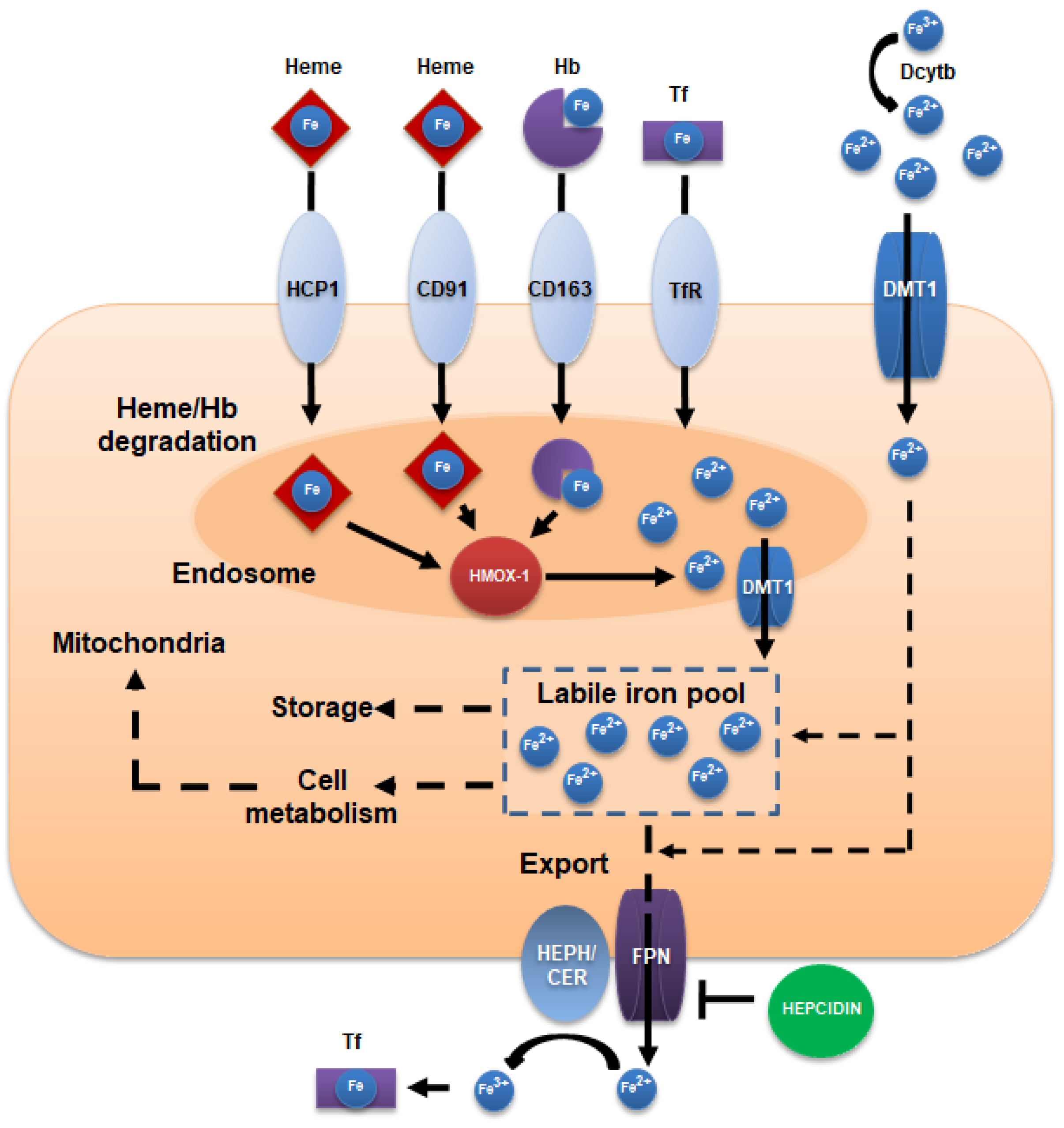
2. Iron Intake, Absorption, and Homeostasis
3. Anti/Pro-Oxidant Activities of Polyphenols
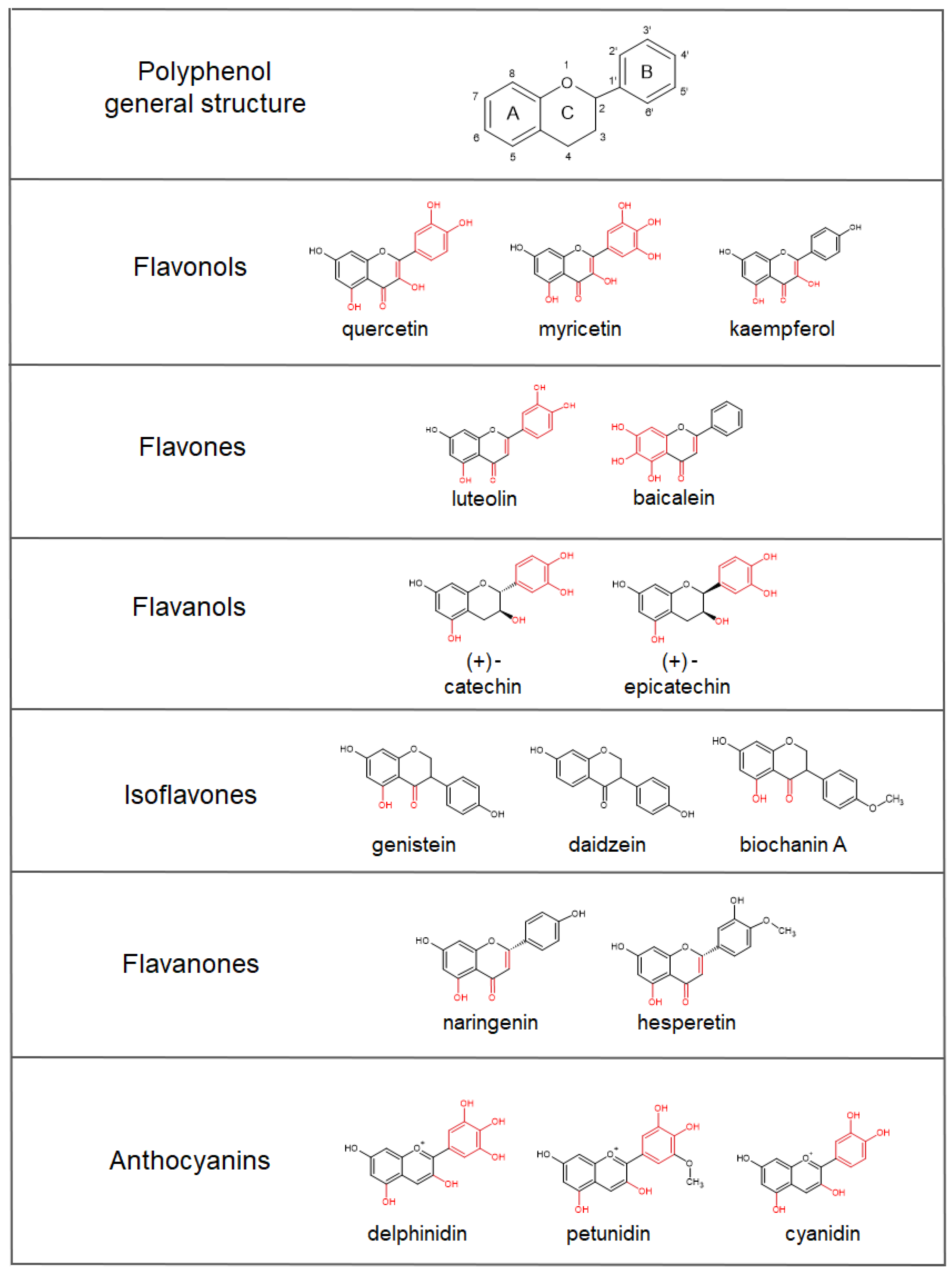
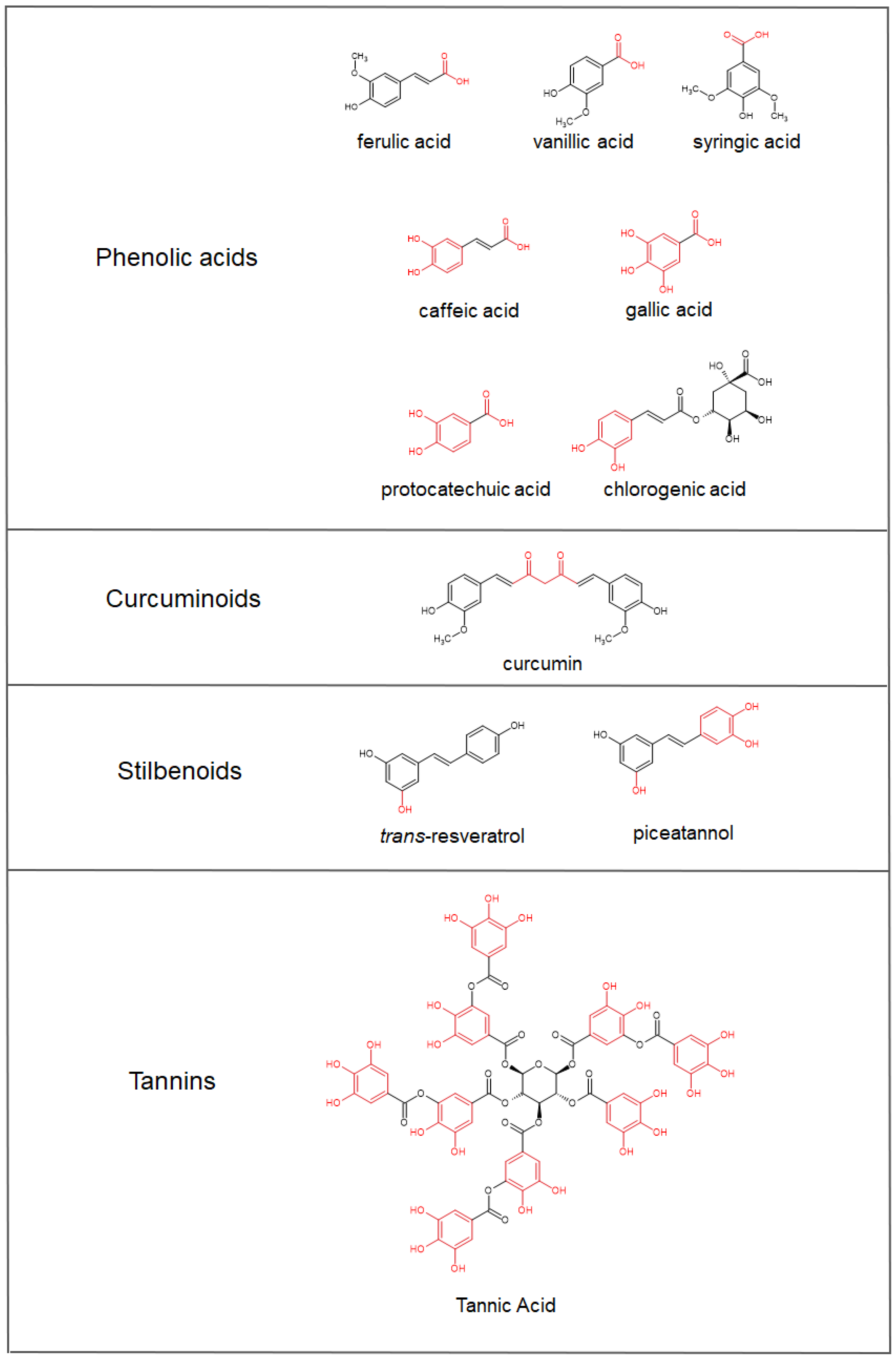
4. Iron-Chelating Abilities of Polyphenols
5. Polyphenols’ Bioavailability
| Polyphenol Compound—Food Source | Dosage | Cmax 1 of the Main Compounds in Plasma (tmax 2) | Main Compounds Excreted in Urine (Time) | Reference |
|---|---|---|---|---|
| Quercetin-4’-O-glucoside | 100 mg | 2.12 µg·mL−1 of quercetin-4’-O-glucoside (0.70 h) | NA 3 | [94] |
| Quercetin-3-O-rutinoside | 200 mg | 0.32 µg·mL−1 of quercetin-3-O-rutinoside (6.98 h) | NA | [94] |
| Fried onions (containing 275 µmol flavonols, principally quercetin-4’-glucoside and quercetin-3,4’-diglucoside) | 270 g | 665 nM of quercetin-3’-sulfate (0.75 h) 351 nM of quercetin-3-glucuronide (0.60 h) 63 nM of quercetin diglucuronide (0.80 h) | 274 nM quercetin diglucuronide (8–24 h) | [95] |
| Fresh blackcurrant (containing 897 mg total anthocyanins) | 100 g | Anthocyanins not detected | 339 µg total anthocyanins (48 h) 17.3 mg hippuric acid (0–24 h) | [84] |
| Elderberry concentrate (containing 1.9 g anthocyanins and equivalent to 235 mL fresh juice) | 11 g | NA | 15.8 µg cyanidin-3-glucoside (1 h) 29.8 µg cyanidin-3-sambubioside (2 h) | [96] |
| Homogenized raspberries (containing 292 µmol anthocyanins, 6.3 µmol ellagic acids, 251 µmol ellagitannins, and 2.5 µmol phenolic acids) | 300 g | 180 nmol·L−1 of 3’,4’-dihydroxyphenylaceticacid (6 h) 78 nmol·L−1 of 4’-Hydroxyhippuricacid (1 h) 47 nmol·L−1 of ferulic acid-4′-sulfate (1.5 h) 18 nmol·L−1 of ferulic acid-4′-O-glucuronide (1.5 h) 14 nmol of isoferulic acid-3’-O-glucuronide (1.5 h) | 19.9 nmol cyanidin-3-O-glucoside (0–48 h) 6.4 nmol 4-Hydroxybenzoic acid (0–48 h) 6.5 nmol ferulic acid-4-sulfate (0–48 h) 16.1 nmol 4-hydroxyhippuricacid (0–48 h) 239 nmol hippuric acid (0–48 h) | [97] |
| Evelor 500 mg tablets (containing trans-resveratrol) | 500 g | 71.18 mg·mL−1 of trans-resveratrol (1.339 h) 1516.014 mg·mL−1 of sulfated resveratrol 4083.900 mg·mL−1 of glucuronated resveratrol | NA | [98] |
| Coffee beverage, containing various doses of chlorogenic acid: 412 µmol (A) 635 µmol (B) 795 µmol (C) | 200 mL | 808 µmol of total chlorogenic acid derivatives (0.5–6 h) (A) 1242 µmol of total chlorogenic acid derivatives (0.5–6 h) (B) 1164 µmol of total chlorogenic acid derivatives (0.5–6 h) (C) | 100.7 µmol of total chlorogenic acid derivatives (0–24 h) (A) 160.0 µmol of total chlorogenic acid derivatives (0–24 h) (B) 125.2 µmol of total chlorogenic acid derivatives (0–24 h) (C) | [99] |
| Curcuminoids as native powder, micronized powder, or liquid micelles (containing 410 mg curcumin, 80 mg demethoxycurcumin, and 10 mg bis-demethoxycurcumin) | 500 mg | 7.1 nmol curcumin (7.5 h) (native powder) 41.6 nmol curcumin (8.8 h) (micronized powder) 3228 nmol curcumin (1.1 h) (liquid micelles) | 5.1 nmol 4 curcumin (0–24 h) (native powder) 70.6 nmol 4 curcumin (0–24 h) (micronized powder) 753 nmol 4 curcumin (0–24 h) (liquid micelles) | [100] |
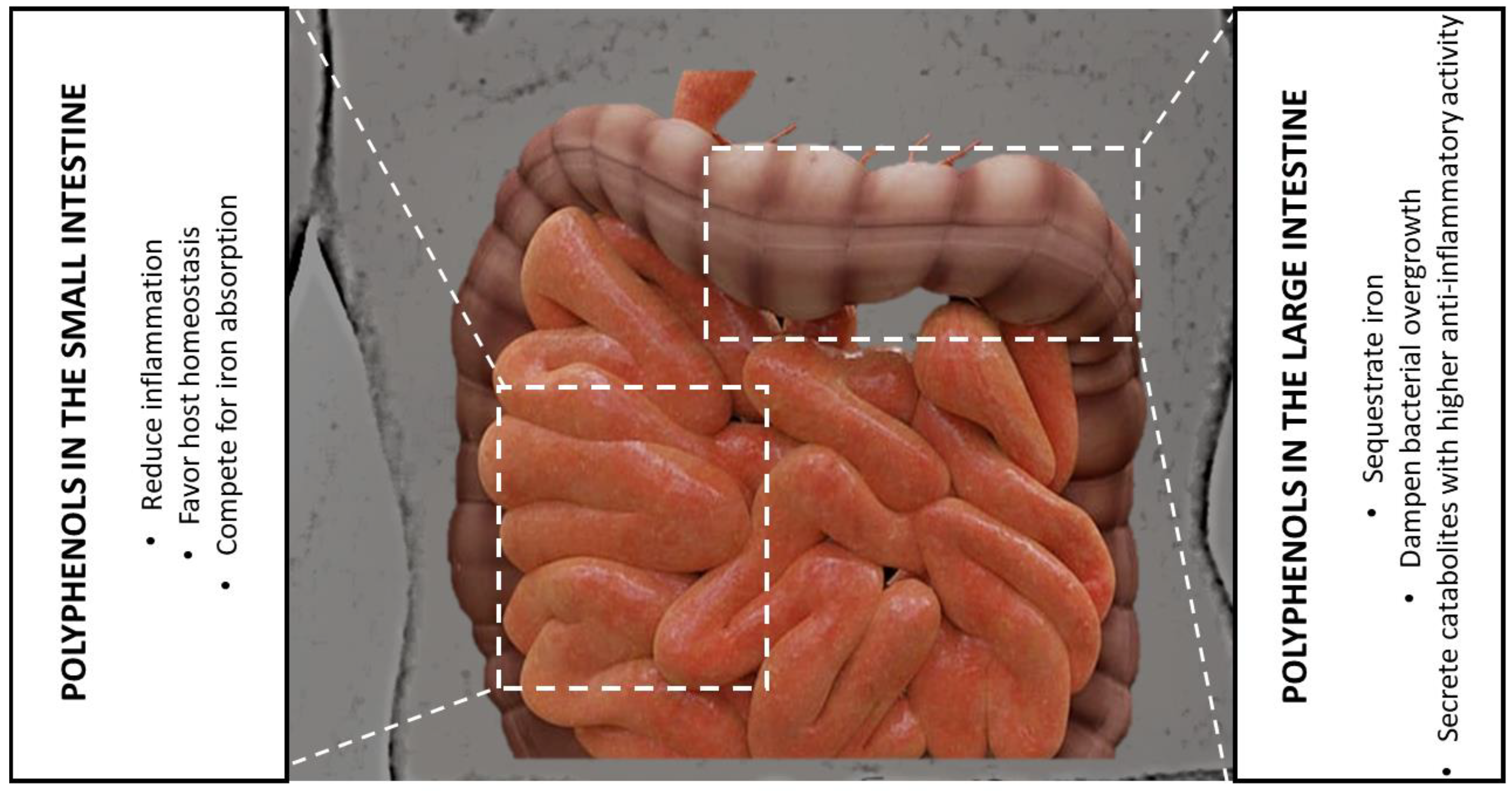
6. Polyphenol-Mediated Iron Sequestration Affects the Inflammatory Response
6.1. The Host Level
| Compound | Model | Effect | Reference |
|---|---|---|---|
| Anthocyanins | C57BL/6 mice fed with a high-fat diet supplemented with purple corn anthocyanins in drinking water | Downregulation of inflammatory mediators, increased expression of iron genes metabolism, and upregulation M2 markers in adipose tissue macrophages | [132] |
| Quercetin | In vitro RAW 264.7 cells; in vivo lipopolysaccharide (LPS)/ovalbumin (OVA)-induced neutrophilic asthma mouse model | Reduction in ferroptosis and inhibition of M1 macrophage polarization. The effect was ferrostatin-like | [134] |
| Quercetin | In vitro bone marrow dendritic cells (BMDCs) | Changes in M2-like phenotype, increased expression in ferroportin, and reduction in inflammatory abilities | [124] |
| Quercetin and baicalin | Kunming mice fed with a diet supplemented with iron-dextran and baicalin 1% or quercetin 1% | Inhibition of iron-overload induced lipid peroxidation and protein oxidation in the liver | [139] |
| Resveratrol | Kunming mice with iron-overload induced liver fibrosis | Regulation of iron homeostasis by reducing the expression of hepcidin, ferritin, TfR, and DMT1, and raising the expression of FPN-1 | [142] |
| Resveratrol | In vitro human β-thalassemic-erythroid cells; in vivo in β-thalassemic mice (Hbbth3/+) | Reduction in ineffective erythropoiesis, increase in hemoglobin levels, and reduction in oxidative stress in circulating red cells | [140] |
| Myricetin | In vitro HepG2 cells; HEK293 cells; C57BL/6 mice | Inhibition of hepcidin expression in vitro; and reduced hepatic hepcidin expression, decreased splenic iron levels, and increased serum iron levels in vivo | [143] |
| Curcumin | C3H/HeNCrl mice fed with different amounts of iron and curcumin | Decreased iron levels in blood, liver, bone marrow, and spleen, induced TfR1, and repressed ferritin and hepcidin | [65] |
| Quercetin, quercetagetin, and patuletin | Sprague Dawley rats fed with low-iron diet for 20 days, followed by a 30-day treatment with 50 mg kg−1 ferrous sulfate supplement combined with quercetin, quercetagetin, and patuletin | Improved hematological parameters and increased splenic tissue availability of iron and ferroportin expression | [141] |
| Curcumin | In vitro T51B cells | Repression of iron-dependent generation of ROS and inhibition of intracellular iron toxicity | [62] |
| Curcumin | Sprague Dawley rats receiving iron and curcumin supplementation in drinking water | Reduction in iron overload-induced lipid peroxidation and reduction in oxidative stress in the liver and spleen | [136] |
| Curcumin | In vitro LLC-PK and NRK52E cells | Stimulation of heme-oxygenase1 expression | [144] |
6.2. The Gut Microbiota Level
7. Polyphenols as Prebiotics—Chelating Iron to Select Beneficial Bacteria
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laddomada, B.; Colella, G.; Tufariello, M.; Durante, M.; Angiuli, M.; Salvetti, G.; Mita, G. Application of a simplified calorimetric assay for the evaluation of extra virgin olive oil quality. Food Res. Int. 2013, 54, 2062–2068. [Google Scholar] [CrossRef]
- Pasqualone, A.; Summo, C.; Laddomada, B.; Mudura, E.; Coldea, T.E. Effect of processing variables on the physico-chemical characteristics and aroma of borş, a traditional beverage derived from wheat bran. Food Chem. 2018, 265, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Di Grigoli, A.; Todaro, M.; Alabiso, M.; Vitale, F.; Di Trana, A.; Giorgio, D.; Settanni, L.; Gaglio, R.; Laddomada, B.; et al. Improvement of oxidative status, milk and cheese production, and food sustainability indexes by addition of durum wheat bran to dairy cows’ diet. Animals 2019, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Chieppa, M.; Santino, A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Chieppa, M.; Santino, A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The effects of dietary polyphenols on circulating cardiovascular disease biomarkers and iron status: A systematic review. Nutr. Metab. Insight 2019, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2021, 61, 1804–1826. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, F.R.; Vadrucci, E.; Cavalcanti, E.; De Santis, S.; Kunde, D.; Vacca, M.; Myers, J.; Allen, F.; Bianco, G.; Huang, A.Y.; et al. Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur. J. Immunol. 2015, 45, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Daheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradha, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.S. Iron deficiency anemia due to excessive green tea drinking. Clin. Case Rep. 2016, 4, 1053–1056. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A review on iron chelators in treatment of iron overload syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Feo, V.; Battistelli, A.; Gomes Da Cruz, A.; Coppola, R. Polyphenols, the new frontiers of prebiotics. In Advances in Food and Nutrition Research, 1st ed.; Gomes da Cruz, A., Prudencio Schwinden, E., Almeida Esmerino, E., da Silva, M.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 35–89. [Google Scholar] [CrossRef]
- Naranjo-Arcos, M.A.; Bauer, P. Iron Nutrition, Oxidative Stress, and Pathogen Defense. In Nutritional Deficiency; Erkekoglu, P., Kocer-Gumusel, B., Eds.; InTechOpen: London, UK, 2016; pp. 63–98. [Google Scholar] [CrossRef] [Green Version]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing Acts: Molecular Control Of Mammalian Iron Metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Kim, E.-Y.; Lindsay, A.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verna, G.; Sila, A.; Liso, M.; Mastronardi, M.; Chieppa, M.; Cena, H.; Campiglia, P. Iron-enriched nutritional supplements for the 2030 pharmacy shelves. Nutrients 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; Giannelli, G. Immune cells and microbiota response to iron starvation. Front. Med. 2018, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Beck, K.L.; Conlon, C.A.; Kruger, R.; Coad, J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: A review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef] [Green Version]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The central role of iron in human nutrition: From folk to contemporary medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging roles of the iron chelators in inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J. Mechanisms of iron loading and toxicity. Am. J. Hematol. 2007, 82 (Suppl. 12), 1128–1131. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2017, 22, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Makácová, K.; Mladěnka, P.; Filipský, T.; Říha, M.; Jahodár, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flvaonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Kung, F.S.; Bohmont, C.W.; Pardini, R.S. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem. Pharmacol. 1986, 35, 2345–2357. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhemová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Puppo, A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry 1992, 31, 85–88. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, S.; Ito, K.; Yamamoto, K.; Kawanishi, S. Caffeic acid causes metal-dependent damage to cellular and isolated DNA through H2O2 formation. Carcinogenesis 1992, 13, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, L.T.; Moreira, D.C.; Andrade Jr, R.; Ginani, J.; Alonso, A.; Hermes-Lima, M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.C. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients 2019, 11, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marì, M.; de Gregorio, E.; de Dios, E.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909. [Google Scholar] [CrossRef]
- Zhitkovich, A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Romano, G.; Del Coco, L.; Milano, F.; Durante, M.; Palombieri, S.; Sestili, F.; Visioni, A.; Abderrazek, J.; Fanizzi, F.P.; Laddomada, B. Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Whole-Meal Flours. Foods 2022, 11, 4070. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reaction has possible biological implications. J. Biol. Chem. 1999, 274, 962–971. [Google Scholar] [CrossRef] [Green Version]
- Andjelković, M.; Camp, J.V.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the health benefits of supplemental propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C. Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 2021, 9, 685364. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.; Dias, C.; Saavedra, M.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Khalili, M.; Ebrahimzadeh, M.; Kosaryan, M. In vivo iron-chelating activity and phenolic profiles of the Angel’s wings mushroom, Pleurotus porrigens (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 847–856. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Galano, A.; Marquez, M.F.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Messner, D.J.; Sivam, G.; Kowdley, K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009, 29, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Wilkinson IV, J.; Pietsch, E.C.; Buss, J.L.; Wan, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wilkinson IV, J.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino Jr, R.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlíčková, J.; Macáková, K.; Říha, M.; Teixeira Pinheiro, L.M.; Filipský, T.; Horňasová, V.; Hrdina, R.; Mladěnka, P. Isoflavones reduce copper with minimal impact on iron in vitro. Oxid. Med. Cell Longev. 2015, 2015, 437381. [Google Scholar] [CrossRef] [Green Version]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hedge, M.L.; Rao, K.S. Polyphenols as potential metal chelation compounds against Alzeimer’s disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. BioMetals 2000, 13, 77–83. [Google Scholar] [CrossRef]
- Perron, N.R.; Hodges, J.N.; Jenkins, M.; Brumaghim, J.L. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg. Chem. 2008, 47, 6153–6161. [Google Scholar] [CrossRef]
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A review on coordination properties of Al(III) and Fe(III) toward natural antioxidant molecules: Experimental and theoretical insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Van Besien, E.; Milhazes, N.; Borges, F.; Marques, M.P.M. Conformational analysis of a trihydroxylated derivative of cinnamic acid—A combined Raman spectroscopy and ab initio study. J. Mol. Struct. 2004, 693, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Walton, M.C. the bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Chieppa, M.; Santino, A. 2022. Microbiota as a metabolic organ processing dietary polyphenols. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 20–26. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 95215. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; InTechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčkova, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Saito, A.; Sugisawa, A.; Umegaki, K.; Sunagawa, H. Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci. Biotechnol. Biochem. 2004, 68, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [Green Version]
- Catalkava, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Capanoglu, E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Holland, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennet, R.; Kroon, P. Processing blackcurrants dramatically reduces the content and does not enhance urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef]
- Kasikci, M.B.; Bagdatlioglu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999, 277, G120–G126. [Google Scholar] [CrossRef]
- Matsukawa, N.; Matsumoto, M.; Hara, H. High biliary excretion levels of quercetin metabolites after administration of a quercetin glycoside in conscious bile duct cannulated rats. Biosci. Biotechnol. Biochem. 2009, 73, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Oh, J.-H.; Lee, Y.-J. Biliary excretion of curcumin is mediated by multidrug resistance-associated protein 2. Biol. Pharm. Bull. 2012, 35, 777–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent development in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef]
- Shen, C.; Chen, R.; Qian, Z.; Meng, X.; Hu, T.; Li, Y.; Chen, Z.; Huang, C.; Hu, C.; Li, J. Intestinal absorption mechanisms of MTBH, a novel hesperetin derivative, in Caco-2 cells, and potential involvement of monocarboxylate transporter 1 and multidrug resistance protein 2. Eur. J. Pharm. Sci. 2015, 78, 214–224. [Google Scholar] [CrossRef]
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Merillon, J.-M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005, 53, 798–803. [Google Scholar] [CrossRef]
- Graefe, E.U.; Witting, J.; Pharm, D.; Mueller, S.; Riethling, A.-K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mülleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Larrosa, M.; Yanez-Gascon, M.J.; Selma, V.M.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Vadrucci, E.; Delvecchio, F.R.; Addabbo, F.; Bettini, S.; Liou, R.; Monsurrò, V.; Huang, A.Y.C.; Pizzaro, T.T.; Santino, A.; et al. Administration of reconstituted polyphenol oil bodies efficiently suppresses dendritic cell inflammatory pathways and acute intestinal inflammation. PLoS ONE 2013, 9, e88898. [Google Scholar] [CrossRef] [Green Version]
- Liso, M.; Sila, A.; Verna, G.; Scarano, A.; Donghia, R.; Castellana, F.; Cavalcanti, E.; Pesole, P.L.; Sommella, E.M.; Lippolis, A.; et al. Nutritional regimes enriched with antioxidants as an efficient adjuvant for IBD patients under infliximab administration, a pilot study. Antioxidants 2022, 11, 1380. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-kappaB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-kB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- D’Introno, A.; Paradiso, A.; Scoditti, E.; D’Amico, L.; De Paolis, A.; Carluccio, M.A.; Nicoletti, N.; De Gara, L.; Santino, A.; Giovinazzo, G. Antioxidant and anti-inflammatory properties of tomato fruit synthesizing different amounts of stilbenes. Plant Biotech. J. 2009, 7, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixão, J.; Nunes, C.; Dinis, T.C.P.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5- aminosalicylic acid. PLoS ONE 2013, 8, e73001-10. [Google Scholar] [CrossRef] [Green Version]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pasqualone, A.; Laddomada, B.; Carluccio, M.A. Phenolic extracts from whole wheat biofortified bread dampen overwhelming 1 inflammatory response in human endothelial cells and monocytes: Major role of VCAM-1 and CXCL-10. Eur. J. Nutr. 2020, 59, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pellegrino, M.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Red grape skin polyphenols blunt matrix metalloproteinase-2 and -9 activity and expression in cell models of vascular inflammation: Protective role in degenerative and inflammatory diseases. Molecules 2016, 21, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Different role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Maffia, M.; Chieppa, M.; Laddomada, B.; Carluccio, M.A. Non-Celiac Gluten Sensitivity and Protective Role of Dietary Polyphenols. Nutrients 2022, 14, 2679. [Google Scholar] [CrossRef]
- Nairz, M.; Therl, I.; Swirski, F.K.; Weiss, G. “Pumping iron”—How macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers. Arch. 2017, 469, 397–418. [Google Scholar] [CrossRef] [Green Version]
- Recalcati, S.; Cairo, G. Macrophages and iron: A special relationship. Biomedicines 2021, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012, 4, 446–453. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Gammella, E.; Correnti, M.; Cairo, G.; Recalcati, S. Iron Availability in Tissue Microenvironment: The Key Role of Ferroportin. Int. J. Mol. Sci. 2021, 22, 2986. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Sonnweber, T.; Weiss, G. The struggle for iron—A metal at the host-pathogen interface. Cell Microbiol. 2010, 12, 1691–1702. [Google Scholar] [CrossRef]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13, e0196921. [Google Scholar] [CrossRef] [Green Version]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Agoro, R.; Mura, C. Inflammation-induced up-regulation of hepcidin and down-regulation of ferroportin transcription are dependent on macrophage polarization. Blood Cells Mol. Dis. 2016, 61, 16–25. [Google Scholar] [CrossRef]
- Galleggiante, V.; De Santis, S.; Cavalcanti, E.; Scarano, A.; De Benedictis, M.; Serino, G.; Caruso, M.L.; Mastronardi, M.; Pinto, A.; Campiglia, P.; et al. Dendritic cells modulate iron homeostasis and inflammatory abilities following quercetin exposure. Curr. Pharm. Des. 2017, 23, 2139–2146. [Google Scholar] [CrossRef]
- Verna, G.; Liso, M.; De Santis, S.; Dicarlo, M.; Cavalcanti, E.; Crovace, A.; Sila, A.; Campiglia, P.; Santino, A.; Lippolis, A.; et al. Iron Overload Mimicking Conditions Skews Bone Marrow Dendritic Cells Differentiation into MHCIIlowCD11c+CD11b+F4/80+ Cells. Int. J. Mol. Sci. 2020, 21, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, S.; Kunde, D.; Serino, G.; Galleggiante, V.; Caruso, M.L.; Mastronardi, M.; Cavalcanti, E.; Ranson, N.; Pinto, A.; Campiglia, P.; et al. Secretory leukoprotease inhibitor is required for efficient quercetin-mediated suppression of TNFα secretion. Oncotarget 2016, 7, 75800–75809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, S.; Galleggiante, V.; Scandiffio, L.; Liso, M.; Sommella, E.; Sobolewski, A.; Spilotro, V.; Pinto, A.; Campiglia, P.; Serino, G.; et al. Secretory Leukoprotease Inhibitor (Slpi) Expression Is Required for Educating Murine Dendritic Cells Inflammatory Response Following Quercetin Exposure. Nutrients 2017, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Du, P.; Song, J.; Qiu, H.; Liu, H.; Zhang, L.; Zhou, J.; Jiang, S.; Liu, J.; Zheng, Y.; Wang, M. Polyphenols Extracted from Shanxi-Aged Vinegar Inhibit Inflammation in LPS-Induced RAW264.7 Macrophages and ICR Mice via the Suppression of MAPK/NF-κB Pathway Activation. Molecules 2021, 26, 2745. [Google Scholar] [CrossRef]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef]
- De Santis, S.; Crupi, P.; Piacente, L.; Mestice, A.; Colabufo, N.A.; Amodio, L.; Pontrelli, P.; Gesualdo, L.; Moschetta, A.; Clodoveo, M.L.; et al. Extra virgin olive oil extract rich in secoiridoids induces an anti-inflammatory profile in peripheral blood mononuclear cells from obese children. Front. Nutr. 2022, 9, 1017090. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.-P.; Tonelli, C.; Petroni, K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019, 17, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, M.; Sadeghi, A.; Hosseini, H.; Teimouri, M.; Khorzoughi, R.B.; Pasalar, P.; Meshkani, R. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci. Rep. 2020, 10, 3791. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 2022, 938, 175407. [Google Scholar] [CrossRef] [PubMed]
- Peréz, S.; Rius-Peréz, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin attenuates iron accumulation and oxidative stress on the liver and spleen of chronic iron-overload rats. PLoS ONE 2015, 10, e134156. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.; Tako, E.; Kochian, L.; Glahn, R. Identification of Black Bean (Phaseolus vulgaris L.) Polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhao, Y.; Gao, Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur. J. Pharmacol. 2006, 535, 263–269. [Google Scholar] [CrossRef]
- Franco, S.; De Falco, L.; Ghaffari, S.; Brugnara, C.; Sinclair, D.A.; Mattè, A.; Iolascon, A.; Mohandas, N.; Bertoldi, M.; An, X.; et al. Resveratrol accelerates erythroid maturation by activation of Foxo3 and ameliorates anemia beta-thalassemic mice. Haematologica 2014, 99, 267–275. [Google Scholar] [CrossRef]
- Mazhar, M.; Faizi, S.; Gul, A.; Kabir, N.; Simjee, S.U. Effects of naturally occurring flavonoids on ferroportin expression in the spleen in iron deficiency anemia in vivo. RSC Adv. 2017, 7, 23238–23245. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jiang, C.; Yang, Y.; Li, J.; Wang, Y.; Wang, C.; Gao, Y. Resveratrol ameliorates iron overload induced liver fibrosis in mice by regulating iron homeostasis. PeerJ 2022, 10, e13592. [Google Scholar] [CrossRef]
- Mu, M.; An, P.; Wu, Q.; Shen, X.; Shao, D.; Wang, H.; Zhang, Y.; Zhang, S.; Yao, H.; Min, J.; et al. The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 2016, 30, 53–61. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem ovygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.; Zimmermann, M. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Ponce, M.C.; Polman, K.; Roos, N.; Wieringa, F.T.; Berger, J.; Doak, C.M. What Approaches are Most Effective at Addressing Micronutrient Deficiency in Children 0-5 Years? A Review of Systematic Reviews. Matern. Child. Health J. 2019, 23 (Suppl. 1), 4–17. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Galla, S.; Golonka, R.M.; Patterson, A.D.; Chassaing, B.; Joe, B.; Vijay-Kumar, M. Lipocalin 2 deficiency-induced gut microbiota dysbiosis evokes metabolic syndrome in aged mice. Physiol. Genom. 2020, 52, 314–321. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends Endocr. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Lu, F.; Inoue, K.; Kato, J.; Minamishima, S.; Morisaki, H. Functions and regulation of lipocalin-2 in gut-origin sepsis: A narrative review. Crit. Care 2019, 23, 269. [Google Scholar] [CrossRef] [Green Version]
- Pi, H.; Helmann, J.D. Ferrous iron efflux systems in bacteria. Metallomics 2017, 9, 840–851. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Gibson, R.S.J.F. The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr. Bull. 2007, 28, S77–S100. [Google Scholar] [CrossRef] [Green Version]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes 2021, 13, e1874855. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174. [Google Scholar] [CrossRef]
- De Santis, S.; Scarano, A.; Liso, M.; Calabrese, F.M.; Verna, G.; Cavalcanti, E.; Sila, A.; Lippolis, A.; De Angelis, M.; Santino, A.; et al. Polyphenols enriched diet administration during pregnancy and lactation prevents dysbiosis in ulcerative colitis predisposed littermates. Front. Cell Infect. Microbiol. 2021, 11, 622327. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Lenucci, M.S.; Fontana, S.; Forgia, F.M.; Minervini, F.; Scarano, A.; Santino, A.; Dalfino, G.; Gesualdo, L.; et al. In vitro selection of probiotics, prebiotics, and antioxidants to develop an innovative symbiotic (NatuREN G) and testing its effect in reducing uremic toxins in fecal batches from CKD patients. Microorganisms 2021, 9, 1316. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Santino, A.; Scarano, A.; De Santis, S.; De Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and consolidated perspectives. Curr. Pharm. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef]
- Kortman, G.; Mulder, M.; Richters, T.; Shanmugam, N.; Trebicka, E.; Boekhorst, J.; Timmerman, H.; Roelofs, R.; Wiegerinck, E.; Laarakkers, C.; et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur. J. Immunol. 2015, 45, 2553–2567. [Google Scholar] [CrossRef] [Green Version]
- Kortman, G.; Dutilh, B.; Maathuis, A.; Engelke, U.; Boekhorst, J.; Keegan, K.; Nielsen, F.; Betley, J.; Weir, J.; Kingsbury, Z.; et al. Microbial metabolism shifts towards an adverse profile with supplementary iron in the TIM-2 in vitro model of the human colon. Front. Microbiol. 2016, 6, 1481. [Google Scholar] [CrossRef] [Green Version]
- Runyen-Janecky, L.J. Role and regulation of heme iron acquisition in gram-negative pathogens. Front. Cell Infect. Microbiol. 2013, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Sousa Gerós, A.; Simmons, A.; Drakesmith, H.; Aulicino, A.; Frost, J.N. The battle for iron in enteric infections. Immunology 2020, 161, 186–199. [Google Scholar] [CrossRef]
- Archibald, F. Lactobacillus Plantarum, an Organism Not Requiring Iron. FEMS Microbiol. Lett. 1983, 19, 29–32. [Google Scholar] [CrossRef]
- Duhutrel, P.; Bordat, C.; Wu, T.-D.; Zagorec, M.; GuerquinKern, J.-L.; Champomier-Vergès, M.-C. Iron sources used by the nonpathogenic lactic acid bacterium Lactobacillus sakei as revealed by electron energy loss spectroscopy and secondary-ion mass spectrometry. Appl. Environ. Microbiol. 2010, 76, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Gutierrez, P.; de Wouters, T.; Werder, J.; Chassard, C.; Lacroix, C. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front. Microbiol. 2016, 7, 1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deriu, E.; Liu, J.; Pezeshki, M.; Edwards, R.; Ochoa, R.; Contreras, H.; Libby, S.; Fang, F.; Raffatellu, M. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe 2013, 14, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdebenito, M.; Crumbliss, A.L.; Winkelmann, G.; Hantke, K. Environmental factors influence the production of enterobactin, salmochelin, aerobactin, and yersiniabactin in Escherichia coli strain Nissle 1917. Int. J. Med. Microbiol. 2006, 296, 513–520. [Google Scholar] [CrossRef]
- Nitert, M.D.; Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Anderson, G.J.; Frazer, D.M.; Callaway, L. Iron supplementation has minor effects on gut microbiota composition in overweight and obese women in early pregnancy. Br. J. Nutr. 2018, 120, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.J.G. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in south African children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Kortman, G.A.M.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef] [Green Version]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020, 31, 115–130.e6. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Moreno-Navarrete, J.M.; Fernández-Real, J. The role of iron in host–microbiota crosstalk and its effects on systemic glucose metabolism. Nat. Rev. Endocrinol. 2022, 18, 683–698. [Google Scholar] [CrossRef]
- Dostal, A.; Chassard, C.; Hilty, F.M.; Zimmermsann, M.B.; Jaeggi, T.; Rossi, S.; Lacroix, C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J. Nutr. 2012, 142, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; Molinaro, A.; Stahlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Christmas, L.M.; Li, R.; Gu, L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Bindels, L.B.; Schmaltz, R.; Neyrinck, A.M.; Cani, P.D.; Walter, J.; Ramer-Tait, A.E.; Delzenne, N.M. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: Proof of concept in germ-free versus conventionalized mice. Mol. Nutr. Food Res. 2015, 59, 1603–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-R.; Liu, X.-M.; Chen, Z.-Y.; Zhang, Y.-S.; Zhang, Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Attri, S.; Goel, G. Influence of polyphenol rich seabuckthorn berries juice on release of polyphenols and colonic microbiota on exposure to simulated human digestion model. Food Res. Int. 2018, 111, 314–323. [Google Scholar] [CrossRef]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Carielo Lima, G.; Veloso Naves, M.M. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzyński, J.; Rój, E.; Zduńczyk, Z. Chemical Composition of Blackberry Press Cake, Polyphenolic Extract, and Defatted Seeds, and Their Effects on Cecal Fermentation, Bacterial Metabolites, and Blood Lipid Profile in Rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Verna, G.; Liso, M.; Salviati, E.; Esposito, T.; Carbone, D.; Pecoraro, C.; Chieppa, M.; Campiglia, P. Hop-derived fraction rich in beta acids and prenylflavonoids regulates the inflammatory response in dendritic cells differently from quercetin: Unveiling metabolic changes by mass spectrometry-based metabolomics. Food Funct. 2021, 12, 12800–12811. [Google Scholar] [CrossRef]
- Verna, G.; Liso, M.; Cavalcanti, E.; Bianco, G.; Di Sarno, V.; Santino, A.; Campiglia, P.; Chieppa, M. Quercetin Administration Suppresses the Cytokine Storm in Myeloid and Plasmacytoid Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 8349. [Google Scholar] [CrossRef]
- Hatcher, H.C.; Singh, R.N.; Torti, F.M.; Torti, S.V. Synthetic and natural iron chelators: Therapeutic potential and clinical use. Future Med. Chem. 2009, 1, 1643–1670. [Google Scholar] [CrossRef] [Green Version]
- Ohara, T.; Tomono, Y.; Boyi, X.; Yingfu, S.; Omori, K.; Matsukawa, A. A novel, nontoxic iron chelator, super-polyphenol, effectively induces apoptosis in human cancer cell lines. Oncotarget 2018, 9, 32751–32760. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants 2023, 12, 630. https://doi.org/10.3390/antiox12030630
Scarano A, Laddomada B, Blando F, De Santis S, Verna G, Chieppa M, Santino A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants. 2023; 12(3):630. https://doi.org/10.3390/antiox12030630
Chicago/Turabian StyleScarano, Aurelia, Barbara Laddomada, Federica Blando, Stefania De Santis, Giulio Verna, Marcello Chieppa, and Angelo Santino. 2023. "The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota" Antioxidants 12, no. 3: 630. https://doi.org/10.3390/antiox12030630
APA StyleScarano, A., Laddomada, B., Blando, F., De Santis, S., Verna, G., Chieppa, M., & Santino, A. (2023). The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants, 12(3), 630. https://doi.org/10.3390/antiox12030630











